Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Effect of using the Suction Above Cuff Endotracheal Tube (SACETT) on postoperative respiratory complications in rhinoplasty: a randomized prospective controlled trial
Received 7 January 2019
Accepted for publication 23 March 2019
Published 17 April 2019 Volume 2019:15 Pages 571—577
DOI https://doi.org/10.2147/TCRM.S200662
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Nureddin Yuzkat,1 Canser Yilmaz Demir2
1Department of Anesthesiology and Reanimation, Faculty of Medicine, Van Yuzuncu Yıl University, Van, Turkey; 2Department of Plastic, Reconstructive and Aesthetic Surgery, Faculty of Medicine, Van Yuzuncu Yıl University, Van, Turkey
Background: The Suction Above Cuff Endotracheal Tube (SACETT) has a dorsal port above the cuff designed to enable the continuous or intermittent suctioning of secretions from the subglottic space. Thus, it facilitates the suctioning of excessive secretions above the cuff and around the glottis.
Objectives: In this study, we investigated the effect of the using the SACETT on laryngospasm and postoperative complications in rhinoplasty operations.
Methods: This randomized controlled clinical trial enrolled 132 patients undergoing rhinoplasty. The patients were randomly divided into two groups: Suction above Cuff Endotracheal Tube (n=66; Group SA) and classic endotracheal tube (n=66; Group C). Complications following general anesthesia were statistically analyzed among the two groups.
Results: The incidence of postoperative laryngospasm (p=0.02) and respiratory complications was found to be lower in Group SA than in Group C. In addition, the incidence of agitation (p=0.035), postoperative nausea, and vomiting (PONV) (p=0.041), which required antiemetic drug administration, swallowing difficulty (p=0.012), and sore throat (p=0.027) were found to be lower in Group SA than in Group C.
Conclusion: We suggest that using the SACETT in rhinoplasty reduces the incidence of postoperative respiratory complications as well as the incidence of agitation, sore throat, swallowing difficulty, and PONV.
Clinical Trial Number: NCT03584503
Keywords: general anesthesia, laryngospasm, postoperative complications, rhinoplasty, suction above cuff endotracheal tube
Introduction
Due the accumulation of blood and secretions in the mouth, the incidence of laryngospasm and aspiration-related respiratory complications increases in rhinoplasty operations.1,2 Cuffed endotracheal tubes are preferred in rhinoplasty operations, because prevent tracheal aspiration and protect the airway by balloon cuff. However, although intraoral secretions are suctioned, secretions above the cuff cannot be completely suctioned.3,4
The Suction above Cuff Endotracheal Tube (SACETT) has a dorsal port above the cuff designed to enable suctioning of secretions from the subglottic space. Because the SACETT facilitates the suctioning of excessive secretions in the subglottic area, is preferred in intensive care units.5,6 However, the use of the SACETT in in rhinoplasty operations has not been adequately investigated.
The use of the SACETT in in rhinoplasty operations has not been adequately investigated. In this study, we investigated the effect of the use of the SACETT on laryngospasm and postoperative respiratory complications in rhinoplasty operations.
Materials and methods
This study was conducted among patients scheduled for rhinoplasty under general anesthesia. The patients who underwent routine preoperative examinations and had no obstacles to the operation were informed about the study. In accordance with the Declaration of Helsinki, before the patients were included in the study, written informed consent was obtained from each patient (Van Yuzuncu Yıl University Ethics Committee IRB approval date: January 17, 2018; decision number: 01). Clinical Trial Number: NCT03584503.
A total of 136 patients, who were American Society of Anesthesiologists (ASA) class I–II and aged 18–65 years, were included in the study. Patients who had upper or lower respiratory tract infections, asthma, or a history of allergies, who received isoflurane or desflurane for maintenance of anesthesia, who were ASA class III–IV, who had a difficult airway (Mallampati score III–IV), or who had a long uvula, gastroesophageal reflux, electrolyte disturbances such as hypomagnesemia and hypocalcemia, or a body mass index (BMI) over 30 kg/m2 were excluded from the study.
