Back to Journals » Journal of Pain Research » Volume 12
Effectiveness and tolerability of THC:CBD oromucosal spray as add-on measure in patients with severe chronic pain: analysis of 12-week open-label real-world data provided by the German Pain e-Registry
Authors Ueberall MA , Essner U , Mueller-Schwefe GHH
Received 25 October 2018
Accepted for publication 18 February 2019
Published 20 May 2019 Volume 2019:12 Pages 1577—1604
DOI https://doi.org/10.2147/JPR.S192174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Michael A Ueberall,1 Ute Essner,2 Gerhard HH Mueller-Schwefe3
1Institute of Neurological Sciences, 90411 Nuernberg, Germany; 2O.Meany Consultancy, 22339 Hamburg, Germany; 3Interdisciplinary Centre for Pain and Palliative Care Medicine, 73033 Goeppingen, Germany
Objective: To evaluate effectiveness, tolerability and safety of an oromucosal spray containing Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), as add-on treatment in patients with severe chronic pain (SCP).
Methods: Exploratory analysis of anonymized 12-week routine/open-label data provided by the German Pain e-Registry (GPR) on adult SCP patients treated with THC:CBD oromucosal spray in 2017.
Results: Among those 30.228 cases documented in the GPR in 2017, 800 (2.6%; 57% female, mean ± SD age: 46.3±9.7 years) received a treatment with THC:CBD. All patients fulfilled the legislative preconditions for a treatment with cannabis as medicine as defined by the German Act Amending Narcotics and Other Regulations. THC:CBD-treatment was followed by an aggregated nine-factor symptom relief (ASR-9) improvement at end of week 12 vs baseline of 39.0±26.5% (95%-CI: 36.9–41.1, median: 42, range −41 to 85). A full ASR-9 response (ie, a 50%-improvement in all 9 factors) was found for 123 patients (15.4%), while 488 patients (56.0%) presented with an ≥50% improvement in at least 5 of 9 ASR factors. With a 54.9±17.2% (median: 56%, range: −6 to 85) improvement was significantly superior in the neuropathic pain subgroup (n=497, 62.1%) vs those with mixed (n=249, 31.1%; ASR-9: 18.2±12.0, median: 19, range: −12 to 42%) or nociceptive pain (n=54, 6.8%; ASR-9: −11.9±10.5, median: −11, range: −41% to 12%; p<0.001 for each). 159 patients (19.9%) reported at least one of 206 TEAEs, most of them of mild intensity (n=81.6%). Most frequently reported TEAEs were increased appetite (n=50, 6.3%) and dysgeusia (n=23, 2.9%). TEAE-related discontinuations were reported for 32 patients (4.0%). 113 (14.1%) patients discontinued due to inadequate pain relief, most of them with nociceptive pain (n=40, 74.1%), least with neuropathic pain (n=1, 0.2%; p<0.001).
Conclusion: THC:CBD oromucosal spray proved to be an effective and well-tolerated add-on treatment for patients with elsewhere refractory chronic pain – especially of neuropathic origin.
Keywords: THC:CBD spray, add-on treatment, severe chronic pain, neuropathic pain, retrospective analysis, German pain e-Registry
Background
Chronic pain (CP) is a long-lasting unpleasant sensorial and emotional experience without the acute warning signals of physiological nociception1 that persists past normal healing time2 and usually lasts or recurs for more than 3 months.3 It is a common and economically important health issue, affecting approximately 20% of people worldwide4–6 and accounting accordingly for 15–20% of medical consultations.7,8
Its phenomenological occurrence with symptoms suggesting the pathophysiological participation of neuropathic processes represents the most common disorder of the peripheral as well as central nervous system. It affects approximately one in forty adults in the general population9 and between 20% and 35% of CP patients.10 Among the group of CP patients, those where nerves are damaged or neuropathic mechanisms are engaged, typically report higher pain intensity scores as well as more and especially more severe disabling pain-related restrictions with respect to daily life activities, social relationships, psychological well-being, and quality-of-life as compared to those suffering from so-called nociceptive pain.11,12
A multitude of treatment guidelines have been developed worldwide aiming to enhance CP management. Despite the recommendation of a broad spectrum of non-pharmacologic strategies and the increased application of multimodal strategies and interdisciplinary approaches incorporating medical, psychosocial, physiotherapeutic, and other disciplines,13–15 pharmacologic treatments still constitute the backbone of the general management. Commended measures for CP usually range from paracetamol, NSAIDs, and opioids, reflecting the stepwise analgesic pain ladder approach of the WHO, supplemented by muscle relaxants in case of a proven increase in muscle tone, and adjuvant agents (eg, tricyclic antidepressants, selective serotonin-norepinephrine reuptake inhibitors, or Ca2+-channel modulating antiepileptic agents) if CP patients present with clinical signs suggestive for a neuropathic component (NC).16–22
However, regardless of its underlying etiology, recommended first and second-line medications as well as nonpharmacological counter measures do not always result in a satisfactory symptom improvement. Additionally, the abundant prescription of opioids and the prolonged use of anti-inflammatory drugs such as nonsteroidal agents (NSAIDs) or selective cox-2 inhibitors for CP have become highly controversial due to their large potential for ab-/misuse and their limited long-term safety. Consequently, CP frequently persists despite all these established measures and even despite additional complementary medicine approaches (such as acupuncture, osteopathy, traditional Chinese medicine, etc.) and finally evolved into one of the major reasons for clinically relevant restrictions with respect to the daily life activities and quality-of-life in industrialized countries, highlighting the need for alternative third-line approaches for those patients with severe CP in whom all other approaches failed.23,24
In response to these (and other) medical challenges, the Federal Parliament adopted the Act Amending Narcotics and Other Regulations25 – based on a proposal developed by the Health Minister and approved by the German Cabinet, which took effect on March 10, 2017. This law bypassed established drug regulatory procedures and entitles physicians to prescribe cannabis-based medicines (CBMs) [eg, dried cannabis flowers, plant-based cannabinoid extracts (such as tetrahydrocannabinol (THC), cannabidiol (CBD), and combination products), as well as synthetic THC analogues] independent of their formal approval status for the no-/off-label medical use in patients suffering from severe diseases according to the definitions given in Code V of the Social Law, and obligates statutory health insurances to reimburse all costs associated with these prescriptions for patients resistant to other treatments.
The available scientific evidence of CBMs for CP supporting this concerted action of German politicians (that transcended any party lines) was extremely low.26 Current systematic reviews and meta-analyses on CBMs underlined the persistent tentativeness if CBMs improve pain and report rather low-quality evidence – confounded by the fact that different cannabinoid-based products with variable purities, doses, and routes are often blended.27,28 Among all currently available CBMs, the oromucosal spray containing Δ9-THC and CBD, is the scientifically best evaluated CBM for CP and presented limited, but fair evidence for a beneficial effect in the treatment of CP (especially of neuropathic origin), while none of the other CBMs showed any conclusive scientific data that support their use for CP patients.29–32 Moreover, it is approved across the European Union (EU) for multiple sclerosis (MS) spasticity management, has a standardized pharmaceutical production and a well-defined and acceptable tolerability and safety profile.33
As CBMs, in general, enjoy an increasing popularity among physicians, the German Pain Association and the German Pain League initiated the present non-interventional evaluation of routine data provided by the German Pain e-Registry (GPR) focussing on patients with severe CP refractory to other analgesics to gain deeper insight into the differential effects and the risks and benefits of the legalized use of the THC:CBD oromucosal spray under real-world conditions. Objectives of this analysis were to assess 1) the analgesic effects by using a composite responder definition that incorporates response- and relief-rates of a combination of nine different patient-reported/relevant parameters (such as pain intensity, pain-related disabilities with respect to daily life activities, sleep, overall well-being, physical and mental quality-of-life, depression, anxiety and stress), and 2) to evaluate prevalence and spectrum of treatment-emergent adverse events (TEAEs) in response to THC:CBD as add-on treatment to pre-existing analgesic medications. Due to the fact that neuropathic pain seemed to be an entity in which the analgesic effects of THC:CBD are superior to those seen with other pain types, effectiveness evaluation focused especially on the differential effects in patients with a “nociceptive”, “mixed”, or “neuropathic” pain phenotype as categorized by their clinical pain phenomenology (and independent of the original pain diagnoses).
Methods
Study design
This is a non-interventional cohort study of all CP patients who started a treatment with THC:CBD oromucosal spray as part of routine care. Anonymized real-world data of the GPR – a national web-based pain treatment registry developed by the Institute of Neurological Sciences on behalf of the German Pain Association – were analyzed that have originally been prospectively sampled for routine care purposes. Data were entered by using electronic case report forms as provided by the GPR and the related online documentation service iDocLive®.
