Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Effect of pre- and post-treatment with Bacopa monnieri (Brahmi) on phencyclidine-induced disruptions in object recognition memory and cerebral calbindin, parvalbumin, and calretinin immunoreactivity in rats
Authors Piyabhan P, Tingpej P, Duansak N
Received 3 November 2018
Accepted for publication 5 March 2019
Published 1 May 2019 Volume 2019:15 Pages 1103—1117
DOI https://doi.org/10.2147/NDT.S193222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
P Piyabhan,1 P Tingpej,2 N Duansak1
1Division of Physiology, Department of Preclinical Science, Faculty of Medicine, Thammasat University, KlongLuang, Pathumthani, Thailand; 2Division of Microbiology, Department of Preclinical Science, Faculty of Medicine, Thammasat University, KlongLuang, Pathumthani, Thailand
Background: Decreased gamma-aminobutyric acid (GABA)-ergic neurons in the brain of both schizophrenic patients and animal models indicates that impairment of GABAergic function is implicated in pathophysiology of the disorder. Decreased GABAergic neurotransmission might be also involved in cognitive impairment, which is developed in schizophrenia. Brahmi (Bacopa monnieri) could be a new treatment and prevention for this cognitive deficit in schizophrenia by increasing GABAergic neurons to a normal level.
Aim: The authors aimed to study cognitive-enhancement- and neuroprotective-effects of Brahmi on novel object recognition memory and GABAergic neuronal density, defined by the presence of calcium binding proteins (CBPs; calbindin (CB), parvalbumin (PV), and calretinin (CR)) in a sub-chronic (2 mg/kg, Bid, ip) phencyclidine (PCP) rat model of schizophrenia.
Materials and methods: In the cognitive-enhancement-effect study rats were assigned to three groups; Group-1: Control, Group-2: PCP-administration, and Group-3: PCP+Brahmi. In the neuroprotective-effect study rats were assigned to three groups; Group-1: Control, Group-2: PCP-administration, and Group-3: Brahmi+PCP. A discrimination ratio (DR) representing cognitive ability was obtained from the novel object recognition task. CB, PV, and CR immunodensity were measured in the prefrontal cortex, striatum, and cornuammonis fields 1–3 (CA1–3) using immunohistochemistry.
Results: Reduced DR was found in the PCP group, which occurred alongside reduced CB, PV, and CR in all brain regions except for CR in the striatum and CA1–3 in the cognitive-enhancement-effect study. PCP+Brahmi showed a higher DR score with increased CB in the prefrontal cortex and striatum, increased PV in the prefrontal cortex and CA1–3, and increased CR in the prefrontal cortex. The Brahmi+PCP group showed higher DR score with increased CB in all areas, increased PV in the striatum, and increased CR in the prefrontal cortex and striatum.
Conclusion: The present study demonstrated the effects, both partial restoration of cognitive deficit and neuroprotection, of Brahmi, and elucidated its underlying mechanism of actions via increasing GABAergic neurons in a PCP-induced schizophrenic-like model.
Keywords: schizophrenia, cognitive impairment, Bacopa monnieri, calbindin, parvalbumin, calretinin
Introduction
Schizophrenia is a chronic neuropsychiatric disorder characterized by positive symptoms (eg, hallucination, delusions, thought disorder, perceptual disturbances, and increased motor function), negative symptoms (eg, alogia, anhedonia, flat affect, avolition, and social withdrawal), and deficits in cognitive abilities.1,2 The cognitive deficits pose the greatest restriction on the quality-of-life and functional performance of schizophrenic individuals.3 Currently, the main management of schizophrenia relies on antipsychotic medication. Conventional antipsychotic treatments, mainly dopamine receptor subtype 2 (D2) antagonists, have been proven to be effective at diminishing positive and negative symptoms, but often ineffective in relieving the cognitive deficits associated with the disease.4,5 These antipsychotics also often cause several serious adverse effects, especially extrapyramidal symptoms such as Parkinsonism. Atypical antipsychotics, which were discovered more recently, have been shown to be effective at attenuating and preventing the cognitive deficits in schizophrenic-like animal models.6–9 However, their efficacy for the improvement of the cognitive dysfunction in schizophrenic patients is still under question, and they are associated with side-effects including weight gain and metabolic dysfunction.4,10 Therefore, it is important to develop a novel medication which is more effective at improving the cognitive deficits and has fewer side-effects.
