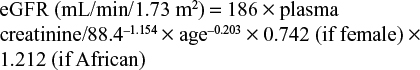Back to Journals » Clinical Epidemiology » Volume 9
Effect of glomerular filtration rate at dialysis initiation on survival in patients with advanced chronic kidney disease: what is the effect of lead-time bias?
Authors Janmaat CJ, van Diepen M, Krediet RT, Hemmelder MH, Dekker FW
Received 15 November 2016
Accepted for publication 10 January 2017
Published 10 April 2017 Volume 2017:9 Pages 217—230
DOI https://doi.org/10.2147/CLEP.S127695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Sørensen
Cynthia J Janmaat,1 Merel van Diepen,1 Raymond T Krediet,2 Marc H Hemmelder,3 Friedo W Dekker1
On behalf of the NECOSAD study group
1Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, 2Department of Nephrology, Academic Medical Center, Amsterdam, 3Department of Internal Medicine, Nefrovisie Foundation, Utrecht, Netherlands
Purpose: Current clinical guidelines recommend to initiate dialysis in the presence of symptoms or signs attributable to kidney failure, often with a glomerular filtration rate (GFR) of 5–10 mL/min/1.73 m2. Little evidence exists about the optimal kidney function to start dialysis. Thus far, most observational studies have been limited by lead-time bias. Only a few studies have accounted for lead-time bias, and showed contradictory results. We examined the effect of GFR at dialysis initiation on survival in chronic kidney disease patients, and the role of lead-time bias therein. We used both kidney function based on 24-hour urine collection (measured GFR [mGFR]) and estimated GFR (eGFR).
Materials and methods: A total of 1,143 patients with eGFR data at dialysis initiation and 852 patients with mGFR data were included from the NECOSAD cohort. Cox regression was used to adjust for potential confounders. To examine the effect of lead-time bias, survival was counted from the time of dialysis initiation or from a common starting point (GFR 20 mL/min/1.73 m2), using linear interpolation models.
Results: Without lead-time correction, no difference between early and late starters was present based on eGFR (hazard ratio [HR] 1.03, 95% confidence interval [CI] 0.81–1.3). However, after lead-time correction, early initiation showed a survival disadvantage (HR between 1.1 [95% CI 0.82–1.48] and 1.33 [95% CI 1.05–1.68]). Based on mGFR, the potential survival benefit for early starters without lead-time correction (HR 0.8, 95% CI 0.62–1.03) completely disappeared after lead-time correction (HR between 0.94 [95% CI 0.65–1.34] and 1.21 [95% CI 0.95–1.56]). Dialysis start time differed about a year between early and late initiation.
Conclusion: Lead-time bias is not only a methodological problem but also has clinical impact when assessing the optimal kidney function to start dialysis. Therefore, lead-time bias is extremely important to correct for. Taking account of lead-time bias, this controlled study showed that early dialysis initiation (eGFR >7.9, mGFR >6.6 mL/min/1.73 m2) was not associated with an improvement in survival. Based on kidney function, this study suggests that in some patients, dialysis could be started even later than an eGFR <5.7 and mGFR <4.3 mL/min/1.73 m2.
Keywords: end-stage renal disease, epidemiology, hazard model, kidney function, lead time, linear interpolation model
Introduction
Current clinical KDIGO (Kidney Disease: Improving Global Outcomes) guidelines recommend to initiate dialysis in the presence of symptoms or signs attributable to kidney failure.1 This often occurs with a glomerular filtration rate (GFR) between 5 and 10 mL/min/1.73 m2. There is little evidence about the optimal kidney function to start dialysis, and the only randomized study so far showed no effect on survival for starting at a GFR around 9 versus 7.2 mL/min/1.73 m2.2 Several observational studies have been performed, with contradictory results. Some studies suggested better survival for patients who started with a high plasma creatinine-based estimated GFR (eGFR), whereas the majority suggested better survival for those who started with a lower eGFR.3–19 However, only four of these studies properly accounted for lead-time bias.5,6,10,18 Nevertheless, all were based on a relatively low number of dialysis patients.