Study groups
For the sake of standardization, this study focused on one type of surgical operation, that is, rhinoplasty. The patients included in the study were randomly divided into two groups using a sealed-envelope system. A total of 136 patients underwent surgery during the study period. Whereas Group SA (n=68) consisted of those undergoing intubation using the Suction Above Cuff Endotracheal Tube (SACETT™, PORTEX®, France), Group C (n=68) consisted of those undergoing intubation using the classic endotracheal tube (Endotracheal Tubes, Bicakcilar, Turkey). A total of four patients, two from each group, were excluded from the study, because they did not agree to participate. In both groups, the intubation procedure was performed similarly by an experienced anesthetist. Flow chart is shown in Figure 1.
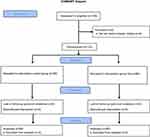 | Figure 1 Flow chart. |
Initially, the patients were monitored after intake to the preoperative care unit. Their vital signs were recorded. After they were taken to the surgery room, standard electrocardiogram (ECG), heart rate (HR), noninvasive blood pressure (NIBP), and peripheral oxygen saturation (SpO2) were monitored. Before the induction of anesthesia, vascular access was performed from the antecubital region or the back of the hand using a 20G IV cannula. Maintenance fluid therapy with 0.9% NaCl was provided.
Applied procedures
For the induction of anesthesia, 1 mg/kg lidocaine, 2 μg/kg fentanyl, 2 mg/kg propofol, and 0.6 mg/kg rocuronium were used. Then, the patients in Group SA were intubated using the SACETT with an internal diameter of 7 or 7.5 mm, and the patients in Group C were intubated using a cuffed endotracheal tube with an internal diameter of 7 or 7.5 mm. The endotracheal tube cuff was inflated with air until the balloon sealed the airway. Cuff pressure was measured in all patients, and the appropriate cuff pressure was determined to be 20–30 cmHg in order to prevent microaspiration. (VBM Cuff Pressure Gauges, VBM Medizintechnik GmbH, Einsteinstrasse, Germany). Moreover, the pack was not inserted into the throat.
When needed for a maintenance dose, 0.5 mcg/kg fentanyl and 0.2 mg/kg rocuronium were used. Throughout the operation, 1% sevoflurane, 40% oxygen, and 60% medical air were used.
The age, gender, height, weight, BMI, ASA score, and duration of operation were recorded for all patients. HR, NIBP, and SpO2 values were recorded at the 0th and 5th minutes preoperatively, at 5 min intervals during surgery, and at the 1st, 5th, and 10th minutes postoperatively. The surgery was performed by the same surgeon with similar technique and washing fluids was not used. Therefore, the fluids in the operation area were accepted as blood and secretions. The amount of blood and secretions suctioned into the surgical aspirator by the surgeon was recorded for all patients during the intraoperative period. For Group SA, the blood and secretions above the cuff was suctioned into the anesthetic aspirator through the aspiration port at 10 min intervals in the intraoperative period and was then recorded by the anesthetist. For both Group C and Group SA, intraoral and pharyngeal area suctioning was performed just before and during the extubation. During aspiration, the negative pressure of the aspirators was kept between 60 and 80 mmHg, which was lower than normal. For postoperative analgesia, 10 mg/kg paracetamol (Perfalgan, Abdi Ibrahim, Istanbul, Turkey) was intravenously administered to all patients 20 mins before the end of surgery.
All patients were placed in a supine position in the intraoperative period, with their heads in a neutral position. The neutral head position of all patients was maintained throughout the operation. The head was not subjected to any repositioning, such as hyperextension or anteflexion. After the patients’ muscle strength and consciousness returned to normal, extubation was performed. All patients were transferred to the PACU after extubation. A standard monitoring system was applied during the intraoperative and postoperative periods. Laryngospasm and other respiratory complications (apnea, desaturation, bronchospasm, cough) were recorded. In addition, agitation, sore throat, swallowing difficulty, postoperative nausea and vomiting (PONV), hypertension, hypotension, bradycardia, and tachycardia were recorded during the postoperative recovery period. The period after extubation and PACU was accepted as the recovery period.