Bacopa monnieri or Brahmi is a medicinal plant which has long been used in Ayurvedic medicines to enhance cognitive ability, memory, learning skills, and improve mental function.11 Several studies have also revealed the cognitive and neuroprotective effects of Brahmi. Long-term administration of bacosides, the active saponins of Brahmi, was shown to prevent age-associated neurodegeneration in female Wistar rats.12 In a transgenic mouse model of Alzheimer’s disease, beta-amyloid levels in the brain were also reduced after Brahmi administration.13 This is consistent with the result showing the enhancement of cognitive performance in elderly with Alzheimer’s disease after receiving the Brahmi extract.14 Interestingly, a recent study reported that 1-month administration of Brahmi extracts (500 mg/day) in complement with antipsychotic drugs could reduce psychopathology without causing additional side-effects in schizophrenic patients.15 By using a rat model of schizophrenia, our previous studies also demonstrated that Brahmi could improve cognitive abilities and prevent cognitive deficits by increasing vesicular glutamate transporters subtype 1–3 (VGLUT1-3) in the brain.16–18 This data is consistent with the glutamate hypothesis of schizophrenia which suggests that hypofunction of the glutamatergic signal is one of the possible causes of schizophrenia.19
Gamma-aminobutyric acid (GABA) has also been known as another neurotransmitter involved in schizophrenia. Post-mortem studies have reported abnormalities of the GABAergic neurotransmission, including a loss of presumptive interneurons in more superficial cortical layers in the prefrontal cortex and a decrease in mRNA expression of the GABA synthesizing enzyme, GAD 67, in this brain region.20,21 A reduction of GABA was also found in the posterior hippocampus and other temporal lobe structures in schizophrenia.22–25 Interestingly, GABAergic neurons are also involved in memory processes.26 A study in non-human primates demonstrated the impairment of working memory tasks in subjects that received a GABA receptor antagonist.26 Alterations of GABA neurotransmission are likely associated with impaired cognitive function in individuals with schizophrenia.27
Phencyclidine (PCP), a noncompetitive glutamate/N-methyl-D-aspartate (NMDA) receptor antagonist, has been acceptably used for inducing animals to become schizophrenic.28 Animals receiving PCP exhibit several schizophrenia-like behaviors, including reduced prepulse inhibition (PPI).28,29 Low-dose PCP produces disinhibition and a state of euphoria, paranoia, and hallucinations, while high-dose PCP can cause general anesthesia, catalepsy, sedation, and seizures.30,31 Acute administration of PCP to animals can increase locomotor activity, which resembles schizophrenia, in a dose-dependent manner.32,33
The main aim of the present study was to investigate the cognitive enhancing effect and the neuroprotective effect of Brahmi in the PCP-induced schizophrenic-like rat model by using the novel object recognition task as an assessment tool for testing the cognitive function. The present study also aimed to elucidate the underlying mechanism of Brahmi, focusing mainly on roles of GABA. The GABAergic neurotransmission in the prefrontal cortex, striatum, and CA1–3 of the hippocampus was evaluated using immunohistochemistry measuring the presence of the calcium-binding proteins (CBPs), including calbindin (CB), parvalbumin (PV), and calretinin (CR), which locate mainly at GABAergic neurons.
Materials and methods
Plant extract
A standardized extract of 225 mg Bacopa monnieri containing 20% of bacosides A+B as a dietary supplement in a tablet formulation was purchased from PlanetaryTM Herbals (Canada). The extract was produced by Dr. Michael Tierra, East West School of Planetary Herbology, Ben Lomond, Canada.
Animals
Male Wistar rats (n=54) from the National Animal Center, Mahidol University, Thailand weighing 224–250 g at the time of first drug administration were used. The animals were single-housed, under a 12 hour/12 hour light/dark cycle with food and water available ad libitum in the home cage. Room temperature (21±2ºC) and humidity (45–55%) were kept constant throughout. All animals were acclimatized for 7 days before experiment. The animal experiments were performed in accordance with Mahidol University Code of Practice and the National Institutes of Health (USA) guidelines for treatment of laboratory animals. The protocol of the present study was approved by the Animal Research Ethical Committee of Thammasat University, Thailand. The number of project license for animal experiment in the present study is AE 015/2015.
Drugs and drug administration
Cognitive enhancement effect study
Animals were assigned to three groups (n=9/group).
Control group
Animals received vehicle solution (0.9% NaCl) ip, bi-daily (08:00 and 16:00 h) for 7 days. Four weeks later, they then orally received vehicle solution (distilled water) daily (08:00 h) for further 14 days.
Sub-chronic PCP group
Animals received 2 mg/kg of PCP (Sigma, USA) ip, bi-daily (08:00 and 16:00 h) for 7 days. Four weeks later, they then orally received vehicle solution (distilled water) daily (08:00 h) for a further 14 days.
PCP+Brahmi group
Animals received 2 mg/kg of PCP ip, bi-daily (08:00 and 16:00 h) for 7 days. Four weeks later, they then orally received 40 mg/kg/day of Brahmi supplement (PlanetaryTM Herbals) daily (08:00 h) for a further 14 days. PCP HCl and Brahmi were dissolved in 0.9% NaCl and distilled water, respectively.
Neuroprotective effect study
Animals were assigned to three groups (n=9/group).
Control group
Animals orally received vehicle solution (distilled water) daily (08:00 h) for 14 days. They then received vehicle solution (0.9% NaCl) ip, bi-daily (08:00 and 16:00 h) for 7 days, beginning 7 days after the first oral administration of vehicle.
Sub-chronic PCP group
Animals orally received vehicle solution (distilled water) daily (08:00 h) for 14 days. They then received 2 mg/kg of PCP ip, bi-daily (08:00 and 16:00 h) for 7 days, beginning 7 days after the first oral administration of vehicle.
Brahmi+PCP group
Animals orally received 40 mg/kg of Brahmi supplement daily (08:00 h) for 14 days. They then received 2 mg/kg of PCP ip, bi-daily (08:00 and 16:00 h) for 7 days, beginning 7 days after the first oral administration of Brahmi. PCP and Brahmi supplement were dissolved in 0.9% NaCl and distilled water, respectively.
