Back to Journals » Journal of Pain Research » Volume 11
Effect of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain management and short-term outcomes after gastric cancer resection: a retrospective analysis of 3,042 consecutive patients between 2010 and 2015
Authors Wang LP, Li X, Chen H, Liang J, Wang Y
Received 21 March 2018
Accepted for publication 11 July 2018
Published 5 September 2018 Volume 2018:11 Pages 1743—1749
DOI https://doi.org/10.2147/JPR.S168892
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Michael Schatman
Liping Wang, Xuan Li, Hong Chen, Jie Liang, Yu Wang
Department of Anaesthesiology, Harbin Medical University Cancer Hospital, Harbin, China
Background: Effective postoperative analgesia is essential for rehabilitation after surgery. Many studies have compared different methods of postoperative pain management for open abdominal surgery. However, the conclusions were inconsistent and controversial. In addition, few studies have focused on gastric cancer (GC) resection. This study aimed to determine the effects of patient-controlled epidural analgesia (PCEA) on postoperative pain management and short-term recovery after GC resection compared with those of patient-controlled intravenous analgesia (PCIA).
Methods: We analyzed retrospectively collected data on patients with non-metastatic GC diagnosed between 2010 and 2015 who underwent resection in a university hospital. PCIA and PCEA documented by the acute pain service team were retrospectively analyzed. A propensity score-matched analysis that incorporated preoperative variables was used to compare the short-term outcomes between the PCIA and PCEA groups.
Results: In total, 3,042 patients were identified for analysis. Propensity score matching resulted in 917 patients in each group. The PCEA group exhibited lower pain scores in the recovery room and on the first and second postoperative days (P=0.0005, P=0.0065, and P=0.0034 respectively). The time to the first passage of flatus after surgery was shorter in the PCEA group than in the PCIA group (P=0.032). The length of the hospital stay was 12.6±7.2 and 11.8±6.6 days in the PCEA and PCIA groups, respectively. No significant differences were observed in the length of hospital stay or the incidence of complications after surgery.
Conclusion: PCEA provided more effective postoperative pain management and a shorter time to the first passage of flatus than PCIA after GC resection. However, it did not have an effect on the length of hospital stay or the incidence of postoperative complications.
Keywords: gastrectomy, gastric cancer, patient-controlled analgesia, epidural analgesia, short-term outcomes, pain treatment, analgesia-related complications
Introduction
Gastric cancer (GC) is one of the 5 most prevalent cancers in China.1 Surgical treatment with distal or total gastrectomy is the most effective but invasive treatment for this cancer. Although surgical techniques have greatly improved, pain control and the recovery after such invasive operations remain prominent clinical problems.
Good postoperative pain control is an important part of adequate postoperative care.2 Poor pain control leads to a delay in postoperative recovery, resulting in a prolonged hospital stay and increased costs. Additionally, it also compromises the patient’s physical and mental health.3 Currently, 2 major postoperative continuous analgesic options exist as follows: patient-controlled epidural analgesia (PCEA) and patient-controlled intravenous analgesia (PCIA). The optimal analgesia technique following open abdominal surgery is still controversial. A thoracic epidural is often recommended according to the enhanced recovery protocol of open abdominal surgery. Many investigations have demonstrated that PCEA provides superior postoperative analgesia compared with PCIA. Additionally, some reports have shown that epidural postoperative analgesia could improve the outcome and survival after major surgery.4–9 However, many other studies have shown that epidural analgesia has no relevant effect on the reduction of morbidity and mortality after major surgery.10–13 Nonetheless, few previously mentioned studies have focused on the effect of PCEA in patients with GC.
Many patients are reluctant to accept epidural anesthesia because they fear the procedure. Similarly, some surgeons do not favor regional anesthesia because of concerns that the total intervention time would increase. In addition, epidural catheters are associated with rare but significant complications.14 PCEA can be recommended for patients who undergo GC resection only when the benefit of PCEA on pain control and postoperative recovery can be demonstrated.
The aim of this study was to compare the quality of pain relief and short-term postoperative outcomes between PCEA and PCIA in patients who underwent open resection of GC.