The presence of apnea, desaturation (SpO2<85%), and inspiratory stridor after extubation was considered laryngospasm. When laryngospasm developed, first an increase of the inspired fraction of oxygen, elimination of irritant stimuli with pharyngeal suctioning, airway-opening maneuvers, and continuous positive airway pressure were primarily planned. When persistent laryngospasm developed, deepening of anesthesia, intravenous succinylcholine, and reintubation were primarily planned.4 The emergence of expiratory wheezing requiring bronchodilator therapy was considered bronchospasm.7
The Riker Sedation-Agitation Scale (Riker-SAS) was used to assess agitation.9 Continuous cough (less than 5 seconds) or persistent cough was regarded as a positive cough, and the presence of nausea and vomiting requiring antiemetic drug administration was regarded as positive PONV.8 PONV (0 - No nausea, 1 - Mild nausea, 2 - Severe nausea, requiring an anti-nausea agent, 3 - Nausea and vomiting) were assessed using a four-point scale.8 If a patient’s nausea and vomiting score was ≥2, i.v. ondansetron 50 µg/kg (Zofran 4 mg, Glaxo Smith Kline, Istanbul, Turkey) was administered.
The presence of postoperative complications was recorded by an anesthesiologist blinded to the study groups.
Whereas a systolic arterial pressure greater than 150 mmHg or an increase in SBP more than 20% from baseline was considered hypertension, a mean arterial pressure less than 60 mmHg or a decrease in SBP more than 20% from baseline was considered hypotension. Whereas a heart rate less than 50 beats/min or a 20% decrease from baseline was considered bradycardia, a heart rate greater than 110 beats/min or a 20% increase from baseline was considered tachycardia.
Statistical analysis
Categorical variables were expressed as numbers and percentages, and continuous variables were expressed as mean, standard deviation, and minimum and maximum values. Repeated measures analysis of variance (ANOVA) was performed to compare the mean values of continuous variables between groups. The paired Student t-test was used to compare the two dependent variables. The Pearson correlation coefficient was individually calculated for groups to determine the relationship between these variables. The hi-square test was used to determine the relationship between categorical variables.
For the number of postoperative complications, previous studies have established 3 as the standard deviation (σ). Effect size (d) was assumed to be 0.9, and a Z value of 1.96 was used for the 0.05 type I error rate. The sample size was found to be 61 using the equation for sample size calculation (n= Z2.σ2/d2), and 66 patients were included in each group. A p-value of <0.05 was considered statistically significant. The Statistical Package for the Social Sciences (SPSS) v. 20.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data.
Results
There were no significant differences between the two groups in terms of demographic data and general characteristics (Table 1).
 | Table 1 Comparison of general characteristics between the two groups |
The incidence of postoperative respiratory complications, such as laryngospasm (p=0.002) and apnea (p=0.017), was found to be lower in Group SA. All laryngospasm episodes were treated with oxygen, positive pressure ventilation, and pharyngeal suctioning. None of the patients needed a muscle relaxant or reintubation. There was no difference in the incidence of cough between the groups (p=0.226) In addition, the incidence of agitation (p=0.035), PONV (p=0.041), swallowing difficulty (p=0.012), and sore throat (p=0.027), was found to be lower in Group SA than in Group C (Table 2).
 | Table 2 Laryngospasm and postoperative complication rates in the groups |
The total blood and secretions volume accumulated in the suction chamber was found to be greater in Group SA (67.8±17.8 mL) than in Group C (60.6±14.5 mL)(p=0.012; Table 3).
 | Table 3 Comparison of blood accumulating in aspirator in groups |
However, the incidence of hypotension (p=0.154) and tachycardia (p=0.645) was similar between the groups (p>0.05). Neither PONV nor swallowing difficulty was observed in Group SA. None of the patients had bradycardia, hypertension, or bronchospasm. There were no significant differences between the two groups in terms of intraoperative mean blood pressure (p=0.154), pulse rate (p=0.154), or peripheral oxygen saturation (p=0.154) values (Table 4).