Novel object recognition test
The novel object recognition test began 6 weeks after the last PCP or saline injection. The test was undertaken following the protocol from Neill et al34 and Piyabhan and colleagues18. In summary, after all animals received drugs or vehicle, they were acclimatized for 1 week, then they were tested in a novel object recognition paradigm. Testing took place in a 360 lux lighting room. The material used in the task was a 63×63×45 cm solid black plastic box which was placed on the floor throughout the experiment. Animal behavior was recorded by a video recorder (Canon), which was located on a movable trolley above the plastic box. The objects to be explored were made of glass, plastic, or ceramic, and had approximately equal heights. All these objects were fixed by adhesive tape at the bottom in order to avoid displacing by the animals. Familiar and novel objects were alternated between the left and right position to prevent bias for a particular location. Three days before starting the novel object recognition test, all rats were initially habituated to the empty plastic box for three sessions of 3 minutes daily. In the novel object recognition test, each rat was placed in the plastic box and exposed for 3 minutes to two identical objects placed approximately 10 cm apart in the center of the box. The rat was then returned to its home cage for an hour. The box and the objects were then thoroughly cleaned with 70% ethanol in an attempt to remove any remaining olfactory cues. Both objects in the box were replaced, one with an identical object and another with a novel object. Rats were then returned to the plastic box and allowed to explore the objects for 3 minutes. All trials were recorded by a video recorder located above the plastic box, and behavioral analysis was carried out blind to treatment. Object exploring was defined as the rat sniffing, licking, or touching the objects with forepaws whilst sniffing, but not by leaning against, standing on, turning around on, or sitting on the objects. The data were expressed as the discrimination ratio (DR) calculated from the following equation; DR=[(time exploring novel object−time exploring familiar object)/total exploration time)]. The data are expressed as mean±SEM.
Kruskal-Wallis non-parametric test was performed to determine the effect of treatment on DR value, followed by multiple pair-wise comparison using Bonferroni-corrected Mann-Whitney U-test. Statistical significances were defined as P<0.05. All statistical analysis was performed using SPSS V13 for windows (SPSS Inc., Chicago, IL, USA).
Immediately following the novel object recognition test, all rats were sacrificed by deep anesthesia with pentobarbital. Whole brains were removed and proceeded to immunohistochemistry.
Analysis of CB, PV, and CR by immunohistochemistry
Brain tissues from the previous experiment were fixed in 4% paraformaldehyde and embedded in paraffin wax and subsequently sectioned at a thickness of 5 µm, then mounted onto 3-aminopropyltriethoxysilane (APES) coated glass slides. The sections were determined using a rat brain atlas.35 Prefrontal cortex sections were taken between Bregma 2.7–2.2 mm, while those of striatum were taken from Bregma 0.7 mm. Sections for hippocampus were sectioned posterior to Bregma 3.3 mm. All sections were dewaxed in xylene then rehydrated in 100%, 90%, and 70% ethanol, consecutively, and washed for 5 minutes in distilled water. The sections were immersed in antigen retrieval solution (1 mM EDTA in 0.1 M Tris-HCl, pH 8.0) and heated in a microwave oven on full power (850 W) for 3×5 minutes. The sections were left at room temperature for 30 minutes to cool down before being washed in Tris-HCl buffer for 2×5 minutes, then incubated with endogenous peroxidase blocking solution (5% H2O2 in absolute methanol) for 30 minutes. The sections were washed in Tris-HCl buffer for 2×5 minutes before incubation for 45 minutes with protein blocking solution (2% normal goat serum in Tris-HCl buffer). For CB immunostaining, the sections were incubated at 4°C overnight with monoclonal antibody against CB, raised in rabbit (Abcam, UK) at a dilution of 1:2,500 in protein blocking solution. For PV immunostaining, the sections were incubated at 4°C overnight with polyclonal antibody against PV, raised in rabbit (Abcam) at a dilution of 1:15,000 in protein blocking solution. For CR immunostaining, the sections were incubated at 4°C overnight with polyclonal antibody against CR, raised in rabbit (Abcam) at a dilution of 1:500 in protein blocking solution. After the antibody incubation, the sections were washed for 2×5 minutes in Tris-HCl buffer before incubation for 1 hour with biotinylated secondary antibody (anti-rabbit IgG) at a dilution of 1:200. Sections were processed by the avidin-biotin method using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) and peroxidase was visualized using the chromogen diaminobenzidine, intensified with nickel chloride (DAB) (Vector Laboratories). The sections were dehydrated in 70%, 90%, 100% ethanol and xylene, then cover slipped with DPX mounting medium for microscopy. No immunoreactivity could be detected in control sections, in which the primary antibody was omitted from the staining protocol. All slides were coded and analyzed blind to treatment.
Following CB, PV, and CR staining, the sections were scanned and their images were captured by a Nikon (DS-Fi2) microscope. CB, PV, and CR optical density of the regions of interest were measured using NIS-Elements microscope imaging software. The analysis of CB, PV, and CR optical density was performed following the methods of Piyabhan et al18 and made blind to the diagnostic category of the cases. Briefly, five regions of interest were measured in each of the subfields of prefrontal cortex, striatum, and CA1-3 of the hippocampus of all sections. Each region of interest was 500×500 µm. The distance between each region of interest was approximately 500 µm. Three brain-sectioned slides were used for optical density measurement of CB, PV, and CR of each animal. The value measured is the sum of the optical densities of all pixels in the region divided by the number of pixels. The average values from five regions of interest in each brain subfield of each subject was used for statistical analysis. Kruskal-Wallis non-parametric test was performed to determine the difference of cerebral CB, PV, and CR optical density among all groups, followed by multiple pair-wise comparison using Bonferroni-corrected Mann-Whitney U-test. Statistical significances were defined as P<0.05. All statistical analysis was performed using SPSS V13 for windows (SPSS Inc.).