Materials and methods
After this retrospective study was approved by the institutional review board of Harbin Medical University Cancer Hospital, a systematic retrospective review of the medical records was performed. The need for written informed consents was waived because this study was part of an audit, conformed to standards for minimal risk research, and did not affect patient safety or clinical care. Moreover, all individual information was securely protected by delinking identifying information from the main data set and was available only to investigators. All the data were analyzed anonymously.
Patients diagnosed with non-metastatic GC between 2010 and 2015 who underwent gastrectomy and received postoperative analgesia were included. Data on these patients were collected in our institutional computer-based documentation system. The following cases were excluded: patients undergoing laparoscopic gastrectomy; those with metastatic disease; and patients who did not complete 3 days of patient-controlled analgesia.
Patients in the PCEA group had 1 catheter inserted at the T8–9 level before the induction of general anesthesia. Intravascular or intrathecal catheter placement was excluded using a test dose of 3 mL lidocaine (1.33%). After confirming the correct placement of the catheter and effectively providing analgesia, general anesthesia was induced. In these patients, postoperative analgesia was provided by the epidural infusion of a local anesthetic and opioid. The standard local anesthetic concentrations used were 0.2% ropivacaine or 0.15% bupivacaine. The standard opioid concentrations used were 2–3 µg/mL fentanyl or 0.3 µg/mL sufentanil. The background continuous rate was 3 mL/h, and the bolus dose was 2 mL with a 15-minute lockout time. Each patient in the PCIA group received intravenous opioids at a continuous basal rate of 3 mL/h (sufentanil 0.5 µg/mL or hydromorphone 0.02 mg/mL), with rescue boluses of 3 mL every 10 minutes if needed. The first evaluation after surgery was performed in the post-anesthesia care unit (PACU), and the efficacy of the analgesia regimen was documented.
During the following postoperative days, the acute pain service recorded the intensity of postoperative pain using a visual analog scale (VAS). Pain was graded from 0 cm (no pain) to 10 cm (worst pain imaginable). The following side effects were evaluated by the acute pain service at least once a day: incidence of hypotension (systolic blood pressure <90 mmHg); excessive sedation; nausea or vomiting; and delirium.
The time to the first passage of flatus after the surgery (days), length of hospital stay, and complications after the operation were collected in the electronic database and analyzed.
Patients who received PCEA were matched to those who received PCIA on a 1:1 ratio using propensity score matching. This matching was used to obtain groups of patients corresponding to the 2 analgesic modalities that were balanced with regard to potential confounding baseline variables. Propensity scores were calculated using a nonparsimonious multivariable logistic regression model in which the mode of analgesia was used as a dependent variable. The independent variables were the following 5 confounding variables for acute pain after GC resection: (1) age, (2) gender, (3) invasiveness (type of surgical procedure), (4) the identity of the surgeon, and (5) the duration of the surgical procedure. The sets of matched patients were compared with regard to the following 3 primary outcomes: (1) the VAS scores on the day of surgery and the first, second, and third days after surgery; (2) the time to the first passage of flatus after surgery; and (3) the length of hospital stay.
Statistical analysis was performed using SPSS version 22.0 for Windows (IBM Corp., Armonk, NY, USA) and STATA (Version 13; StataCorp LLC, College Station, TX, USA). Nominal scale variables were described using frequencies and analyzed using the chi-squared test. Fisher’s exact test was used for small sample sizes (expected frequencies <5). Quantitative data are presented as mean ± SD or medians (inter-quartile range) according to the distribution of each. Student’s t-test was used for comparisons when variables were normally distributed and variances were equivalent. Otherwise, Wilcoxon rank-sum tests were used. P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
We obtained data on 3,312 patients who underwent gastrectomy for GC and were treated by the acute pain service of the Department of Anesthesiology between 5 January 2010 and 31 January 2015. Using the exclusion criteria described, 80 patients were excluded because metastasis was discovered during the operation, and 102 were excluded due to laparoscopic gastrectomy. In the PCEA group, the epidural catheter was removed in 88 patients within 72 hours. Of these, epidural analgesia was discontinued in 17 and 71 patients due to continuous hypotension and epidural malfunction, respectively. Patients aged >65 years had an increased risk of early epidural discontinuation (Table 1). We identified 3,042 patients for analysis, of whom 42.6% (n=1,297) and 57.4% (n=1,745) received PCEA and PCIA, respectively (Figure 1). Of the 3,042 records that remained, 1,834 were successfully matched on a 1:1 ratio based on the predetermined confounding variables and baseline characteristics, with 917 patients included in each group. The patients in the matched groups were similar with respect to both the matched characteristics and other baseline characteristics (Table 2).