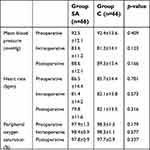 | Table 4 Comparison of hemodynamic data in the groups. |
Discussion
In this study, we found that using the SACETT in rhinoplasty enabled the aspiration of secretions above the cuff and reduced the incidence of laryngospasm. In addition, using the SACETT significantly reduced the incidence of agitation, PONV requiring antiemetic drug administration, sore throat, and swallowing difficulty.
Adequate suctioning of secretions in the oral and pharyngeal region is essential after intraoral and nasal interventions. Blood and secretions in the mouth and pharynx or above the cuff balloon that cannot be fully suctioned after rhinoplasty may cause postoperative complications. In this respect, the SACETT provided better results than the classical endotracheal tube, because it can suction blood and secretions above the cuff balloon.
This study has several major limitations. First, blood and other secretions could not be differentiated in the aspirated mine content. This limitation was evaluated. In addition, the time of extubation after operation, intraoperative infusion volume, patient satisfaction is seen as other limitations. The length of the study was considered. These issues may be the subject of other studies.
Laryngospasm is commonly perceived to be a significant problem by anaesthetists. Nasal, buccal, pharyngeal, or laryngeal irritations or manipulations play a role in the etiology of laryngospasm.10 The presence of blood and secretions in the mouth, laryngoscopy under light anesthesia, and irritation of the vocal cords with an aspiration catheter increase the incidence of laryngospasm.2,4,11
There is a close association between laryngospasm and the type of surgery.4,11 Nasal, oral, or pharyngeal surgery increases the risk of laryngospasm. The incidence of laryngospasm reported in literature ranges between 0.8% and 25%.3,11 This study found a 12.1% incidence of laryngospasm, but no laryngospasm was observed during the use of the SACETT. The absence of laryngospasm may be due to the SACETT’s adequate suctioning of secretions above the cuff, the reduced need for an aspiration catheter, and thereby a decrease in the irritation associated with it.
Irritation is the main factor that triggers laryngospasm.4,10 For this reason, we chose propofol and sevoflurane, which cause less laryngospasm, for the induction of anesthesia in patients. We avoided barbiturates (which are among the intravenous induction agents thought to trigger laryngospasm)12 and desflurane.13,14 Thus, we focused on the effects of secretions and local irritation by excluding other factors that trigger laryngospasm.
However, the type of extubation has a significant effect on the occurrence of laryngospasm. Research has suggested that extubation under deep anesthesia or upon awakening reduces laryngospasm.15 When extubation is performed under deep anesthesia, ventilatory support is usually required via mask ventilation. During placement of the mask on the face in rhinoplasty operations, compression on the surgical field and difficulty in ventilation are likely. In this study, we chose waking extubation because of the risk of aspiration after extubation and the difficulty of mask ventilation.
In this study, we found that the incidence of laryngospasm in the control group was lower than that reported in the literature (12.1%). Gulhas et al16 chose waking extubation and reported that the incidence of laryngospasm was 25%. In studies with a similar extubation plan, Aljonaieh17 reported that the incidence of laryngospasm was 19.5% in the control group, and Lee et al18 reported that it was 23.7% in the control group. The reason for the lower incidence of laryngospasm in the control group of the present study may be due to the fact that our study was performed in adult age groups.