Results
Cognitive enhancement effect study
Novel object recognition test
In the novel object recognition test, rats receiving sub-chronic administration of PCP and rats receiving PCP+Brahmi had a significant decrease of DR score compared with control (P<0.001 and P<0.05, respectively). Interestingly, there was a significant increase of DR score among rats receiving PCP+Brahmi compared with PCP alone (P<0.05). DR score of PCP+Brahmi was significantly decreased compared with control (P<0.05) (Table 1).
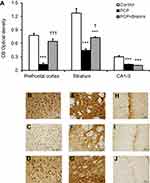
CB immunohistochemistry
As shown in Figure 1, CB immunoreactivity in the prefrontal cortex, striatum, and CA1–3 of rats with sub-chronic PCP administration were significantly decreased compared with the control group (P<0.001). In the PCP+Brahmi treatment group, there were significant increases of CB immunoreactivity in the prefrontal cortex (P<0.001) and in the striatum (P<0.05) compared with rats receiving PCP alone. However, there was no significant difference of CB immunoreactivity in CA1–3 between rats receiving PCP+Brahmi and rats receiving PCP alone. CB immunoreactivity of the prefrontal cortex was not significantly different between PCP+Brahmi and control. However, there were significant decreases of CB immunoreactivity in the striatum and CA1–3 of PCP+Brahmi compared with control (P<0.001).
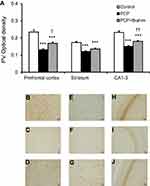
PV immunohistochemistry
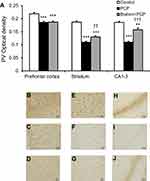
Similar to CB, PV immunoreactivity was also found to diminish in the prefrontal cortex, striatum, and CA1–3 among rats receiving PCP administration when compared with the control group (P<0.001) (Figure 2). When treated with PCP+Brahmi, the PV immunoreactivity was significantly increased in the prefrontal cortex (P<0.05) and in CA1–3 (P<0.01), but not in the striatum, compared with rats treated with PCP alone. However, it should be noted that the PV immunoreactivity of rats treated with PCP+Brahmi remained significantly lower in all three brain regions than the control group (P<0.001) (Figure 2).
CR immunohistochemistry
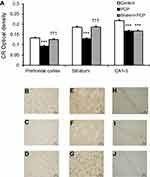
Unlike CB and PV, CR immunoreactivity was significantly reduced only in the prefrontal cortex of rats treated with PCP compared with the control group (P<0.001) (Figure 3). When treated with PCP+Brahmi, the CR immunoreactivity in the prefrontal cortex was significantly higher than rats treated with PCP only (P<0.01). CR immunoreactivity in the striatum and in CA1–3 was not significantly different between the PCP administration group and the control group. Interestingly, it was found that rats in the PCP+Brahmi group had significantly lower CR immunoreactivity in the striatum than both the control group and PCP alone group (P<0.001).
Neuroprotective effect study
Novel object recognition test
The novel object recognition test was also applied to evaluate the neuroprotective effect of Brahmi. Rats with sub-chronic administration of PCP had significantly decreased DR score compared with controls (P<0.001) (Table 2). Rats treated with Brahmi+PCP had a significantly higher DR score than rats treated with PCP alone (P<0.05); however, this DR score was still lower than the control group (P<0.01) (Table 2).
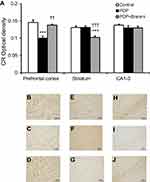
CB immunohistochemistry
To study the neuroprotective effect of Brahmi, experimental rats were given Brahmi prior to the administration of PCP. As expected, rats receiving PCP alone had significant reduction of CB immunoreactivity in the prefrontal cortex, striatum, and CA1–3 when compared to the control group (P<0.001). It was interesting to find that rats receiving Brahmi+PCP had significantly higher CB immunoreactivity in all three brain sections than rats treated with PCP alone (P<0.001 for the prefrontal cortex and CA1–3 and P<0.05 for the striatum) (Figure 4). Nevertheless, the CB immunoreactivity in the striatum and in CA1–3 among rats treated with Brahmi+PCP remained significantly lower than the control rats (P<0.001) (Figure 4).
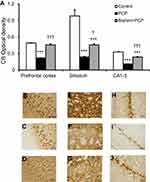
PV immunohistochemistry
Similar with CB, PV immunoreactivity was significantly lower in the prefrontal cortex, striatum, and CA1–3 in rats with PCP administration than the control rats (P<0.001) (Figure 5). Only in the striatum and in CA1–3 did the PV immunoreactivity appear significantly increased in the rats treated with Brahmi+PCP group when compared with PCP alone (P<0.01, P<0.001, respectively) (Figure 5). There was no significant difference of PV immunoreactivity in the prefrontal cortex between the PCP group and the Brahmi+PCP group. Compared to control, PV immunoreactivity of Brahmi+PCP was significantly decreased in the prefrontal cortex (P<0.001), striatum (P<0.001), and CA1–3 (P<0.01).
CR immunohistochemistry
Figure 6 reveals a significant decrease of CR immunoreactivity in the prefrontal cortex, striatum, and CA1–3 of PCP-administration rats compared with control (P<0.001). The CR immunoreactivity of the Brahmi+PCP group was found to be significantly higher in the prefrontal cortex and striatum when compared with the PCP-only group (P<0.001). However, there was no significant difference of CR immunoreactivity in CA1–3 of Brahmi+PCP rats and PCP-alone rats. CR immunoreactivity of Brahmi+PCP rats was not significantly different from control in the areas of the prefrontal cortex and striatum. However, it was significantly reduced in CA1–3 compared with control (P<0.001).