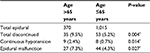  | Table 1 Comparison of early discontinued epidural analgesia Note: *P<0.05. |
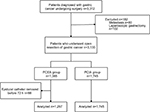  | Figure 1 Patient identification and exclusion. Abbreviations: PCEA, patient-controlled epidural analgesia; PCIA, patient-controlled intravenous analgesia. |
  | Table 2 Patient characteristics after propensity score matching Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; i.v., intravenous. |
Efficacy of pain treatment
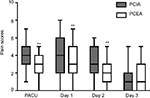
The postoperative pain intensity of all patients was determined using a VAS score (0–10). Analysis of the VAS scores between the propensity-matched groups demonstrated that patients treated with PCEA experienced less pain than those treated with PCIA in the recovery room on the day of surgery and days 1 and 2 after surgery. The median VAS scores on the day of surgery and days 1 and 2 after surgery were 2.9, 3.0, and 2.1 in the PCEA group, respectively, while the scores in the PCIA group were 4.0, 4.0, and 3.1, respectively. No superior analgesic effect was observed in the PCEA group on day 3 (Figure 2).
Recovery
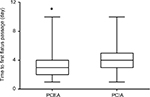
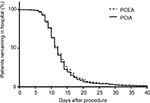
The time to the first passage of flatus after surgery was 3.5±1.5 and 4.3±1.5 days in the PCEA and PCIA groups, respectively; the time in the PCEA group was significantly shorter than the PCIA group (P<0.05, Figure 3). The length of hospital stay was 12.6 and 11.8 days in the PCEA and the PCIA groups, respectively. No significant difference was found between the 2 groups with regard to the length of hospital stay (P>0.05, Figure 4).
Analgesia-related complications
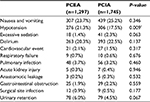
We observed a significantly higher rate of hypotension in patients who received PCEA (21.3%) than in those who received PCIA (17.5%). No differences were observed between the 2 groups with respect to other side effects, including nausea, vomiting, and excessive sedation (Table 3).
Postoperative complications
Postoperative complications and their incidences are listed in Table 3. A cardiovascular event was defined as acute myocardial infarction, angina, congestive heart failure, cardiac shock, third-degree heart block, or major (supra) ventricular tachyarrhythmia. Respiratory failure was defined as the need for prolonged ventilation or reintubation. Acute kidney injury was diagnosed by an increase in serum creatinine of >100 µmol/L or a serum creatinine level >300 µmol/L. Urinary retention was defined as the need for catheterization after removing the urinary drainage tube. No significant difference was observed in the incidence of complications between the 2 groups.
Discussion
Based on valid concerns, epidural analgesia is not preferred by some patients and doctors. Epidural administration of anesthesia and analgesia is considered a technique with a risk of serious complications. Although the incidence of neuraxial hematoma and abscess is <1 in 100,000 patients, use of a thoracic epidural catheter in surgical patients carries a 10- to 100-fold higher risk.15,16 So far, such serious complications have not occurred in our institution. PCEA is a safe technique for postoperative pain control. Additionally, in contrast to the subjective experience of many anesthetists, failure of epidural anesthesia and analgesia is a common clinical problem. On account of the development of technology that has increased the primary and secondary success rates, the failure of epidural anesthesia has decreased. Finally, epidural catheters may be incorrectly placed, migrate after initial correct placement due to body movement or deviate from the midline. In this study, 71 (5%) patients did not complete 3 days of analgesia because of epidural malfunction. The incidence of malfunction was in the acceptable range.