Lead-time bias often occurs when evaluating the efficacy of a treatment in observational studies, especially in dialysis initiation, and stems from a difference in timing of treatment initiation.20 Specifically, lead time is the added time of survival attributable to the fact that a selected group of patients starts earlier with dialysis than a later-starting comparative group. When comparing survival time starting from treatment initiation, early starters will show a survival benefit (Figure 1). Any potential survival benefit of early dialysis initiation may then be due to lead-time bias instead of representing an improvement in the course of the disease and effect on survival. In the IDEAL study,2 in which lead-time bias was no issue due to randomization, no difference was observed in survival rates associated with a time difference of 6 months between early and late starts. However, this randomized controlled trial (RCT) does not help to set the optimal kidney function to initiate dialysis. Furthermore, RCTs are hard to conduct and time-consuming, and thus we are still bound by observational studies.
Interpretation of results is further complicated, since most studies used only eGFR instead of true measurements of kidney function.6 It has been argued that eGFR is less valid, because of artificial low plasma creatinine levels in patients with fluid overload or low muscle mass, especially in low ranges of kidney function, when initiation of dialysis is near.21,22 Kidney function may be better reflected by the mean of measured creatinine and urea clearance (CCr–U) based on 24-hour urine collections (measured GFR [mGFR] by CCr–U). This study aimed to examine the effect of kidney function (both eGFR and mGFR) at dialysis initiation on survival in chronic kidney disease (CKD) patients, and the role of lead-time bias therein.
Materials and methods
Study design
Netherlands Cooperative on the Adequacy of Dialysis-2 (NECOSAD) was a multicenter prospective observational cohort study in which 38 dialysis centers throughout the Netherlands participated.23 Inclusion of patients took place between 1997 and 2007, and follow-up data on death were available until February 2015. Patients were followed until time of death or censored, due to kidney transplantation, recovery of kidney function as reason to stop dialysis therapy, withdrawal from the study, transfer to a dialysis center that did not participate in the study, loss to follow-up, or end of the study period (February 2015), whichever came first. Available data on mGFR and eGFR during the predialysis period collected from medical records were added retrospectively to the prospective NECOSAD cohort for a convenient sample of patients included before 2003. The study was approved for all participating hospitals by the Medical Ethics Committee of the Academic Medical Center in Amsterdam, as coordinating center of the NECOSAD study, and all hospitals involved (Supplementary material) approved participation. The study was conducted according to the Declaration of Helsinki. All patients gave written informed consent.
Patient inclusion
For the present analysis, incident-dialysis patients aged ≥18 years with no history of renal replacement therapy (RRT; ie, starting dialysis or renal transplantation) were included at the start of dialysis treatment. Patients were excluded when they had a hemodialysis catheter. The latter ensured we excluded patients with acute renal impairment. The current study population included patients studied by Korevaar et al.5
Exposure and outcome
The effect of GFR at dialysis initiation on survival in CKD patients was investigated using time to death as outcome. The GFR at dialysis initiation was based on tertiles of GFR at the moment of dialysis initiation, and included the categories late, intermediate, and early dialysis initiation (ie, low, intermediate, and high levels of GFR). Starting groups were based on two measures: mGFR (mL/min/1.73 m2, by CCr–U) and eGFR (mL/min/1.73 m2). The first is calculated by the mean of endogenous CCr–U in 24-hour collected urine, corrected for body-surface area, and the latter was calculated by the ‘186’ 4-item Modification of Diet in Renal Disease (MDRD) formula (Supplementary material).24 Plasma creatinine concentration was measured per dialysis center using the local method, which was predominately the alkaline picrate (Jaffe) method. A pilot study comparing these measurements with more precise enzyme-mediated methods found that differences were negligible for the very high concentrations present in patients with end-stage renal disease. For all patients included in the present analysis, the start date of dialysis was regarded as baseline. The GFR value at dialysis initiation was used as baseline measurement. For eGFR, plasma creatinine was drawn before the first dialysis session. For mGFR, urine and blood samples were collected either before or until 1 month after the first dialysis session.23
Estimating kidney function decline for lead-time bias correction
Lead-time correction was achieved by using two approaches: mean annual decline rate in kidney function, and individual decline rates imputed from data available for a subgroup in NECOSAD. Both approaches were used to estimate the date when individuals would have had a specific predetermined GFR level before dialysis start (ie, GFR 20 mL/min/1.73 m2). Survival time was then counted from this date onward, thereby eliminating the added survival time associated with starting dialysis early, when counting survival time from dialysis initiation. For the first approach, we used our calculated average annual rates of kidney function decline for eGFR and mGFR in the year prior to dialysis initiation based on predialysis data from the Dutch PREdialysis PAtient REcord-1 (PREPARE-1) study.25–27 PREPARE-1 was a Dutch retrospective follow-up study with incident-predialysis patients with CKD stages 4–5 (for more details, see Supplementary material). PREPARE-1 and NECOSAD were performed during the same period.