Discussion
Schizophrenia is a debilitating mental disorder that limits a patient’s ability to perform ordinary daily life. Individuals with schizophrenia usually experience symptoms such as hallucinations or delusions (also described as positive symptoms), lack of motivation or social engagement (known as negative symptoms), and impaired cognitive function. Although the pharmaceutical treatments are continuously developed, they usually tackle the positive and negative symptoms. The cognitive dysfunction is often neglected. Brahmi has been previously shown to restore cognitive deficit and to protect the brain from memory impairment. The present study again demonstrated the partial restoration of cognitive deficit and neuroprotective effects of Brahmi in a rat model of schizophrenia. The underlying mechanism of Brahmi was also elucidated.
Administration of PCP to the rats has been acceptably used to generate a schizophrenic-like animal model.6–9,16–18,36–38 In the present study, sub- chronic administration of PCP resulted in significant deficits in object recognition memory in all rats. This finding is consistent with several previous studies.9,16–18,36–38 Remarkably, the rats receiving Brahmi either after or before the PCP administration demonstrated a significant improvement in the novel object recognition task, indicating the partial restoration of cognitive deficit and the neuroprotective effects of Brahmi.
We further attempted to elucidate the possible mechanism of action of Brahmi that underlined the memory effects in the schizophrenic-like model. GABA is one of the neurotransmitters that is involved in the memory pathway. Decreased GABA level has been shown to be associated with cognitive impairment, both in normal and in schizophrenic individuals.22–27 We, thus, investigated the presence of GABAergic neurons in the prefrontal cortex and hippocampus (CA1–3), and also GABAergic interneurons in the striatum. Calcium-binding proteins (CBPs) which localize mainly in GABAergic neurons include calbindin (CB), parvalbumin (PV), and calretinin (CR), and these proteins express in non-overlapping populations.39 By measuring CBPs-immunoreactive neurons, virtually all GABAergic neurons in the cortex were explored.
It was found that the schizophrenic-like model in the neuroprotective effect study and in the cognitive enhancement effect study had decreases in CB and PV immunoreactivity neurons in the prefrontal cortex, striatum, and CA1–3. Deficits of both CB and PV immunoreactivity were previously reported in the frontal cortex from the schizophrenic-like model.40–42 Deficits in PV immunoreactivity were also investigated in the hippocampus of both schizophrenic-like models and patients.42–44 However, the study in the striatum has not been reported previously. Interestingly, CR immunoreactivity was reduced in all brain areas in the schizophrenic-like model from the neuroprotective effect study, but it was found to be reduced only in the prefrontal cortex among rats from the cognitive enhancement effect study. Overall, these CBPs immunoreactivity indicated regional differences of GABAergic neuronal density in the studied areas. While diminishing numbers of CB and PV were found throughout the prefrontal cortex, striatum, and CA1–3, the selective depletion of CR found in the prefrontal cortex suggested that the vulnerability of this CBP was probably more prominent in that region. This finding was consistent with the previous study using a Methamphetamine-induced schizophrenic-like model which reported that cortical CR immunoreactivity was more vulnerable than those in hippocampal regions.42 It was suggested that GABAergic neurons expressing CR were not changed in schizophrenia.45 Our present study at least showed that CBPs-containing GABAergic neurons are likely to be one mechanism implicated in cognitive deficits in schizophrenia.
In the present study, the cognitive enhancement effect of Brahmi was tested in rats receiving PCP administration followed by Brahmi. After treatment, CB immunoreactivity was significantly increased in the prefrontal cortex and striatum, while increased PV immunoreactivity was observed in the refrontal cortex and CA1–3. CR immunoreactivity was significantly increased only in the prefrontal cortex compared to the rats receiving only PCP administration. Overall, the increases of all three CBPs immunoreactivity in the prefrontal cortex, especially the CB and CR in which their immunoreactivity nearly returned to the similar densities found in the control rats, indicate the almost complete restoration of GABAergic neurons in this brain region after treating with Brahmi.
The neuroprotective effect of Brahmi was also investigated using the rats receiving Brahmi before PCP administration. An increased CB immunoreactivity was found in all brain areas. PV immunoreactivity was significantly increased in the striatum and CA1–3, while CR immunoreactivity was significantly increased in the prefrontal cortex and striatum when compared to the rats receiving only PCP. Findings suggest that CB neurons in all three brain regions are likely to be protected from PCP by Brahmi. The striatum was also the only area that all CBPs immunoreactivity demonstrated the significant neuroprotective effect in the rats receiving Brahmi prior to PCP administration.
Several studies have demonstrated the connection of GABA and the cognitive function and memory.46,47 GABA plays important roles in encoding and maintaining information in working memory.48 According to the protein synthesis theory, the memory formation is the result of various proteins in the neuronal synaptic networks.49–51 GABA was found to be involved in this de novo protein synthetic process by stimulating growth hormone release, which subsequently increases the brain protein synthesis.52–54 Reduced GABAergic neurons in the prefrontal cortex led to delayed-task performance in monkeys.47 Even though the mechanism underlying the reduction of GABAergic neurons is yet to be investigated, it has been shown that administration of GABA to male rats could increase their novel object recognition task.55 Brahmi is known for its reputation of memory boosting and cognitive facilitating effects.56 A study showed that Brahmi could recover spatial recognition memory deficits in epileptic rats by increasing GABA receptors in the striatum close to normal level.57 Our study also showed the increased numbers of GABAergic neurons in the rats receiving Brahmi both before and after the PCP administration. This histological finding was correlated with the final outcomes – the improvement of cognitive function – in the rats treated with Brahmi. Overall, the present study demonstrated the effects of Brahmi on partial restoration of object recognition memory loss and neuroprotection. This study also elucidated Brahmi’s possible underlying mechanism of actions via the GABAergic neurons.