The GPR has been developed to provide patients and physicians with a standardized electronic documentation program to gather and evaluate patient-reported information on their demography, history, pretreatment, pain characteristics, treatment response, etc. in daily practice. Data were prospectively self-documented by patients as part of routine use of the electronic documentation tools provided by this online service and supplemented by related physician information where appropriate and needed.
Patient questionnaires provided by this system were those recommended by the German Pain Association, the German Pain Society and the German Pain League and covered a broad spectrum of validated instruments addressing amongst other parameters such as the stage of pain chronification (on basis of the Mainz Pain Staging System) and the severity of pain (with the von Korff questionnaire), pain phenomenology, pain intensity, pain-related disabilities in daily life, quality-of-life, overall well-being, depression, anxiety and stress, as well as data on treatment and treatment-related adverse events, etc.34,35
There was no formal sample size calculation for this analysis. All patient data sets for whom a treatment with the study medication have been newly initiated between March 10 and December 31, 2017 were selected for this analysis. Treatment initiation was defined as no study medication use in the prior 12 weeks, and the date of first dose of the THC:CBD oromucosal spray was set as the starting date for the definition of the 12-week data evaluation period. Based on that sample, a blinded endpoints analysis has been performed aiming towards a combination of several patient-reported effectiveness endpoints known to be important for patients. Analgesic treatment with THC:CBD followed medical requirements according to the previous decision of the participating physicians and based exclusively on individual patient needs without any external specifications.
After the baseline evaluation (ie, prior onset of treatment with the newly prescribed medication), patients completed standardized pain diaries on a weekly basis and provided information on their current health status and their response to the medication using the GPR via the web application iDocLive®. No predefined study visits were scheduled, and interim visits were possible at any times according to individual patient needs and/or established routines (eg, if patients had to be closely monitored due to commencement of treatment, inadequate pain control, tolerability issues, and/or adverse events).
Study medication
Study medication of this analysis is Sativex (ATC Code: N02BG10),33 an oromucosal spray that contains a standardized, fixed (nearly) 1:1 quotient of Δ9-THC and CBD derived from strains of Cannabis sativa plants developed to produce high and reproducible yields of both cannabinoids in a liquid with ethanol, propylene glycol, and peppermint oil flavoring. Originally developed and currently approved in 31 countries for the add-on treatment of adults with moderate to severe spasticity due to MS, this THC:CBD oromucosal spray is so far the CBM with the best scientific evidence for CP and the only pharmaceutical industry drug product carrying the cannabinoid therapeutic principle with a regulatory approval in Canada and Israel for neuropathic pain in MS and the treatment of adult cancer patients with opioid-refractory pain. While THC exerts beneficial as well as adverse effects (such as perceptual alterations, emotional processing, delusions, hallucinations, and paranoia), as partial agonist of the cannabinoid 1 and 2 receptors (CB1R and CB2R), CBD acts probably as a positive modulator of the endocannabinoid system with the potential to negate most of the detrimental and potentiate the positive effects of THC – if given concomitantly.36–43
The THC:CBD oromucosal spray allows patients an easy, flexible, and individualized self-titration according to their response to, and tolerance of, the medication – one of the peculiarities of this CBM qualifying it for routine use. Each actuation of the spray releases 100 µl of the THC:CBD extract, containing 2.7 mg THC and 2.5 mg CBD. Optimal dosages reported for the approved indication range between 8 and 12 sprays (ie, 22–32 mg THC and 20–30 mg CBD) per day and are usually reached during a titration phase of 2–4 weeks. However, in this evaluation, patients received THC:CBD in course of routine clinical practice for a non-approved (off-label) indication (according to the national cannabis prescribing legislation) and initial dosing as well as further dose adjustments were done at the discretion of the physician and due to the individual patient needs, but not necessarily according to the recommendations given in the product information for the approved indication.
Pain type differentiation
Pain phenotype was evaluated with the modified 7-item version of the validated Pain Detect questionnaire (PDQ7).44 The PDQ7 consists of seven individual items covering positive as well as negative cardinal symptoms of neuropathic pain and asks patients to record the intensity of each individual item based on a 6-grade Likert-like scale [ranging from “none” (0) to “extreme” (5)] and clinical pain phenomenology was classified as either “nociceptive” (in case of a PDQ7 score of ≤10 at baseline), “mixed” (with scores 11–18), and “neuropathic” (with scores ≥19). This evaluative approach has been specifically chosen, as latest research on neuropathic pain recommended that treatments should primarily be based on the underlying pathophysiology and clinical appearance instead of formal ICD-10 diagnoses or etiology.45
Study assessments
Effectiveness evaluation
Assessment of the effectiveness of THC-CBD based solely on patient perceptions and based on patient-reported information on pain intensity, pain-related disabilities in daily life activities/functionality, sleep, overall well-being, quality-of-life and related psychological factors (eg, depression, anxiety, and stress). Pain intensity measures based on the pain intensity index (PIX), calculated as arithmetic mean of the lowest, average and highest 24-hr pain intensities reported by patients on basis of an 100 mm VAS (0=“no pain” and 100=“worst pain conceivable”). CP-related disabilities in daily life were assessed with a modified version of the pain disability index (mPDI), which recorded the degree of functional restrictions in daily life activities on the basis of an 100 mm VAS (with 0=“none” and 100=“worst conceivable”) with respect to seven distinct domains (related to “home and family activities”, “recreation”, “social activities”, “occupation”, “self-care/personal maintenance”, “sleep”, and “overall QoL”).46,47 Quality of sleep was evaluated on the basis of the respective mPDI subdomain. Quality-of-life was measured using the physical and mental component summary (PCS/MCS) of the SF-12 Health Survey version 2.48 The short form of the Depression Anxiety and Stress self-report Scale (DASS-21)49 was used to evaluate the response of these comorbidities to the THC:CBD treatment and the Marburg Questionnaire on Habitual Health Findings (MQHHF) to assess subjective overall well-being.50 Further efficacy endpoints were the global impression of change assessed by the patient, using the seven-grade Patient Global Impression of Change (PGI-C) scale51 (a 7-point Likert-type scale ranging from “very much better” to “very much worse”). The assessment of the overall efficacy of the study treatment based on the six-grade German school mark (VRS6: very good, good, satisfying, adequate, inadequate, insufficient), and the subjective feeling of CP patients to relief their pain with THC:CBD based on a five-grade verbal rating scale (ranging from “very strong” to “none”) that is part of the quality-of-life impairment by pain inventory of the German Pain Questionnaire.34,35
Safety and tolerability measures
Safety analyses based on TEAE reporting’s, collected via respective patient questionnaires provided by the GPR. For this evaluation, TEAEs were defined as any untoward medical occurrence reported by a patient receiving study medication and did not necessarily confirm a causal relationship with the treatment under evaluation.
Concomitant medication
Since patients were treated according to their individual needs, no specifications were in place on the use of concomitant medications. Physicians could prescribe, and patients could take any medications and/or non-pharmacological measures necessary to provide adequate supportive care for any condition required. All changes in analgesic medication were analyzed at end of the 12-week evaluation period vs baseline, to evaluate their influence on pain intensity and pain-related factors of interest in this study.
Statistical analysis
The aim of this non-interventional treatment evaluation was to gain further insight into the effectiveness and tolerability of the THC:CBD oromucosal spray for CP treatment under real-life conditions and to correlate treatment response with the clinical pain phenomenology (nociceptive vs mixed vs neuropathic). Primary criteria for this effectiveness evaluation were the treatment contrasts for an aggregated nine-factor symptom relief score (ASR-9) in patients who recorded a nociceptive vs mixed vs neuropathic pain phenomenology. Both, the frequency of patients reporting at least a ≥50% relief vs baseline in any of the nine different efficacy endpoints important for a successful treatment under real-life conditions [pain intensity (PIX), pain-related daily life disabilities (mPDI), sleep (subdomain #6 of the mPDI), overall wellbeing (MQHHF), physical and mental quality-of-life (SF12-PCS and -MCS), depression, anxiety and stress (each assessed with the DASS)] as well as the relative average symptom relief over all ASR-9 dimensions (in percent vs baseline) have been evaluated.
The primary effectiveness endpoint of this analysis was the average symptom relief in response to the study medication based on the ASR-9. Secondary endpoints were the percentage of respondents – ie, patients who showed at least a 50% symptom relief vs baseline in any of the ASR-9 dimension. Primary safety endpoints were the TEAE spectrum and the proportion of TEAE-related treatment discontinuations.