Statistical analyses
Data are presented as mean values with standard deviations or medians with interquartile ranges for continuous variables, depending on the distribution. Categorical variables are presented as numbers and percentages. P-values are two-tailed, and P<0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 20.
Missing values of potential confounders were imputed with multiple imputation methods using a fully conditional specification with ten repetitions.28–30 All available baseline variables and outcomes were used for imputation. Follow-up time was logarithmically transformed; age, baseline GFR values, and BMI were square-root-transformed before entry in the imputation model. Estimates and standard deviations were calculated in each imputation set, pooled into one overall estimate and standard deviation according to Rubin’s rules.31,32
Kidney function decline
Individual kidney function declines prior to dialysis initiation were calculated following the two approaches described earlier. For the first approach, average annual eGFR/mGFR rates from PREPARE-1, used for lead-time correction, were based on calculated individual annual GFR rates using linear regression. The assumption of a linear decline is considered safe, given the relatively short follow-up period of 1 year. At least two GFR measurements had to be available to estimate the rate of decline. Furthermore, a minimum of 30 days between first and last predialysis GFR values was applied, as too short a time frame would give an unreliable estimation of the decline. For the second approach, individual annual GFR decline rates prior to dialysis initiation were first calculated for those individuals in NECOSAD with available predialysis GFR data, and linear regression analysis was used for this purpose. With these available predialysis GFR decline data, GFR decline rates were imputed for individuals with missing predialysis data in NECOSAD.
Survival analysis
In our cohort of NECOSAD, we first performed a regular survival analysis for the effect of GFR at dialysis initiation on survival from dialysis initiation. Cumulative survival rates for early, intermediate, and late starters were calculated using the Kaplan–Meier method. Crude and adjusted hazard ratios (HRs) for timing of dialysis initiation were obtained using Cox proportional-hazard regression analyses, adjusted for the confounders age, sex, primary kidney disease, ethnicity, and comorbidities using the Khan comorbidity score.33 The Khan comorbidity score includes the following risk groups: low risk, defined as age <70 years and no comorbid illness; medium risk, defined as age 70–80 years or age <80 years with any one of cardiac, pulmonary, or liver disease or age <70 years with diabetes mellitus; and high risk, defined as age >80 years or any age with two or more organ dysfunctions in addition to end-stage renal disease or any age with visceral malignancy.33 Information on comorbidities included in the Khan score was collected by using questionnaires completed by clinicians, and was based on clinical diagnosis and information on comorbidities from patient records. Primary kidney disease was classified according to the codes of the European Renal Association–European Dialysis and Transplantation Association.34
Survival analysis corrected for lead-time bias
Next, the aforementioned survival analyses were repeated with correction for lead-time bias. This was achieved by measuring survival from the predetermined point before dialysis (ie, eGFR/mGFR of 20 mL/min/1.73 m2), rather than from the start of dialysis (Figure 1), based on the method used by Traynor et al.18 The date of this common starting point was calculated back from the start of dialysis, using a linear interpolation model with either the previously computed mean annual GFR slopes prior to dialysis commencement from PREPARE-1 or the computed individual predialysis GFR slopes from NECOSAD. Then, these lead-time-corrected results were compared to the previous uncorrected results of survival analyses. The difference in HRs between survival rates for the timing of dialysis initiation, corrected and uncorrected for lead-time bias, showed the impact of lead-time bias. Finally, the length of lead time was estimated by calculating the difference in baseline GFR value between early versus late and intermediate versus late dialysis initiation, divided by the annual GFR decline from PREPARE-1.