The nootropic activity of Brahmi has been attributed to the presence of two saponins, namely bacoside A and B.58,59 Besides the function on the cognition, these active compounds have been shown to possess an anxiolytic effect, antidepressant activity, anticonvulsive action, and antioxidant activity.60 Brahmi can inhibit in vitro free radical formation and DNA damage in a dose-dependent manner.61 Moreover, Brahmi has also been recognized as being effective in the treatment of mental illness and epilepsy.61 Interestingly, its action was mediated via an increase of GABA receptors in the cerebral cortex.62 More studies also demonstrated the actions of Brahmi that improved behavioral deficits in epileptic rats by increasing GABA receptors in the hippocampus and cerebellum.63,64 Moghaddam et al65 suggested that ketamine, as well as other NMDA receptor antagonists, may produce disinhibition of GABAergic or other inhibitory inputs. Additionally, there is evidence to support the suggestion that a PCP/ketamine model of schizophrenia may incorporate GABAergic dysfunction. Yonezawa et al66 found that an acute PCP administration may reduce cortical GABAergic function in rats, suggesting that the NMDA receptor antagonists selectively reduce GABAergic transmission. Abdul-Monim et al67 found significant deficits of PV in CA2/3 and DG of the hippocampus following sub-chronic PCP (2 mg/kg) administration to rats. Therefore, the partial cognitive restoration and neuroprotection observed in the present study might be due to Brahmi affecting on increase in GABAergic transmission.
As we discussed earlier, animals receiving PCP exhibits schizophrenic-like symptoms including memory loss (poorer object recognition), attention deficit, and increased locomotor activity.32–34 It is possible that the poorer object recognition in schizophrenic-like rats observed in our present study was due to impaired attention to the objects, possibly induced by increased animals’ locomotor activity. However, there are no reports on the direct effect of Brahmi on this locomotor function, and we did not use any task to observe this activity and the consequent attention ability of rats in our present study. However, Brahmi has been reported as the herbal medicine to directly ameliorate attention deficit through the reduction of Acetyl choline esterase (AChE) activity.68 The memory improvement (as seen in our present study) and the attention improvement (as seen in the study by Peth-Nui et al68) could be mediated through an increased GABAergic function and through an increased cholinergic function, respectively. It is likely that the decreased locomotor function could be another possible underlying mechanism of both memory and attention improvement, and it should be the subject of further exploration.
In order to know the Brahmi effect differs on a schizophrenia model compared to that on controls, a Control+Brahmi group should be investigated; however, this was not undertaken in our present study. Prabhakar et al69 found that Brahmi has no effects on memory in normal animals, which was different when compared to human studies, which have been reported by Stough et al70 and Roodenrys et al71. Therefore, the findings from our present study may suggest the therapeutic and neuroprotective effects of Brahmi in schizophrenic-like rats. However, a study in a Control+Brahmi group is needed for this to be confirmed in future study.
Conclusion
In conclusion, our results suggested that decreased GABAergic neurons, assessed by reduced CB, PV, and CR immunoreactivity, in the brain were responsible for the cognitive deficit in schizophrenic subjects, which are consistent with reports from several studies. The present study also showed that Brahmi potentiated a partial cognitive enhancement and neuroprotective effects against cognitive deficit in schizophrenia by reversing the alterations in cerebral CB, PV, and CR. Overall, it is suggested that Brahmi could be a valuable alternative medicine for partial treatment of cognitive deficit developed in patients with schizophrenia. The mechanisms of its actions would be via glutamatergic neurotransmission from Piyabhan and colleagues16–18 studies and via GABAergic neurotransmission from the present study.
Acknowledgments
The present study is funded by Thammasat University, Thailand. The grant number is AE 015/2015.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Braff DL, Heaton R, Kuck J, et al. The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry. 1991;48(10):891–898. doi:10.1001/archpsyc.1991.01810340023003
2. Steinpreis RE. The behavioral and neurochemical effects of phencyclidine in humans and animals: some implications for modeling psychosis. Behav Brain Res. 1996;74:45–55. doi:10.1016/0166-4328(95)00162-X
3. Bobes J, Garcia-Portilla MP, Bascaran MT, Saiz PA, Bousoño M. Quality of life in schizophrenic patients. Dialogues Clin Neurosci. 2007;9(2):215–226.
4. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi:10.1016/S0140-6736(13)60733-3
5. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi:10.1037/0894-4105.12.3.426
6. Abdul-Monim Z, Reynolds GP, Neill JC. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res. 2006;169(2):263–273. doi:10.1016/j.bbr.2006.01.006
7. Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res. 2007;184(1):31–38. doi:10.1016/j.bbr.2007.06.012
8. McLean SL, Beck JP, Woolley ML, Neill JC. A preliminary investigation into the effects of antipsychotics on sub-chronic phencyclidine-induced deficits in attentional set-shifting in female rats. Behav Brain Res. 2008;189(1):152–158. doi:10.1016/j.bbr.2007.12.029
9. McKibben CE, Jenkins TA, Adams HN, Harte MK, Reynolds GP. Effect of pretreatment with risperidone on phencyclidine-induced disruptions in object recognition memory and prefrontal cortex parvalbumin immunoreactivity in the rat. Behav Brain Res. 2010;208(1):132–136. doi:10.1016/j.bbr.2009.11.018
10. Walters Y, Agius M. Do atypical antipsychotics improve cognition? Psychiatr Danub. 2014;26(Suppl 1):285–288.
11. Shinomol GK, M.S. Bharath M. Exploring the role of “Brahmi” (Bacopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:33–49. doi:10.2174/187221411794351833
12. Rastogi M, Ojha RP, Prabu PC, Devi BP, Agrawal A, Dubey GP. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology. 2012;13:183–195. doi:10.1007/s10522-011-9367-y
13. Holcomb LA, Dhanasekaran M, Hitt AR, Young KA, Riggs M, Manyam BV. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J Alzheimers Dis. 2006;9(3):243–251. doi:10.3233/JAD-2006-9303
14. Dhanasekaran M, Tharakan B, Holcomb LA, Hitt AR, Young KA, Manyam BV. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother Res. 2007;21:965–969. doi:10.1002/(ISSN)1099-1573
15. Sarkar S, Mishra BR, Praharaj SK, Nizamie SH. Add-on effect of Brahmi in the management of schizophrenia. J Ayurveda Integr Med. 2012;3(4):223–225. doi:10.4103/0975-9476.104448
16. Piyabhan P, Wetchateng T. Cognitive enhancement effects of Bacopa monnieri (Brahmi) on novel object recognition and VGLUT1 density in the prefrontal cortex, striatum, and hippocampus of sub-chronic phencyclidine rat model of schizophrenia. J Med Assoc Thai. 2013;96(5):625–632.
17. Piyabhan P, Wetchateng T. Bacopa monnieri (Brahmi) enhanced cognitive function and prevented cognitive impairment by increasing VGLUT2 immunodensity in prefrontal cortex of sub-chronic phencyclidine rat model of schizophrenia. J Med Assoc Thai. 2015;98(Suppl 3):S7–15.
18. Piyabhan P, Wannasiri S, Naowaboot J. Bacopa monnieri (Brahmi) improved novel object recognition task and increased cerebral vesicular glutamate transporter type 3 in sub-chronic phencyclidine rat model of schizophrenia. Clin Exp Pharmacol Physiol. 2016;43(12):1234–1242. doi:10.1111/cep.2016.43.issue-12
19. Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmuller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379–382. doi:10.1016/0304-3940(80)90178-0
20. Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi:10.1001/archpsyc.1991.01810350036005
21. Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi:10.1001/archpsyc.1995.03950160008002
22. Toru M, Watanabe S, Shibuya H, et al. Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr Scand. 1988;78(2):121–137. doi:10.1111/j.1600-0447.1988.tb06312.x
23. Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi:10.1001/archpsyc.57.3.237
24. Reynolds GP, Czudek C, Andrews HB. Deficit and hemispheric asymmetry of GABA uptake sites in the hippocampus in schizophrenia. Biol Psychiatry. 1990;27(9):1038–1044. doi:10.1016/0006-3223(90)90039-5
25. Simpson MD, Slater P, Deakin JF, Royston MC, Skan WJ. Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neurosci Lett. 1989;107(1–3):211–215. doi:10.1016/0304-3940(89)90819-7
26. Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese Macaque Monkeys. Exp Brain Res. 1989;75:457–469. doi:10.1007/BF00249897
27. Volk DW, Lewis DA. GABA targets for the treatment of cognitive dysfunction in schizophrenia. Curr Neuropharmacol. 2005;3(1):45–62. doi:10.2174/1570159052773396
28. Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi:10.1016/S0893-133X(00)00137-8
29. Nicholi AM
30. Smith DE. A clinical approach to the treatment of phencyclidine (PCP) abuse. Psychopharmacol Bull. 1980;16:67–70.
31. Liden CB, Lovejoy FH
32. Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi:10.1126/science.281.5381.1349
33. Hanania T, Hillman GR, Johnson KM. Augmentation of locomotor activity by chronic phencyclidine is associated with an increase in striatal NMDA receptor function and an upregulation of the NR1 receptor subunit. Synapse. 1999;31:229–239. doi:10.1002/(ISSN)1098-2396
34. Neill JC, Barnes S, Cook S, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128(3):419–432.
35. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego: Academic Press; 1998.
36. Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2007;32:514–521. doi:10.1038/sj.npp.1301047
37. Hashimoto K, Fujita Y, Shimizu M, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur J Pharmacol. 2005;219:114–117. doi:10.1016/j.ejphar.2005.07.002
38. Hashimoto K, Ishima T, Fujita Y, et al. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the novel selective alpha7 nicotinic receptor agonist SSR180711. Biol Psychiatry. 2008;63:92–97. doi:10.1016/j.biopsych.2007.04.034
39. Reynolds GP, Zhang ZJ, Beasley CL. Neurochemical correlates of cortical GABAergic deficits in schizophrenia: selective losses of calcium binding protein immunoreactivity. Brain Res Bull. 2001;55(5):579–584. doi:10.1016/S0361-9230(01)00526-3
40. Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24(3):349–355. doi:10.1016/S0920-9964(96)00122-3
41. Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52(7):708–715.