This retrospective analysis showed that the effect of PCEA was better than that of PCIA in patients undergoing resection for GC. The VAS score in the PCEA group was significantly lower than that in the PCIA one. The absolute difference was <2 cm on a 10-cm VAS. Some investigators believe this small statistically significant difference is not clinically relevant.17,18 A commonly accepted minimum difference to determine the clinical superiority is 20–30 mm on a 100-mm VAS, which is equal to a 2–3 cm difference on a 10-cm VAS. In contrast, a recent study Myles et al demonstrated that analgesic interventions that result in a change of 10 on a 100-mm VAS (a change of 1 on the 10-cm scale) indicate a clinically important improvement or deterioration.19 Pain was most severe in the PACU and on the first day after surgery. The VAS score was 4.0 in the PCIA group, which was higher than a score of 3.3 in the PCEA group (33 for the 100-mm scale). PCIA failed to provide a good pain control in the first stage after surgery. The difference in the VAS score between the 2 groups in the PACU and on postoperative days 1 and 2 was >1 (10 on the 100-mm scale). This result demonstrated that PCEA was superior to PCIA for postoperative pain management for patients who underwent gastric resection.
An intimate connection exists between the recovery speed and postoperative analgesic effect.20–22 This study showed a faster time to recovery of bowel functioning when the epidural was evaluated using the time to the first passage of flatus. Gastrointestinal dysfunction after abdominal surgery may have numerous causes, including the inflammatory response, autonomic nervous system dysfunction, and anesthetic and opioid administration. Owing to a decrease in sympathetic tone, stress response, and inflammatory processes, epidurals are associated with a faster postoperative return of gut function. The systemic absorption of local anesthetic may also be a factor in improving gastrointestinal motility based on its direct excitatory effect on intestinal smooth muscle.23,24
Generally, sympatholytic effects of epidural analgesia that cause vasodilation increase the incidence of postoperative hypotension, especially for patients undergoing gastrectomy. A total of 17 patients receiving epidurals required early discontinuation due to continuous hypotension. Nearly 22% of patients developed postoperative hypotension in the PCEA group, which is a much higher number than in the PCIA one. The consequences of the induced hypotension and the correct method with which to treat it are less clear. Standard therapy for treating hypotension typically starts with fluid loading. Excessive fluid loading to regain hemodynamic stability has largely unknown effects on splanchnic blood flow, particularly the supply and drainage of anastomotic regions for abdominal surgery.25 Aggressive fluid resuscitation may lead to bowel wall edema and subsequent anastomotic breakdown.11 Some studies have determined that PCEA does not influence the incidence of anastomotic leakage.26 In this study, the incidence of anastomotic leakage and ileus were similar regardless of the postoperative analgesia technique used. There was no significant difference in the incidence of other complications between the 2 groups.
PCEA can provide superior pain management and enhanced recovery of gastrointestinal function; therefore, PCEA should shorten the hospital stay. However, PCEA did not affect the length of hospital stay in this study. The duration of postoperative hospitalization is multifactorial and sometimes depends on factors other than medical factors, including the social situation and the patients’ and surgeons’ desire for continued hospital-based observation. These observations might explain the lack of difference in the hospital stay observed between the PCEA and PCIA groups.
There were certainly limitations to our study. Because this was a retrospective study performed at a single institution, our results may not be generalizable. First, specific discharge criteria were not pre-established. Surgeons’ professional habits and patients’ desires may have influenced the duration of the hospital stay. Second, administrative data sources did not adequately capture postoperative complications, which could have helped in evaluating the effect of PCEA on postoperative recovery. Third, patient satisfaction data were not collected in this study, which may have allowed the determination of whether improved patient pain scores translated into improved patient satisfaction.
In conclusion, our findings reveal that PCEA can provide superior postoperative pain management and shorten the time to the first passage of flatus. No difference was observed between groups regarding postoperative complications and the length of the hospital stay. PCEA is a safe technique that could improve short-term outcomes after gastrectomy for GC. However, older patients were more likely to have a risk of serious hypotension and epidural malfunction when PCEA was applied. In our opinion, epidural analgesia is a valid option for postoperative analgesia to improve short-term outcomes that can be used for patients undergoing gastrectomy after individual risk assessment.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Acknowledgment
This study was supported by the Innovation Research funding of the Fundamental Research Funds for the Provincial Universities (Grant#2017LCZX90 [YW]).