Sensitivity analyses
To validate the robustness of our results, we performed several sensitivity analyses. First, to confirm that early starters did not decline faster than late starters, mean GFR decline rates prior to dialysis initiation were calculated for late-, intermediate-, and early-starting groups in both PREPARE-1 and a selection of patients in NECOSAD with available data on GFR decline rates prior to dialysis initiation. Early-, intermediate-, and late-starting groups were based on the same GFR tertiles as used in the main analyses in NECOSAD. Second, correction for lead-time bias was also achieved by using the lowest and highest values of decline in kidney function extracted from a review of the literature on GFR decline in the year prior to dialysis initiation.23,35,36 Third, we repeated the analyses in subjects with both mGFR and eGFR values at dialysis initiation available to enable a direct comparison between mGFR and eGFR results. Fourth, we varied the cutoff point of the GFR value for dividing the study population into three categories. Fifth, we performed additional adjustment in the survival analysis for possible additional confounders or variables that are potentially in the causal pathway: smoking, systolic and diastolic blood pressure, and blood pressure medication.
Results
Patient characteristics at baseline
In total, 852 patients with an mGFR measurement and 1,143 patients with an eGFR measurement at dialysis initiation were included for the present analyses. See Figure 2 for a flowchart of patient inclusion. Individual predialysis decline rates were available for 150 of the 852 patients with mGFR data and for 363 of the 1,143 patients with eGFR data. Baseline characteristics for the total population under study and for early, intermediate, and late starters, based on either mGFR or eGFR data, are shown in Table 1. Mean baseline mGFR was 2.5 (±1.4) for late starters, 5.4 (±0.7) for intermediate, and 8.9 (±2.1) mL/min/1.73 m2 for early starters. Late, intermediate, and early starters based on eGFR data had higher mean baseline eGFRs of 4.4 (±1.2), 6.7 (±0.6), and 10.2 (±2.3) mL/min/1.73 m2, respectively. Median time from dialysis initiation and baseline plasma creatinine measurement used to calculate eGFR was 6 (interquartile range 1–14) days. In general, diabetes was the underlying cause of kidney disease in a larger proportion of early starters compared to later starters. A total of 21 variables were used to impute the missing values of potential confounders at baseline for both mGFR and eGFR. Most confounders had no missing values. From variables with missing values, the percentage of missing values varied between 0.5% and 11.2%.
  | Figure 2 Patient-inclusion flowchart for patients with data on mGFR (A) and data on eGFR (B). Abbreviations: mGFR, measured glomerular filtration rate; eGFR, estimated glomerular filtration rate. |
Survival analyses with and without lead-time correction
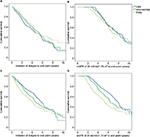
Using the first approach, for the starting groups based on mGFR data, an unadjusted Kaplan–Meier analysis suggested incrementally increased survival of early starters compared to late starters without lead-time correction (Figure 3A). However, after correction for lead-time bias, the Kaplan–Meier analysis suggested a reversed survival benefit of patients initiating dialysis later (Figure 3B). These analyses were also performed for starting groups based on eGFR data. In contrast, without lead-time correction, increased cumulative survival was observed for late starters (Figure 3C), and after correction for lead-time bias this survival benefit increased (Figure 3D). These results were reflected by crude Cox analyses, with and without correction for lead-time bias, as shown in Table 2.