Data analyses were performed for the complete set of anonymized data provided by the GPR for patients fulfilling the criteria mentioned above and followed a modified intent-to-treat approach as any patients who (1) took at least one dose of the study medication and (2) had at least one post-baseline/post-dose measure were evaluated. Linear interpolation was used to impute intermittent missing scores and the conservative last observation carried forward method to impute missing scores after early discontinuation. The corresponding completed data set built the basis for all primary and secondary endpoint analyses.
Descriptive and inferential statistical analyses were performed. For continuous variables, descriptive statistics were summarized by the number of patients (n), the mean, SD, 95% CIs of the mean, median, and range (minimum–maximum) values. For categorical and ordinal variables data were summarized by frequency number (n) and percentage (%) of participants in each category; where appropriate, 95%CIs were added. For between groups comparisons of continuous/categorical variables, Student t/Pearson’s chi-squared tests were used. For within-group (eg, pre-post) comparisons paired samples t-tests were performed. All statistical tests were carried out using a two-sided significance level of 0.05. Since all comparisons, except those for the primary endpoint, were considered secondary, respective analyses were classified as exploratory and subsequently not adjusted for multiplicity.
Ethics
This non-interventional treatment evaluation was conducted in accordance with the principles of the Declaration of Helsinki, conformed to relevant national and regulatory requirements and approved by the independent ethics committee of the German Pain Association. All patients provided written informed consent prior participation in the GPR and this study was registered in the electronic data base of the European Medicine Agency for non-interventional studies (ENCEPP: EUPAS 25,799). All analyses were performed with anonymized data to comply with national guidelines on protection of data privacy and the EU General Data Protection Regulation. Data selection based on a temporary selection key list as defined by the initiation date of the studied treatment.
Results
Patient disposition
In 2017, 30,228 pain patients actively participated in the GPR and used 13,946,222 validated documentation tools (on average 461.4 per patient) to report on their pain problems and their response to treatments. 1,224 patients, 4.1% of the registry population, recorded a treatment with any type of CBM and 800 of them (65.4%) with THC-CBD oromucosal spray, formally fulfilling the inclusion criteria of this evaluation. The 12-week attrition rate was 18.1% (n=145). Most patients prematurely discontinued their THC:CBD treatment due to an insufficient analgesic efficacy, only 4% (n=32) reported discontinuation in response to a TEAE (see Figure 1).
 | Figure 1 Patient disposition.Abbreviations: THC, Δ9-tetrahydrocannabinol; CBD, cannabidiol; TEAE, treatment-emergent adverse event; Efficacy↓, inadequate analgesic efficacy. |
Missing/imputed data
Overall, 7.2% of the data evaluated for this analysis had to be imputed to complete missing information due to premature treatment discontinuations and/or incomplete data entries.
Baseline characteristics
Demographics and baseline data characterized a group of patients suffering from severe CP (see Tables 1 and 2). Mean age (±SD) was 46.3±9.7 (median: 47, range: 19–77) years and 57.0% (456/800) were female. Average pain duration was 1,002±692.3 (median: 911, range: 150–2,580) days. With 87.5% (700/800) of the sample prevalence, nine out of ten patients suffered for longer than 6 months and with 67.9% (543/800) nearly seven out of ten for longer than 12 months prior baseline. On average, patients were treated by 8.7±1.4 (median: 9, range: 3–13) physicians and reported an analgesic pre-treatment with 9.7±2.3 (median: 10, range: 4–17) analgesic medications. With 51.0%, more than half of patients recorded 10 or more pain treatments. Non-opioid analgesics were the most frequently used treatments, reported by 99.8% (n=798/800) of patient’s prior study medication, followed by antidepressants (88.0%, n=704/800), strong opioids (86.9%, n=695/800), muscle relaxants (72.5%, n=580/800), mild opioid analgesics (70.9%, n=567/800), and antiepileptic agents (65.5%, n=525/800). With 87.6% (n=701/800), nearly nine of ten patients recorded five or more non-pharmacological pain treatments, most prevalently transcutaneous electric nerve stimulation (TENS; 79.4%), acupuncture (79.3%), physiotherapy (77.6%), and psychological measures (75.5%).
 | Table 1 Patient demographics |
 | Table 2 Patient baseline characteristics |
On average patients recorded 3.6±1.9 (median: 3, range: 1–12) concomitant diseases, most prevalently allergies (47.9%) and cardiovascular problems (44.0%), and 110 subjects (13.8%) suffered from cancer, not always painful (31/110, see Table 1). Due to these comorbidities, patients took on average 2.0±1.3 (median: 3, range: 1–12) pharmacological non-pain management treatments.
The spectrum of diagnoses given for the conditions underlying CP was broad and ranged from (low) back pain, reported by 29.3% (n=234/800), failed back surgery syndrome (18.5%, n=148/800), and shoulder/neck pain (11.4%) to osteoarthritis (1.9%), phantom pain (2.4%), peripheral nerve lesions (2.8%) and fibromyalgia (3.3%). Only 31 of the 110 cancer patients registered (28.2%) took THC:CBD for cancer-related pain. Overall, 248 patients (31.0%) reported conditions and ICD-10 diagnoses usually categorized as neuropathic, 446 patients (55.8%) noted diagnoses usually associated with a mixed pain phenomenology, and only 15 (1.9%) presented with typical nociceptive pain diagnoses.
Average PDQ7 scores at baseline were 19.0±5.4 (median: 19) and ranged from 0 to 35. With 62.1% (497/800), six out of 10 patients presented with PDQ7 scores ≥19 (and suffered therefore per definition from a neuropathic pain at baseline), while 31.1% (n=249/800) scored 11–18 (and their pain was therefore formally classified as of “mixed” or “unclear” pathophysiology), and the remaining 6.8% (n=54/800) scored equal to or less than 10 (and suffered therefore per PDQ7 definition from a “nociceptive” type of CP). Pain type phenomenology assessed via PDQ7 and diagnoses given as underlying cause for CP showed only a minor correlation, supporting our concept to rely on PDQ7 scores and related phenomenological pain clusters instead of conventional diagnoses.
With 58.5% (n=461/800) six out of ten patients presented with an advanced stage of pain chronification (stage III) according to the Mainz Pain Staging System, and 93.1% (n=745/800) suffered from a high disability with either moderate (grade III; n=284/800, 35.5%) or even severe (grade IV; n=461/800, 57.6%) limitations according to the von Korff pain grading scale. Baseline pain scores for lowest, average and highest 24-hr. pain intensities were 18.6±18.8 (median: 12), 48.6±20.4 (median: 48), as well as 73.3±22.1 (median: 80) mm VAS, and the corresponding 24-hr. PIX score was 46.8±16.2 (median: 48) mm VAS. Pain related disabilities in daily life were recorded to be 66.2±17.4 (median 68) mm VAS on average and 78.3% of patients presented with mPDI scores of ≥50 mm VAS, indicating severe restrictions. Pain-related sleep problems were documented to be 66.3±23.0 (median: 69) mm VAS and seven of ten patients (70.1%) reported significant pain-related sleep problems. Overall well-being was significantly impaired in 78.3% and 78.6/54.0% of patients presented with significant pain-related impairments of their physical/mental quality of life. According to the DASS-21 scale, average depression, anxiety, and stress scores were 16.5±4.1, 14.4±4.5, and 17.9±2.1 corresponding to 83.0%, 86.6%, and 68.4% of patients with extreme scores for the respective items.
THC:CBD dose titration and dose exposure
On average patients started their THC:CBD treatment as add-on to other ongoing analgesic medications with 2.6±0.7 (median: 3, range: 1–4) sprays per day and titrated slowly upwards until they reached a plateau at the end of week 9 with 7.1±1.4 (median: 7, range: 3–11) sprays per day (see Figure 2). Average daily dose exposure on treatment day 1 was 7.0±1.9 (median: 8.1, range: 2.7–10.8) mg THC and 6.5±1.8 (median: 7.5, range: 2.5–10.0) mg CBD, which increased up to 19.2±3.8 (median: 18.9, range: 8.1–29.7) mg THC and 17.8±3.5 (median: 17.5, range: 7.5–27.5) mg CBD at end of treatment week 12. Corresponding cumulative dose exposure over the whole 12-week evaluation period was 1,143.5±479.0 (median: 1,266, range: 56.7–2,135.7) mg THC and 1,058.8±443.5 (median: 1,173, range: 52.5–1,977.5) mg CBD.