The cuff pressure of an endotracheal tube varies according to patient-related factors, environmental circumstances, and therapeutic interventions. Factors leading to increased cuff pressure include positive-pressure ventilation, ventilation with nitrous oxide, and pathologic processes such as bronchoconstriction, laryngeal spasms, and edema. Little information, however, is available regarding the effect of changes in body position on the cuff pressure of endotracheal tubes. Changing the position of the head results in a displacement of the tube.19
Studies have reported an incidence of cough in septorhinoplasty operations between 6.6% and 45%.18,20 This study found that the incidence of cough was 7.5% in general. Our results are consistent with the literature.
Agitation is one of the most common complications in the early postoperative period. Patients have an increased risk of self-injury because agitation causes behavioral changes. It can lead to serious complications such as hypoxia, aspiration, self-injury, touching the surgical field, and falling associated with self-extubation.21
Agitation is often seen especially after ear, nose, throat, and septoplasty operations. The blockage of the nasal passages in these operations causes the patient to feel a choking sensation and may increase the incidence of agitation. Kim et al22 showed that the incidence of agitation for intranasal operations was 22.2%. In this study, the agitation rate was 16.6% in the classical endotracheal tube group and 4.5% in the SACETT group. This substantial reduction may be due to the prevention of irritation in the patients’ subglottic area with the use of the SACETT.
Postoperative nausea and vomiting is one of the most common problems in the postoperative period. It is reported in 10–50% of patients. The causes of PONV include blood reaching the stomach and blood and secretions accumulating in the mouth during the operation.23 In this study, no PONV (requiring antiemetic drug administration) was observed in the SACETT group. This may be due to the fact that adequate suction with the SACETT may decrease blood and secretions in the mouth, thus reducing the amount of blood that escapes into the stomach.
A prospective study found a 40% incidence of sore throat, and the presence of a blood‐stained tracheal tube on extubation, suction, or postendotracheal intubation were associated with risk of sore throat.24,25 In this study, sore throat decreased significantly with the use of the SACETT, and no swallowing difficulty was observed. This may be due to lowered trauma to the patient with the use of the SACETT and better tolerability of the tube.
In conclusion, we suggest that the use of the SACETT in rhinoplasty reduces the incidence of respiratory complications. In addition, it reduces the incidence of other postoperative complications, such as agitation, sore throat, swallowing difficulty, and PONV, when compared with the classic endotracheal tube. Further studies of the role of the SACETT in rhinoplasty operations and other intraoral or nasal surgical procedures may be warranted.
Data sharing statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Acknowledgments
We would like to thank Sıddık Keskin MD. Prof. who carried out a statistical analysis of the data in this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Doyle DJ. Anesthesia for ear, nose, and throat surgery. In: Miller RD, editor. Miller’s Anesthesia.
2. Punj J, Darlong V, Pandey R. Paradoxical vocal cord motion–another cause to differentiate from laryngospasm. Pediatr Anesth. 2008;18(10):979–980. doi:10.1111/j.1460-9592.2008.02683.x
3. Alalami AA, Ayoub CM, Baraka AS. Laryngospasm: review of different prevention and treatment modalities. Pediatr Anesth. 2008;18(4):281–288. doi:10.1111/j.1460-9592.2008.02448.x
4. Visvanathan T, Kluger M, Webb R, Westhorpe R. Crisis management during anaesthesia: laryngospasm. BMJ Qual Saf. 2005;14(3):e3–e3. doi:10.1136/qshc.2002.004275
5. Gunjan A, Shekhar S, Akhileshwar PK. Would “suction above cuff” be a better option than the “standard” endotracheal tube for the prevention of ventilator-associated pneumonia: A randomized study in postoperative neurological patients. Anesth Essays Res. 2018;12(2):480. doi:10.4103/aer.AER_39_18
6. Wang F, Bo L, Tang L, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: an updated meta-analysis of randomized controlled trials. J Trauma Acute Care Surg. 2012;72(5):1276–1285. doi:10.1097/TA.0b013e318247cd33
7. Looseley A. Management of bronchospasm during general anaesthesia. Update Anaesth. 2011;27(1):17–21.
8. Polat R, Peker K, Baran I, Aydın GB, Gülöksüz ÇT, Dönmez A. Comparison between dexmedetomidine and remifentanil infusion in emergence agitation during recovery after nasal surgery. Anaesthesist. 2015;64(10):740–746. doi:10.1007/s00101-015-0077-8
9. Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329.