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Zheng R, Zeng H, Zhang S, Chen T, Chen W. National estimates of cancer prevalence in China. Cancer Lett. 2016;370(1):33–38. | ||
Wu CL, Rowlingson AJ, Partin AW, et al. Correlation of postoperative pain to quality of recovery in the immediate postoperative period. Reg Anesth Pain Med. 2005;30(6):516–522. | ||
Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97(4):1078–1085. | ||
Wu CL, Rowlingson AJ, Herbert R, Richman JM, Andrews RA, Fleisher LA. Correlation of postoperative epidural analgesia on morbidity and mortality after colectomy in Medicare patients. J Clin Anesth. 2006;18(8):594–599. | ||
Schneemilch CE, Schilling T, Bank U. Effects of general anaesthesia on inflammation. Best Pract Res Clin Anaesthesiol. 2004;18(3):493–507. | ||
Wu CL, Anderson GF, Herbert R, Lietman SA, Fleisher LA. Effect of postoperative epidural analgesia on morbidity and mortality after total hip replacement surgery in medicare patients. Reg Anesth Pain Med. 2003;28(4):271–278. | ||
Oh TK, Lim MC, Lee Y, Yun JY, Yeon S, Park SY. Improved Postoperative Pain Control for Cytoreductive Surgery in Women With Ovarian Cancer Using Patient-Controlled Epidural Analgesia. Int J Gynecol Cancer. 2016;26(3):588–593. | ||
Cummings KC, Xu F, Cummings LC, Cooper GS. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: a population-based study. Anesthesiology. 2012;116(4):797–806. | ||
Yanagimoto Y, Takiguchi S, Miyazaki Y, et al. Comparison of pain management after laparoscopic distal gastrectomy with and without epidural analgesia. Surg Today. 2016;46(2):229–234. | ||
Cummings KC, Patel M, Htoo PT, Bakaki PM, Cummings LC, Koroukian S. A comparison of the effects of epidural analgesia versus traditional pain management on outcomes after gastric cancer resection: a population-based study. Reg Anesth Pain Med. 2014;39(3):200–207. | ||
Hughes MJ, Ventham NT, Mcnally S, Harrison E, Wigmore S. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg. 2014;149(12):1224–1230. | ||
Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet. 2008;372(9638):562–569. | ||
Neuman MD, Rosenbaum PR, Ludwig JM, Zubizarreta JR, Silber JH. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA. 2014;311(24):2508–2517. | ||
Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101(4):950–959. | ||
Cook TM, Counsell D, Wildsmith JA; Royal College of Anaesthetists Third National Audit Project. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102(2):179–190. | ||
Cameron CM, Scott DA, Mcdonald WM, Davies MJ. A review of neuraxial epidural morbidity: experience of more than 8,000 cases at a single teaching hospital. Anesthesiology. 2007;106(5):997–1002. | ||
Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4(7):407–414. | ||
Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88(3):287–294. | ||
Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118(3):424–429. | ||
Zhu Z, Wang C, Xu C, Cai Q. Influence of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain control and recovery after gastrectomy for gastric cancer: a prospective randomized trial. Gastric Cancer. 2013;16(2):193–200. | ||
Bartha E, Carlsson P, Kalman S. Evaluation of costs and effects of epidural analgesia and patient-controlled intravenous analgesia after major abdominal surgery. Br J Anaesth. 2006;96(1):111–117. | ||
Servicl-Kuchler D, Maldini B, Borgeat A, et al. The influence of postoperative epidural analgesia on postoperative pain and stress response after major spine surgery--a randomized controlled double blind study. Acta Clin Croat. 2014;53(2):176–183. | ||
Person B, Wexner SD. The management of postoperative ileus. Curr Probl Surg. 2006;43(1):6–65. | ||
Shi WZ, Miao YL, Yakoob MY, et al. Recovery of gastrointestinal function with thoracic epidural vs. systemic analgesia following gastrointestinal surgery. Acta Anaesthesiol Scand. 2014;58(8):923–932. | ||
Axelrod TM, Mendez BM, Abood GJ, Sinacore JM, Aranha GV, Shoup M. Peri-operative epidural may not be the preferred form of analgesia in select patients undergoing pancreaticoduodenectomy. J Surg Oncol. 2015;111(3):306–310. | ||
Piccioni F, Mariani L, Negri M, et al. Epidural analgesia does not influence anastomotic leakage incidence after open colorectal surgery for cancer: A retrospective study on 1,474 patients. J Surg Oncol. 2015;112(2):225–230. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.