Treatment response
Overall pain and pain-related symptoms relief
THC:CBD treatment triggered a significant pain intensity relief (see Figure 3). LPI/API/HPI dropped from 18.6±18.8/48.6±20.4/73.3±22.1 (median: 12/48/80) mm VAS at baseline to 8.9±15.9/22.5±19.8/28.9±24.6 (median: 0/17/24) mm VAS at end of treatment week 12 (p<0.001 for each intensity). In parallel, the 24-hr. PIX showed an absolute improvement of −26.8±17.2 (median: −27) mm VAS at end of observation vs baseline, corresponding to a relative pain intensity relief of 57.3±36.3 (median: −64) percent. Proportion of patients recording a pain intensity improvement of 50% or more vs baseline increased from 13.1% at end of week 1–67.5% at end of week 12. Corresponding ≥30/70% pain relief response rates at end of week 12 were 82.3/43.3%.
The degree of THC:CBD-related change of the 24-hr PIX varied with the clinical pain phenomenology (see Figure 4). While patients with neuropathic CP recorded on average an absolute improvement of −33.6±14.1 mm VAS vs baseline (corresponding to a relative change of −75.5±21.8%; p<0.001), and those suffering from mixed CP an improvement of −14.2±10.5 mm VAS/-28.4±19.5%, those with a nociceptive type of CP presented with an 8.5±12.4 mm VAS a 27.2±42.7% deterioration under THC:CBD treatment. Consequently, the differential evaluation of patients with different pain phenomenology according to PDQ7 revealed significant differences with respect to the ≥50% PIX response, with highest rates for those patients suffering from PDQ7 defined neuropathic pain (94.8%), in comparison to those with a mixed (24.9%, OR: 54.6, 95%-CI: 33.5–89.0; RR: 3.8; p<0.001; positive/negative predictive value: 75.1/94.8%) or nociceptive pain (13.0%; OR: 121.6, 95%-CI: 50.1–295.2; RR: 7.3; p<0.001; positive/negative predictive value: 87.0/94.8%).
Corresponding ≥50% improvement rates were also found for all remaining eight factors of the ASR-9 (see Figure 5). The range of nociceptive pain patients who presented an at least 50% improvement vs baseline for any of the nine factors of the ASR-9 ranged from 3.7% (for sleep and mental quality-of-life) to 14.8% (for depression) and contrasts significantly vs those seen for mixed pain CP patients – in whom the ≥50% responder rates ranged from 7.6% (for mental quality-of-life) to 14.8% (for depression), and especially those for patients presenting with neuropathic CP, who recorded ≥50% response rates between 23.7% (for mental quality-of-life) and up to 94.8% (for pain intensity and stress).
In an aligned way, the proportion of patients who recorded no ≥50% response in any of the nine ASR factors was with 90.7% (n=49/54) for patients with nociceptive CP significantly higher, compared to those presented by mixed CP (58.2%; n=145/249) and especially neuropathic CP patients (1.4%; n=7/497; p<0.001 for each comparison). While nine out of ten patients with neuropathic CP (90.5%, n=450/497) showed a ≥50% response in at least four of nine ASR factors, 91.2% (n=227/249) of mixed CP patients presented with a ≥50% response in only two or even less of ASR-9 factors, and in nociceptive CP patients nine out of ten (90.7%, n=49/54) showed absolutely no significant improvement in any of the ASR-9 items.
Dose-response analyses failed to find any significant correlation (see Figure 6). Despite a trend towards numerically higher/better average ASR-9 symptom relief scores, correlation analyses failed to prove a statistically relevant correlation between the daily number of THC:CBD sprays and the treatment response achieved (R2=0.0647). Even more important, between-group analyses based on the clinical pain phenomenology revealed absolute comparable average number of sprays per day for patients with nociceptive (6.5±1.9), mixed (6.4±1.9) and neuropathic CP (6.8±1.7) despite significant differences with respect to the achieved treatment effect.
 | Figure 6 Scatterplot of the number of THC:CBD sprays per day (X-axis) vs the aggregated nine-factor symptom relief score (ASR-9; Y-axis).Abbreviations: THC, Δ9-tetrahydrocannabinol; CBD, cannabidiol. |
Primary effectiveness endpoint
The overall sample symptom relief in response to the THC:CBD study medication based on the ASR-9 was 39.0±26.5 (median: 42, range −41 to 85) percent (see Figure 7). Overall, 123 patients (15.4%) recorded an average improvement of ≥50% at end of week 12 vs baseline with respect to all ASR-9 factors. Highest ≥50% relief rates were seen for stress (78.8%), followed by pain intensity (67.5%), depression (66.5%), overall well-being (61.3%), anxiety (57.6%), disabilities in daily life (56.3%), sleep (47.0%), physical (42.1%), and mental quality-of-life (17.4%).
The differential ASR-9 analysis with respect to the pain phenomenology revealed significant differences for all nine ASR-9 factors with highest improvement rates for patients suffering from neuropathic CP, intermediate response rates for those suffering from mixed CP and virtually no effects for patients suffering from nociceptive CP. Corresponding ASR-9 overall symptom relief/improvement scores were with 54.9±17.2 (median: 56, range: −6–85) percent for patients with neuropathic CP significantly higher, than those recorded by patients with a mixed type of CP (18.2±12.0, median: 19, range: −12% to 42%; p<0.001) or those suffering from nociceptive CP (−11.9±10.5; median: −11, range: −41% to 12%; p<0.001). Worst THC:CBD treatment-related symptom changes were recorded by nociceptive CP patients for overall well-being, mental and physical quality-of-life (worsening of −48.5%, −30.5%, and −29.3% vs baseline) (see Figure 7). In contrast to that highest improvements vs baseline were reported by patients suffering from neuropathic CP for pain intensity (75.8%), stress (72.7%), depression (72.1%), anxiety (70.0%), pain-related disabilities in daily life (69.1%), and sleep (60.3%).
A correlation analysis between the clinical pain phenomenology assessed via the PDQ7 and the ASR-9 based symptom relief score (see Figure 8) revealed a close correlation of the THC:CBD effects with the degree of neuropathic symptomatology (R2=0.7702). Maximum effects were seen for patients with PDQ7 scores above 30 (81.1±4.2, median: 82.2, range: 65.7–84.8%), lowest for patients with PDQ7 scores of ten or less (−11.9±10.5, median: −11.1, range: −41.3% to 11.6%; p<0.001).
Further effectiveness analyses
Inadequate analgesic response was the primary reason for a treatment discontinuation of THC:CBD (Figure 9). With 77.8% (n=42/54) discontinuation rates were significantly higher for patients suffering from nociceptive CP compared to those seen for patients with a mixed (32.9%, n=82/249) or neuropathic pain phenomenology (4.2%, n=21/497; p<0.001 for each comparison). Overall, 145 patients (18.1%) discontinued their treatment with THC:CBD prematurely, more than half of them (52.4%, n=76/145) within the titration phase of the first 4 weeks. Based on this data, the cumulative number of treatment days was calculated to be 64,386 days, equating to 176.4 patient years of THC:CBD exposure.
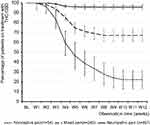 | Figure 9 Proportion of patients (percent) on THC:CBD treatment.Abbreviations: THC, Δ9-tetrahydrocannabinol; CBD, cannabidiol; BL, baseline; W1-W12, weeks 1–12. |
Corresponding to the ASR-9 response/symptom relief rates, the THC:CBD-associated subjective feeling of patients to relieve their pain differed significantly between the three pain phenomenology groups (Figure 10). While 65% (n=323/497) of patients with neuropathic CP reported that their THC:CBD-related ability to relief pain was “strong” or “very strong”, less than one third of patients with a mixed type of CP (32.5%, n=81/249) and only one of those 54 patients with nociceptive CP (1.9%) recorded a comparable capability (p<0.001 for each comparison). Furthermore, a significantly higher percentage of patients with neuropathic CP rated the overall efficacy of THC:CBD as “excellent” or “good” (66.0%, n=328/497), in comparison to those with a mixed (36.5%, n=91/249) or nociceptive type of CP (1.9%, n=1/54; p<0.001 for each comparison).