42. Veerasakul S, Thanoi S, Reynolds GP, Nudmamud-Thanoi S. Effect of methamphetamine exposure on expression of calcium binding proteins in rat frontal cortex and hippocampus. Neurotox Res. 2016;30(3):427–433. doi:10.1007/s12640-016-9628-2
43. Zhang Z, Sun J, Reynolds GP. A selective reduction in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia patients. Chin Med J (Engl). 2002;115(6):819–823.
44. Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114(7):893–898. doi:10.1007/s00702-007-0627-6
45. Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia–postmortem studies and animal models. Neurotox Res. 2004;6(1):57–61. doi:10.1007/BF03033297
46. Katz RJ, Liebler L. GABA involvement in memory consolidation: evidence from posttrial amino-oxyacetic acid. Psychopharmacology. 1978;56:191–193. doi:10.1007/BF00431848
47. Michels L, Martin E, Klaver P, et al. Frontal GABA levels change during working memory. PLoS One. 2012;7:1–8. doi:10.1371/journal.pone.0031933
48. Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30:319–333. doi:10.1016/S0896-6273(01)00285-9
49. Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. doi:10.1037/0033-2909.96.3.518
50. Rossato JI, Bevilaqua LRM, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi:10.1101/lm.422607
51. Balderas I, Chavez-Hurtado J, Rodriguez-Ortiz CJ, McGaugh JL, Salgado-Tonda P, Bermudez-Rattoni F. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem. 2008;15:618–624. doi:10.1101/lm.1028008
52. Tujioka K, Okuyama S, Yokogoshi H, et al. Dietary g-aminobutyric acid affects the brain protein synthesis rate in young rats. Amino Acids. 2007;32:255–560. doi:10.1007/s00726-006-0358-2
53. Gibney J, Wallace JD, Spinks T, et al. The effects of 10 years of recombinant human growth hormone (GH) in adult GH-deficient patients. J Clin Endocrinol Metab. 1999;84:2596–2602. doi:10.1210/jcem.84.8.5916
54. Deijen JB, de Boer H, van der Veen EA. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology. 1998;23:45–55. doi:10.1016/S0306-4530(97)00092-9
55. Thanapreedawat P, Kobayashi H, Inui N, et al. GABA affects novel object recognition memory and working memory in rats. J Nutr Sci Vitaminol. 2013;59(2):152–157.
56. Singh HK, Dhawan BN. Drugs affecting learning and memory. In: Tandon PN, Bijiani V, Wadhwa S, editors. Lectures in neurobiology, vol. 1. New Delhi: Wiley Eastern; 1992:189–207.
57. Mathew J, Soman S, Sadanandan J, Paulose CS. Decreased GABA receptor in the striatum and spatial recognition memory deficit in epileptic rats: effect of Bacopa monnieri and bacoside-A. J Ethnopharmacol. 2010;130(2):255–261. doi:10.1016/j.jep.2010.04.025
58. Singh HK, Dhawan BN. Neuropsycho-pharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi). Ind J Pharmcol. 1997;29:S359–365.
59. Singh HK, Rastogi RP, Srimal RC, Dhawan BN. Effect of bacoside A and B on the avoidance responses in rats. Phytother Res. 1988;2:70–75. doi:10.1002/ptr.2650020205
60. Reas SK, Amee K, Paulose CS. Glutamate receptor gene expression and binding studies in pilocarpine induced epileptic rat: neuroprotective role of Bacopa monnieri extract. Epilep Behav. 2008;12:54–60. doi:10.1016/j.yebeh.2007.09.021
61. Russo A, Izzo AA, Borrelli F, Renis M, Vanella A. Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother Res. 2003;17:46–54. doi:10.1002/ptr.1061
62. Mathew J, Balakrishnan S, Antony S, Abraham PM, Paulose CS. Decreased GABA receptor in the cerebral cortex of epileptic rats: effect of Bacopa monnieri and Bacoside-A. J Biomed Sci. 2012;24:19–25.
63. Mathew J, Gangadharan G, Kuruvilla KP, Paulose CS. Behavioral deficit and decreased GABA receptor functional regulation in the hippocampus of epileptic rats: effect of Bacopa monnieri. Monnieri Neurochem Res. 2011;36(1):7–16. doi:10.1007/s11064-010-0253-9
64. Mathew J, Peeyush Kumar T, Khan RS, Paulose CS. Behavioral deficit and decreased GABA receptor functional regulation in the cerebellum of epileptic rats: effect of Bacopa monnieri and bacoside A. Epilepsy Behav. 2010;17(4):441–447. doi:10.1016/j.yebeh.2009.12.014
65. Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi:10.1523/JNEUROSCI.17-08-02921.1997
66. Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol. 1998;341(1):45–56. doi:10.1016/S0014-2999(97)01435-0
67. Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol. 2007;21:198–205. doi:10.1177/0269881107067097
68. Peth-Nui T, Wattanathorn J, Muchimapura S, et al. Effects of 12 week bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evid Based Complement Alternat Med. 2012;2012:606424. doi:10.1155/2012/606424
69. Prabhakar S, Saraf MK, Pandhi P, Anand A. Bacopa monniera exerts antiamnesic effect on diazepam-induced anterograde amnesia in mice. Psychopharmacology (Berl). 2008;200(1):27–37. doi:10.1007/s00213-007-1049-8
70. Stough C, Lloyd J, Clarke J, et al. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl). 2001;156:481–484. doi:10.1007/s002130100815
71. Roodenrys S, Booth D, Bulzomi S, Phipps A, Micallef C, Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002;27:279–281. doi:10.1016/S0893-133X(02)00301-9
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.