10. Popat M, Mitchell V, et al;
11. Olsson G, Hallen B. Laryngospasm during anaesthesia. A computer‐aided incidence study in 136 929 patients. Acta Anaesthesiol Scand. 1984;28(5):567–575.
12. Horita A, Dille J. Observations on the action of thiopental (pentothal®) on the laryngeal reflex. Anesthesiology. 1955;16(6):848–853. doi:10.1097/00000542-195511000-00002
13. Oberer C, von Ungern-Sternberg BS, Frei FJ, Erb TO. Respiratory reflex responses of the larynx differ between sevoflurane and propofol in pediatric patients. J Am Soc Anesthesiolog. 2005;103(6):1142–1148.
14. Soyalp C, Dostbil A, Çelik M, et al. The effects of desflurane and remifentanyl anaesthesia compared to lumbar epidural analgesia combined with desflurane on recovery. Dicle Med J. 2014;41(4). doi:10.5798/diclemedj.0921.2014.04.0491
15. Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anesth. 2010;57(9):843–848. doi:10.1007/s12630-010-9338-9
16. Gulhas N, Durmus M, Demirbilek S, Togal T, Ozturk E, Ersoy MO. The use of magnesium to prevent laryngospasm after tonsillectomy and adenoidectomy: a preliminary study. Pediatr Anesth. 2003;13(1):43–47.
17. Aljonaieh KI. Effect of intravenous lidocaine on the incidence of postextubation laryngospasm: a double-blind, placebo-controlled randomized trial. Saudi J Anaesth. 2018;12(1):3. doi:10.4103/sja.SJA_440_17
18. Lee C, Chien T, Hsu J, et al. The effect of acupuncture on the incidence of postextubation laryngospasm in children. Anaesthesia. 1998;53(9):917–920.
19. Lizy C, Swinnen W, Labeau S, et al. Cuff pressure of endotracheal tubes after changes in body position in critically ill patients treated with mechanical ventilation. Am J Crit Care. 2014;23(1):e1–e8. doi:10.4037/ajcc2014617
20. Baftiu N, Krasniqi I, Haxhirexha K, Domi R. Survey about the extubation practice among anaesthesiologists in kosovo. Maced J Med Sci. 2018;6(2):350–354.
21. Mizuno J, Nakata Y, Morita S, Arita H, Hanaoka K. Predisposing factors and prevention of emergence agitation. Masui Jpn J Anesthesiol. 2011;60(4):425–435.
22. Kim H-J, Kim D-K, Kim H-Y, Kim J-K, Choi S-W. Risk factors of emergence agitation in adults undergoing general anesthesia for nasal surgery. Clin Exp Otorhinolaryngol. 2015;8(1):46–51. doi:10.3342/ceo.2015.8.1.46
23. Galway U, Alam D. Anesthesia for septoplasty and rhinoplasty. In: Abdulmalak B, Doyle DJ, editors. Anesth Otolaryngolog Surg. New York, NY: Cambridge University Press; 2012:113–120.
24. Yang H, Liu F, Tsai S, Tsay P, Lin H, Liu H. Ketorolac tromethamine spray prevents postendotracheal-intubation-induced sore throat after general anesthesia. Biomed Res Int. 2016;2016:1–5. doi:10.1155/2016/4582439
25. El-Boghdadly K, Bailey C, Wiles M. Postoperative sore throat: a systematic review. Anaesthesia. 2016;71(6):706–717. doi:10.1111/anae.13438
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