Concomitant analgesic medication
Add-on treatment with THC:CBD was followed by a significant decrease of analgesic medications (see Table 3), especially in patients with neuropathic pain of whom 61.2% (n=304/497) reported a decrease in rescue analgesics and 46.7% (n=232/497) in maintenance treatments (p<0.001 for each vs baseline) compared to 30.5/19.3% (n=76/48 of 249) with mixed (p=0.002/0.043 vs baseline) and 5.6/9.3% (n=3/5 of 54) with nociceptive pain (p=ns for each vs baseline).
 | Table 3 Summary of analgesic medications taken at baseline (ie, before) and at the end of the 12-week observation period with THC:CBD |
Overall, the number of patients using analgesic rescue medications before/with THC:CBD decreased significantly from 651 (81.4%) patients at baseline to 473 (50.1%) at end of week 12 of the observation period (p<0.001). NSAIDs and non-opioid analgesics were the rescue treatments with the highest stop rates in response to THC:CBD (41.3/41.2%), followed by mild (37.9%) and strong opioids (28.3%). Patients with neuropathic pain were significantly more often able to reduce their rescue treatments with any of the aforementioned medications (on average 40.9%) compared to those with mixed pain (18.8%) or patients with nociceptive pain (who reported an increase in 6.2%; p<0.001 for each comparison). The proportion of patients without any rescue analgesics at baseline vs end of week 12 with THC:CBD increased significantly from 15.5% to 42.7% for neuropathic pain (p<0.001), and from 23.3% to 41.0% for mixed pain (p<0.001) but remained stable for nociceptive pain (25.9% to 24.1%, p=n.s.).
In parallel, average number of analgesic maintenance treatments dropped from 3.2±1.0 at baseline to 2.8±1.2 at end of week 12 (p<0.001) with highest THC:CBD-related decrements seen in patients suffering from neuropathic (−0.5±0.6; p<0.001), vs mixed (−0.2±0.5; p=0.043), and vs nociceptive pain (+0.2±0.6; p=0.353). Highest discontinuation rates of previous analgesic maintenance treatments were reported by neuropathic pain patients for nonopioid analgesics (−30.5%), followed by strong opioids (−21.8%), mild opioids (−16.7%), and antidepressants (−13.4%). Average discontinuation rates of maintenance treatments were 16.9±6.7% for patients with neuropathic pain, and 6.4±3.5% for patients with mixed pain, whereas those with nociceptive pain reported an increased use of analgesics drugs.
Global impression of change
With 76.1%, three of four patients, with neuropathic CP (n=378/497) reported that their overall situation was “much better” or even “very much better” because of the THC:CBD treatment vs one in four patients with mixed CP (24.1%, n=60/249) and only one of 54 patients (1.9%) with nociceptive CP (p<0.001 for each comparison; Figure 11).
 | Figure 11 Patient global impression of change (PGIC) ratings for THC:CBD at end of the 12-week observation period.Abbreviations: THC, Δ9-tetrahydrocannabinol; CBD, cannabidiol. |
Tolerability analyses
Overall, THC-CBD treatment was well tolerated. As shown in Table 4, 19.1% of patients (n=159/800) reported at least one TEAE. Two or more TEAEs were reported by 5.0% (40/800) of patients. Most prevalent TEAEs were recorded by 6.6% (n=52/800) in form of a symptoms complex related to the ´bad´ taste of the THC:CBD spray such as dysgeusia (2.9%, n=23/800), pharyngo-laryngeal pain (1.8%, n=14/800), mouth discomfort (1.1%, n=9/800), and pain at the application site (0.8%, n=6/800), followed by an “increased appetite” reported by 6.3% (n=50/800). Most TEAEs were mild (81.6%) or moderate in intensity (16.5%) and either of a self-limiting nature or easily tolerated by patients in case of their persistence over time. Only four events (2 times fatigue, dysgeusia, and dizziness) were classified as severe. TEAE-related treatment discontinuations were seen in only 4.0% of patients (n=32/800), most frequently due to dysgeusia/mouth discomfort (2.3%, n=18), followed by dizziness/fatigue (each 0.8%, n=6), and somnolence (0.3%, n=2).
 | Table 4 Overall treatment-emergent adverse event (TEAE) experience |
Safety analysis
Based on the available GPR data, we found neither any evidence of abuse, continuous patterns of deliberate overdose or intentional misuse in these 800 cases evaluated. Furthermore, no patient died within the 12-week evaluation period.
Discussion
This study evaluated the effects of THC:CBD in elsewhere refractory patients with severe CP, who had a mean pain disease duration of 2.7 years, an average 24-hr pain intensity of 46.8 mm VAS, daily peak pain intensities of up to 73.3 mm VAS and significant restrictions in all aspects of their physical and mental existence despite an ongoing combination treatment with potent opioid and non-opioid as well as adjuvant analgesics. Based on our data, THC:CBD provides maximum analgesic effects in CP patients with a high neuropathic symptom load (as assessed with the PDQ7). A clinically relevant symptom relief has been also seen for patients with a so-called “mixed” pain phenomenology, however, the majority of nociceptive CP patients who presented without clinical signs or correlates of neuropathic mechanisms failed to respond and 74.1% of them stopped THC:CBD treatment prematurely as a consequence of its ineffectiveness.
Severe chronic pain (SCP) constitutes one of the most difficult-to-treat disease entities – especially if it originates in a lesion or disease of the somatosensory nociceptive system.52,53 Usually half of CP patients with neuropathic pain do not achieve a clinically meaningful pain relief from those pharmacological measures recommended by current treatment guidelines and in the majority of patients not only pain, but especially pain-related health issues persist over time and are frequently followed by a broad spectrum of psychosocial comorbidities.21,44–54,56,57 In face of that, the analgesic effects and treatment response rates due to THC:CBD oromucosal spray, presented in this analysis of real-world data provided by the GPR on a population of 800 patients suffering from severe CP, dimension the potential of this special cannabinoid as add-on measure for patients with elsewhere refractory neuropathic pain.
While there is some clinical evidence from randomized controlled trials that cannabis and its extracts provide some kind of efficacy in patients suffering from neuropathic pain, the current analysis of real-world data suggests that this efficacy is not only significant from a biometrical point of view, but even clinically relevant, as it is not only followed by a significant improvement in pain, pain-related disabilities of daily life activities, sleep and physical quality-of-life, but also translates into a significant improvement in associated psychological dimensions such as mental quality-of-life, depression, anxiety, stress and overall well-being.
This outcome is somewhat in contrast to the efficacy data of few randomized controlled trials with THC:CBD in patients with different types of neuropathic pain, which reported only minor, or even no difference to the effects seen with placebo.58–61 However, in contrast to our analysis, the definition of neuropathic pain in these studies relied primarily on the diagnosis of the original etiological/pathological condition and not – as done by us – on the clinical pain phenomenology, which is known to reflect the underlying pain mechanisms significantly better than the corresponding ICD10 diagnoses. The major problem is that although all neuropathic pain disorders are per definition characterized by a damage of the nociceptive system, the pathogeneses at the bottom of this damage are different. And even more important, subsequent sensory patterns as well as clinical symptoms (eg, the pain phenotype) may not only differ between variable etiologies, but even between individuals who experienced comparable damages.62–64 The spectrum of neuropathy-related cardinal sensory symptoms (ie, the syndrome of hyperalgesia, allodynia, dys-/paresthesia, hyperpathia and sensory loss) mirrors the underlying pathophysiology and opens in some way a diagnostic window into the underlying CP nature.64 Even though the pure clinical evaluation of reports given by patients is only a raw construct to differentiate clinically among the heterogeneity of pain mechanisms responsible for a distinct individual – even if it grounds (as in our case) on validated self-assessment tools, the close relationship between the observed analgesic effects of THC:CBD with the quantitative sensory correlates of the underlying neuropathic pain mechanisms (as assessed with the PDQ7 scores) – as shown in our analyses – supports the rationale for specific treatment approaches aligned towards mechanisms rather than diseases or ICD-10 diagnoses.45,65–68
To the knowledge of the authors, a composite response definition such as those chosen in this analysis to reflect the complexity of daily life considerations has never been used before to evaluate THC:CBD-related treatment effects observed with routine data gathered under real-life conditions. For this reason, our rationale for the choice of the response criteria underlying our primary ASR-9 endpoint deserves some discussion. The combined consideration of different factors addressing different treatment efficacy aspects – something common in daily routine care for pain patients – has not gained much attention in clinical trials. Reasons for that are few methodological concerns pointed to by authorities and scientists, most of them focusing on a potential bias caused by competing risks between different outcomes in different endpoints. Therefore, it is recommended to analyze not only the composite endpoint, but also all its components separately to reassure that the treatment under evaluation affects virtually all components and not only just a single outcome69,70 – a postulate fulfilled by our current analysis showing no negative parameter compensation by positive patterns.
From a practical point of view, the evaluation of treatment effects via a composite endpoint is warranted if the individual components are clinically meaningful and of comparable relevance for the patient and its health problem, as well as the anticipated effects connatural.71–73 Due to the fact that CP is not only a symptom but also a crucial source of functional disability, sleep problems, restrictions in physical and mental quality-of-life and psychological comorbidities such as depression, anxiety and especially stress, we found that the combination of all of these nine dimensions important to define the response for the evaluation of routine data gathered under real-life conditions. Separate analyses for each of these nine efficacy endpoints as well as each individual component of the aggregated nine-factor symptom relief score (ASR-9) provided detailed insight into the effects of THC:CBD for patients suffering from CP. All components and the responder definitions used were clinically meaningful, considered daily life procedures and facilitated the combined evaluation for individual patients reflecting real-world processes.
Overall, and irrespective of the underlying pain mechanisms, THC:CBD treatment was well tolerated. Its side effects/tolerability profile was good, and no safety signals were identified. Most prevalent TEAEs reported for THC:CBD were related to the “bad” taste of the THC:CBD spray (noted by 6.6%) and “increased appetite” (reported by 6.3%). Dizziness and fatigue – the TEAEs most frequently noted in randomized controlled trials with the THC:CBD spray74–84 were reported only by 1.3% and 1.0% of patients, reflecting differences either with respect to the patient populations studied as well as to the reporting modalities between RCTs and registry studies. Importantly, no adverse effects related to cognitive impairment, inadequate effects on mood or suicidality, suicidal behavior, and/or suicidal ideation were observed, supporting the findings from a previous long-term RCT77 as well as those from registry analyses of patients with various indications treated with THC:CBD in the United Kingdom, Germany, and Switzerland82 or Italy.86 Analysis of the ASR-9 efficacy scores revealed a minor worsening of the anxiety scores of the DASS-21 scale in patients with nociceptive CP (−10.2% vs baseline). However, from our point of view this change (as well as those seen for overall well-being and physical/mental quality-of-life) must be primarily seen as a reaction to the inadequate analgesic effects and the corresponding persistence of pain and pain-related disabilities in daily life activities among those non-responders, but not as a specific THC:CBE-related side effect.
In general, TEAE prevalence was lower in our analysis compared to those reported by randomized controlled trials. However, in contrast to these RCTs, the assessment of side effect data in the GPR based solely on spontaneous self-reports of patients, known to account not only for a significantly lower percentage of adverse effect instances reported, but also to cover primarily more relevant or more debilitating events in comparison to assessments with standardized adverse event questionnaires in clinical trials – non-interventional in nature or not.87 The majority of TEAEs documented in our dataset were mild or moderate in intensity, resolved spontaneously without specific counter measures or were well tolerated in case of their persistence over time. Overall, only 4% of patients stopped the THC:CBD treatment because of some adverse effects.
Dose titration was significantly longer in this population, than those reported/recommended for the approved indication. According to the documented data, optimal daily dosages were reached until 8 weeks after treatment initiation without major dose adjustments or any tendency for non-treatment effect-related dose increments thereafter. Daily THC:CBD dosages in short-term RCTs for MS reported a dosage range between 8.3 and 9.4 sprays per day,63–65 while longer-term open-label studies and those incorporating also non-MS patients tended towards lower dosages in the range of 4–7.6 sprays per day,66–71 which correspond nicely with the average dosages found in our analysis of the registry data and which seem to be more typical for the longer-term treatment in general.
Like the findings in MS spasticity studies, none of our dose-response analyses revealed a relevant correlation between distinct dosages or dosage ranges and specific response rates. Therapeutic efficacy was mainly driven by the underlying pain symptomatology and individual response to cannabinoids within the therapeutic window and not by distinct THC:CBD dosages. In front of this, patients should initiate THC:CBD add-on treatment with one spray per day and should then be recommended to increase their daily dose gradually dependent on their individual efficacy and tolerability threshold, but not faster than every third to fourth day – as slower up-titration regimens were generally associated with a significant lower number of adverse events.75,76 If clinically meaningful beneficial effects occur and further dose increments show no additional effects or are followed by adverse events, daily dose should be reduced to the number of sprays with the best effect and without any side effects. If patients experience only minor benefits but relevant side effects with increasing dosages, treatment should be reduced to the best tolerated number of sprays (even if it is only one or two) and kept stable for 2–4 weeks, as there is some rationale found in our study that efficacy increases over time – even with lowest dosages. However, if patients undergo no beneficial effects in response to THC:CBD (even after the waiting time mentioned before) they should be recommended to stop treatment.
The analgesic effects experienced with THC:CBD allowed a significant proportion of patients to reduce otherwise ineffective maintenance treatments as well as critical rescue medications, such as opioid analgesics (which carry the risk of addiction and drug dependence) and/or NSAIDs (known to be associated among others with gastrointestinal bleeding, cardiovascular dysfunction, hypertension, myocardial infarction, stroke, and renal failure). It can be speculated that these treatment discontinuation rates seen at the end of the 12-week observation period further increase over time, due to the increasing confidence of patients that THC:CBD provides an analgesic effectiveness previously not seen with their conventional analgesic multidrug regimens.
Strengths and limitations
Since this treatment effect evaluation based on observational and open-label real-world data gathered via an electronic treatment registry as part of daily routine care, several limitations should be considered.
The most obvious limitation of this analysis is the lack of a control (active or placebo) group (responsible for our inability to differentiate between treatment effects that are the consequence of the study treatment vs those due to other unrecognized reasons) and the fact that entering data into the GPR requires the active participation of physician and pain treatment centers and the implementation of the online documentation service iDocLive® as part of routine care (which might result in some kind of selection bias). While the lack of a placebo/active control and the restricted ability to correlate treatment effects with specific measures are salient features in daily life evaluations of routine medical care and the subsequent problems of internal vs external validity are intensively discussed between specialists representing the bench and the bedside view of the problem, analyses of non-representative or artificial data samples might significantly impair the portability of the results obtained. However, the 572 physicians, 616 non-medicinal specialists and 136 centers constituting the GPR-network represent the whole spectrum of medical and associated disciplines involved in pain management and are homogeneously distributed among Germany, representing about one-fourth of all pain centers in the country, with different sizes and settings (urban, rural) – hence minimizing the risk of geographical or other systemic patient selection biases. All participants were board certified pain specialists, well experienced with pharmacological and non-pharmacological measures and their differential use in patients with CP. This special qualification is probably the reason for the overall low attrition rate as well as the high percentage of data collected, and scales completed and should be kept in mind when THC:CBD treatment strategies are adopted by less experienced physicians.
Patient selection and treatment decisions based solely on the discretion of the physicians and their clinical judgments, eliminating any form of selection bias as far as possible. Nevertheless, it should be considered that CP patients treated by these specialists may differ from (probably less complex) patients who consult general practitioners or other primary care physicians, before the results of this evaluation are transcribed to the general population. However, the different response rates between different pain phenotypes, with no effect in nociceptive pain patients and limited in the mixed pain patients compared to the improvements seen for neuropathic CP patients could be considered as an indirect control and reflect reduced or no placebo effect in absence of a verum effect.
Another potential limitation of this analysis was the inclusion of multiple CP etiologies resulting in a considerable data heterogeneity. However, sensory profiling with the PDQ7 based on the pain phenotype allowed us to build specific as well as harmonized sub-groups of patients.
Because of the use of routine data, we were neither able to perform a systematic monitoring of treatment compliance nor a formal recording of possible THC:CBD misuse or treatment abuse. However, the evaluated study medication is known to have an extremely low risk of mis-/abuse, especially if compared with inhaled cannabinoids or traditional WHO step III analgesics after large numbers of patients treated since start of commercialization in EU in 2011 and studies published. And in fact, we found no signals for any critical or serious adverse events associated with the legalized use of THC:CBD.
With respect to the prior history of refractory CP reported by the patients in this analysis, a 12-week evaluation period is too short to draw final conclusions on the long-term effectiveness of THC:CBD for this indication. Longer treatment observations are necessary, both to gain further insight into the endurance of the effectiveness seen in our cohort of patients as well as into the ability of THC:CBD to replace alternative drugs, either ineffective and/or with a critical safety profile. Additionally, randomized controlled trials are necessary to substantiate our results and to further increase our knowledge about this special type of treatment. However, this analysis of real-world data on the effectiveness and tolerability in patients suffering from severe CP is to our knowledge the largest study on THC:CBD in this special patient population so far and delivers (despite its obvious limitations) a lot of important information on the differential effects obtainable in daily practice.
A formal issue interfering with common Good Clinical Practice standards for conducting clinical trials and non-interventional studies is that none of the patient-reported data derived from the GPR for evaluation purposes like this one allowed a confirmation via paper records or other sources, laboratory results or treatment schedules, simply due to the fact that the direct electronic data entry performed under the conditions of daily routine care does not provide evaluable materials for independent source data verification processes. Moreover, German data protection laws, the EU General data protection guideline and the GPR standard operating procedures forced us to perform any analyses with completely anonymized data sets only, which excludes any possibilities for backward tracing or the identification of individual patients, pain management centers or pain specialists. In any case, the overall study could be reproduced relatively easily again in the future (with an even larger sample) aiming to replicate confirm our findings.
Vice versa, this special design is a unique strength of our analysis, as it focuses almost exclusively on patient-relevant and especially patient-reported outcomes, sampled as part of an electronic routine data registry established to improve patient care under real-life conditions.
Limitations with respect to the range of variables collected was – in comparison to most other routine data collecting systems and registries – not really a problem, as most of the information generated by administrative and clinical GPR processes based on standardized documentation tools (eg, German Pain Questionnaire and German Pain Diary) mutually developed, agreed and recommended for routine use by respective medical associations in Germany (the German Pain Association and the German Pain Society) and the German Pain League (Germany’s largest umbrella group for self-regulating communities of pain and palliative care patients) in 2006.88 Both tools cover a broad range of validated self-assessment instruments (incorporating physical, mental, psychological and social aspects of pain, previous and current pain treatments, comorbidities as well as concomitant medication, etc.) sensitive for baseline as well as follow-up evaluations during the longitudinal course of a pain treatment and fulfil all official requirements for a quality assured standard documentation tool for pain medicine as defined by the National Association of Statutory Health Insurance Physicians.89
The most important factors in favor of this registry-based treatment evaluation is that in contrast to usual studies (interventional or not), neither physicians nor patients received any type of compensations for their data collection activities and that all data recorded via the registry were only entered to improve patient–physician interaction – two factors eliminating virtually any data reported due to economic reasons. GPR participation and the disposal of the online documentation tool iDocLive® is complimentary for physicians who are members of the German Pain Association and free of charge for all patients – irrespective of their health insurance coverage.
Conclusions
In this exploratory analysis of real-world data on 800 patients with elsewhere refractory SCP provided by the GPR, THC:CBD oromucosal spray, a cannabinoid-based fixed dose low THC high CBD medication (originally developed and approved for the symptomatic treatment of adult patients with moderate to severe spasticity due to MS), proved to be an efficacious add-on treatment for the relief of pain, especially if it was neuropathic in nature. The legalized treatment with THC:CBD was well tolerated – especially in comparison with other cannabis products and usually recommended analgesics – and showed a good safety profile without any evidence of abuse, persistent patterns of deliberate overdose, misuse, psychiatric complications or tolerance development. Beneficial effects found for CP patients in this analysis clearly outweighed the potential risks of treatment and confirmed that THC:CBD oromucosal spray provides an effective add-on measure for patients suffering either from elsewhere refractory neuropathic pain conditions as well as those presenting with a pain phenotype suggestive for underlying neuropathic mechanisms.
Transparency
Declaration of financial/other relationships
The concept for this evaluation of routine data provided by the GPR has been developed by M.A.U. at the Institute of Neurological Sciences (IFNAP) on behalf of the German Pain Association (Deutsche Gesellschaft für Schmerzmedizin, DGS) and the German Pain League (Deutsche Schmerzliga, DSL) and its realization has been funded in part (~25%) by an unrestricted scientific grant from Almirall Hermal, Germany. Neither Almirall, nor any of its employees exerted any influence on the data acquisition, the conduct of this analysis, or on the interpretation and publication of the results.
M.A.U and G.H.H.M.-S. are physicians and independent of any significant/relevant financial or other relationship to the sponsor, except for minor reimbursements for occasional lecture or consulting fees. Both are honorary members of the management boards of the German Pain Association and the German Pain League. U.E. is a veterinarian and works as a scientific and medico-legal consultant for various pharmaceutical companies.
The GPR is hosted by an independent contract research organization by order of the German Pain Association and under control of the Institute of Neurological Sciences and collects standardized real-world data from daily routine medical care since January 2000.
Acknowledgments
Data of this analysis have been presented at the annual Congress of the German Pain Society, October 18–20, 2018, Mannheim, Germany and at the annual conference of the German Neurological Association, October 30–November 3, 2018, Berlin, Germany.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
MAU received financial support and/or expenses in form of research money, consultancy fees, and/or remunerations for lecture activities from Almirall, Archimedes, Aristo pharma, Bene-Arzneimittel, Grünenthal, HAPA Medical, Janssen-Cilag, Kyowa Kirin, Lilly, Menarini, MSD, Mucos, Mundipharma, Omnia Med, Pfizer, PharmAllergan, ProStrakan, Servier, Shionogi, TEVA, and Tilray. UE received financial support and/or expenses in form of consultancy fees from Roche, Almirall, MedDay, and Granzer Regulatory Consulting & Services. GHHM-S received financial support and/or expenses in the form of research money, consultancy fees and/or remunerations for lecture activities from Allergan Ltd., Almirall, Grünenthal, Mundipharma, Pfizer, PharmAllergan, ProStrakan, and TEVA. The authors report no other conflicts of interest in this work.
References
1. Bonica JJ. The Management of Pain. Philadelphia: Lea & Febiger; 1953.
2. Treede RD. Entstehung der Schmerzchronifizierung [Development of pain chronification]. In: Baron R, Koppert W, Strumpf M, Willweber-Strumpf A, editors. Praktische Schmerztherapie. Heidelberg: Springer; 2011:3–13. German.
3. Merskey H, Bogduk N. Classification of Chronic Pain.
4. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287. doi:10.1016/j.ejpain.2005.06.009
5. Goldberg DS, Summer JM. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi:10.1186/1471-2458-11-770
6. Gureje O, von Korff M, Kola L, et al. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. Pain. 2008;135:82–91. doi:10.1016/j.pain.2007.05.005
7. Koleva D. Pain in primary care: an Italian survey. Eur J Public Health. 2005;15:475–479. doi:10.1093/eurpub/cki033
8. Mäntyselkä P, Kumpusalo E, Ahonen R, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001;89:175–180.
9. Leger JM. Diagnosis of chronic neuropathy. J Neurol. 1999;246:156–161.
10. Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185–190.
11. Beith ID, Kemp A, Kenyon J, Prout M, Chestnut TJ. Identifying neuropathic back and leg pain: a cross-sectional study. Pain. 2011;152:1511–1516. doi:10.1016/j.pain.2011.02.033
12. Smart KM, Blake C, Staines A, Doody C. Self-reported pain severity, quality of life, disability, anxiety and depression in patients classified with ‘nociceptive’, ‘peripheral neuropathic’ and ‘central sensitisation’ pain. The discriminant validity of mechanisms-based classifications of low back (±leg) pain. Man Ther. 2012;17:119–125. doi:10.1016/j.math.2011.10.002
13. Cherkin DC, Sherman KJ, Balderson BH, et al. Comparison of complementary and alternative medicine with conventional mind-body therapies for chronic back pain: protocol for the Mind-body Approaches to Pain (MAP) randomized controlled trial. Trials. 2014;15:211–215. doi:10.1186/1745-6215-15-211
14. Lee C, Crawford C, Buckenmaier C, Schoomaker E, Delgado R, York A. Active, self-care complementary and integrative medicine therapies for the management of chronic pain symptoms: A rapid evidence assessment of the literature. J Altern Complement Med. 2014;20:A137–A138. doi:10.1089/acm.2014.5368.abstract
15. Breen J. Transitions in the concept of chronic pain. ANS Adv Nurs Sci. 2002;24:48–59.
16. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence based recommendations. Pain. 2007;132(3):237–251. doi:10.1016/j.pain.2007.08.033
17. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain consensus statement and guidelines from the Canadian pain society. Pain Res Manage. 2007;12(1):13–21. doi:10.1155/2007/730785
18. Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22(5):467–474. doi:10.1097/WCO.0b013e3283311e13
19. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 Suppl):S22–S32. doi:10.1016/j.amjmed.2009.04.007
20. Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. doi:10.1111/j.1468-1331.2010.02999.x
21. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–S14. doi:10.4065/mcp.2009.0649
22. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
23.
24.
25. Gesetz zur Änderung betäubungsmittelrechtlicher und anderer Vorschriften. Bundesgesetzblatt 2017 Nov 1. Available from:
26. Häuser W, Finnerup NB, Moore RA. Systematic reviews with meta-analysis on cannabis-based medicines for chronic pain: a methodological and political minefield. Pain. 2018;159:1906–1907. doi:10.1097/j.pain.0000000000001295
27. Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64:e78–94.
28. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159:1932–1954. doi:10.1097/j.pain.0000000000001293
29. Häuser W, Fitzcharles MA, Radbruch L, Petzke F. Cannabis in der Schmerz- und Palliativmedizin [Cannabinoids in pain management and palliative medicine]. Dtsch Arztebl Int. 2017;114(38):627–634. German. doi:10.3238/arztebl.2017.0627
30. Friemel CM, von Keller R, Kabisch J. Cannabis: potential und Risiken. Eine wissenschaftliche Analyse (CaPRis). Available from:
31.
32. Romero-Sandoval EA, Kolano AL, Alvarado-Vazquez PA. Cannabis and cannabinoids for chronic pain. Curr Rheumatol Rep. 2017;11:67. doi:10.1007/s11926-017-0693-1
33. Sativex EMA label. 2015
34.
35.
36. Karniol IG, Carlini EA. Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia. 1973;33(1):53–70.
37. Fernandes M, Schabarek A, Coper H, Hill R. Modification of delta9-THC-actions by cannabinol and cannabidiol in the rat. Psychopharmacologia. 1974;38(4):329–338.
38. Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28(1):172–177.
39. Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology. 1982;76(3):245–250.
40. Hollister LE, Gillespie H. Interactions in man of delta-9-tetrahydrocannabinol. II. Cannabinol and cannabidiol. Clin Pharmacol Ther. 1975;18(1):80–83.
41. Morgan CJ, Gardener C, Schafer G, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological wellbeing. Psychol Med. 2012;42(2):391–400. doi:10.1017/S0033291711001322
42. Wright MJ
43. Hindocha C, Freeman TP, Schafer G, et al. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. 2015;25(3):325–334. doi:10.1016/j.euroneuro.2014.11.014
44. Cappelleri JC, Bienen EJ, Koduru V, Sadosky A. Measurement properties of painDETECT by average pain severity. Clinicoecon Outcomes Res. 2014;6:497–504. doi:10.2147/CEOR.S68997
45. Baron R, Maier C, Attal N, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158(2):261–272. doi:10.1097/j.pain.0000000000000753
46. Tait RC, Chibnall JT, Krause S. The pain disability index: psychometric properties. Pain. 1990;40:171–182.
47. Casser HR, Hüppe M, Kohlmann T, et al. Deutscher Schmerzfragebogen (DSF) und standardisierte Dokumentation mit KEDOQ-Schmerz [German pain questionnaire and standardised documentation with the KEDOQ-Schmerz]. Der Schmerz. 2012;26(2):168–175. German. doi:10.1007/s00482-011-1142-0
48. Hayes CJ, Bhandari NR, Kathe N, Payakachat N. Reliability and validity of the medical outcomes study short form-12 version 2 (SF-12v2) in adults with non-cancer pain. Healthcare (Basel). 2017;5(2):E22. doi:10.3390/healthcare5020022
49. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther. 1995;33(3):335–343.
50. Basler HD. Marburger Fragebogen zum habituellen Wohlbefinden - Untersuchung an Patienten mit chonischem Schmerz [The Marburg questionnaire on habitual health findings - A study on patients with chronic pain]. Schmerz. 1999;13(6):385–391. German. doi:10.1007/s004829900047
51. Guy W. ECDEU Assessment Manual for Psychopharmacology.
52.
53. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi:10.1038/nrdp.2017.2
54. Attal N, Cruccua G, Haanpää M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13(11):1153–1169. doi:10.1111/j.1468-1331.2006.01511.x
56. Turk DC, Audette J, Levy RM, Mackey SC, Stanos S. Assessment and treatment of psychosocial comorbidities in patients with neuropathic pain. Mayo Clin Proc. 2010;85:S42–S50. doi:10.4065/mcp.2009.0648
57. Cruccu G, Truini A. A review of neuropathic pain: from guidelines to clinical practice. Pain Ther. 2017;6(Suppl.):S35–S42. doi:10.1007/s40122-017-0087-0
58. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438–449. doi:10.1016/j.jpain.2012.01.003
59. Langford RM, Mares J, Novotna A, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260(4):984–997. doi:10.1007/s00415-012-6739-4
60. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
61. Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
62. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi:10.1016/S1474-4422(10)70143-5
63. Maier C, Baron R, Toelle TR, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi:10.1016/j.pain.2010.05.002
64. Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi:10.1016/j.neuron.2006.09.021
65. Max MB. Towards physiologically based treatment of patients with neuropathic pain. Pain. 1990;42:131–137.
66. Woolf CJ, Bennett GJ, Doherty M, et al. Towards a mechanism-based classification of pain? Pain. 1998;77:227–229.
67. Hansson P. Difficulties in stratifying neuropathic pain by mechanisms. Eur J Pain. 2003;7:353–357. doi:10.1016/S1090-3801(03)00051-X
68. Jensen TS, Baron R. Translation of symptoms and signs into mechanisms in neuropathic pain. Pain. 2003;102:1–8.
69. Lauer MS, Topol EJ. Clinical trials - multiple treatments, multiple end points, and multiple lessons. JAMA. 2003;289:2575–2577. doi:10.1001/jama.289.19.2575
70.
71.
72. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials. Greater precision but with greater uncertainty? JAMA. 2003;289:2554–2559. doi:10.1001/jama.289.19.2554
73. Montori VM, Permanyer-Miralda G, et al. Validity of composite endpoints in clinical trials. Br Med J. 2005;330:594–596. doi:10.1136/bmj.330.7491.594
74. Collin C, Davies P, Mutiboko IK, Ratcliffe S. Sativex spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14(3):290–296. doi:10.1111/j.1468-1331.2006.01639.x
75. Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32(5):451–459. doi:10.1179/016164109X12590518685660
76. Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols (Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18(9):1122–1131. doi:10.1111/j.1468-1331.2010.03328.x
77. Vachová M, Novotná A, Mareš J, et al. A multicentre, double-blind, randomised, parallel-group, placebo-controlled study of effect of long-term Sativex® treatment on cognition and mood of patients with spasticity due to multiple sclerosis. J Mult Scler. 2014;1(2):10000122.
78. Koehler J, Feneberg W, Meier M, Pöllmann W. Clinical experience with THC:CBD oromucosal spray in patients with multiple sclerosis-related spasticity. Int J Neurosci. 2014;124(9):652–656. doi:10.3109/00207454.2013.877460
79. Oreja-Guevara C, Casanova B, Ordás CM, Silván CV, Asensio D, Massana M. Observational Safety Study of THC: CBD oromucosal spray (Sativex) in multiple sclerosis patients with spasticity. Clin Exp Pharmacol. 2015;5(5):184.
80. Flachenecker P, Henze T, Zettl UK. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur Neurol. 2014;72(1–2):95–102. doi:10.1159/000360285
81. Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice – results of a multicenter, non-interventional study (MOVE2) in patients with multiple sclerosis spasticity. Eur Neurol. 2014;71(5–6):271–279. doi:10.1159/000357427
82. Etges T, Karolia K, Grint T, et al. An observational postmarketing safety registry of patients in the UK, Germany, and Switzerland who have been prescribed Sativex® (THC:CBD, Nabiximols) oromucosal spray. Ther Clin Risk Manag. 2016;12:1667–1675. doi:10.2147/TCRM.S115014
83. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10(4):434–441. doi:10.1191/1352458504ms1082oa
84. Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol. 2013;260(1):285–295. doi:10.1007/s00415-012-6634-z
86. Patti F, Messina S, Solaro C, et al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J Neurol Neurosurg Psychiatry. 2016;87(9):944–951. doi:10.1136/jnnp-2015-312591
87. Robinson CB, Fritch M, Hullett L, et al. Development of a protocol to prevent opioid-induced constipation in patients with cancer: A research utilization project. Clin J Oncol Nurs. 2000;4(2):79–84.
88. Nagel B, Pfingsten M, Lindena G, Nilges P. Handbuch Deutscher Schmerzfragebogen [Handbook German pain questionnaire]. Available from:
89. Bundesvereinigung K. Qualitätssicherungsvereinbarung zur schmerztherapeutischen Versorgung chronisch schmerzkranker Patienten gem. § 135 Abs. 2 SGB V. (Qualitätssicherungsvereinbarung Schmerztherapie). Available from:
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