In the adjusted Cox analyses based on mGFR data, both intermediate and early starters had a lower risk of death compared to late starters, with HRs of 1 (0.77–1.28, early) and 0.8 (0.62–1.03, late). When corrected for lead-time bias, an inverse association was present, with HRs of 1.23 (0.95–1.58) and 1.21 (0.93–1.56) for intermediate and early starters versus late starters, respectively (Table 2). In contrast, this observed inverse association of adjusted HRs after correction for lead time was not found for starting groups based on eGFR data at dialysis initiation. Without lead-time bias correction, the adjusted Cox analyses based on eGFR data at dialysis initiation showed no difference in mortality risk between early and late dialysis initiation. However, after correction for lead-time bias, the early starters had a higher risk of death, with an HR of 1.33 (1.05–1.68) (Table 2). With the second approach with individual decline rates prior to dialysis initiation from NECOSAD to correct for lead-time bias, adjusted Cox analyses based on mGFR data showed no substantial difference between early and late starters (Table 3). The HR was approximately equal to 1. Based on eGFR data, the early and intermediate starters still had a higher risk of death compared to late starters after correction for lead-time bias, with HRs of 1.1 (0.81–1.5) and 1.1 (0.82–1.48) (Table 3), respectively.
Length of lead time
The first approach, with computed annual GFR declines derived from the predialysis cohort in PREPARE-1, as shown in Table 4, yielded a lead time of 13.9 months for early versus late starters and 6.3 months for intermediate versus late starters, based on mGFR data (Table 2). For starting groups based on eGFR data, shorter lead times of 9.2 and 3.6 months were shown for early- versus late- and intermediate- versus late-starting groups, respectively (Table 2). Under the second approach, with individual decline rates from NECOSAD to correct for lead-time bias, even longer lead times were calculated for early and intermediate versus late starters, both based on mGFR and eGFR data (Table 3). Mean rates of kidney function decline for the three starting groups, used to compute the length of lead time based on the second approach, are shown in Table 5.
Sensitivity analyses
Calculated annual GFR declines prior to dialysis initiation in PREPARE-1 and a selection of patients of NECOSAD (with available data) showed that early/intermediate starters had a less rapid decline then late starters (Table S1). Repeating the crude and adjusted Cox analyses with correction for lead-time bias based on the lowest and highest value of GFR decline extracted from literature, adjusted and corrected risk of mortality for early compared to late starters ranged between 1.14 (0.88–1.47) and 1.61 (1.24–2.09), based on mGFR data (Table 6). This was accompanied by lead time between 11.5 and 23.6 months. For starting groups based on eGFR values, adjusted and corrected HRs between 1.22 (0.96–1.54) and 1.52 (1.21–1.92) were calculated for early- versus late-dialysis initiation, accompanied by lead time of 6–15.3 months (Table 6).
Additional subgroup analyses in subjects (n=577) with both eGFR and mGFR measurement available at dialysis initiation were similar and in line with results obtained in the main analyses. Classification among late, intermediate, and early starters was tested by additional analyses in which the study population was divided into two groups based on the median GFR value at dialysis initiation, in quartiles, and in categories of GFR value at dialysis initiation <5, 5–10, and >10 mL/min/1.73 m2(data not shown). All classifications showed the same patterns of association, and confirmed the stability of our results. Adding additional confounders to the Cox proportional-hazard model did not alter our conclusions (Table S2).
Discussion
This study on the effect of lead-time bias when examining the effect of both eGFR and mGFR at dialysis initiation on survival in CKD patients underlines the impact of lead-time bias herein. Without lead-time bias correction, we demonstrated no substantial effect of GFR levels at dialysis initiation, ie, early versus late start, on survival in CKD patients, although a borderline survival benefit for early dialysis initiation was observed based on mGFR. However, after lead-time correction, early dialysis initiation yielded no survival benefit and seemed rather harmful, irrespective of whether early start was based on eGFR or mGFR. The start time for dialysis differed by about a year between early and late starters. Our results underline the importance to correct for lead-time bias, and showed that early dialysis initiation was not associated with an improvement in survival.
To our knowledge, this is one of the first studies accounting for lead-time bias in survival of CKD patients starting dialysis in an observational study design, based on both eGFR and mGFR. The only performed RCT, in which lead-time bias was no issue, showed no difference between early- and late-initiation strategies.2 However, in this RCT the mean difference in eGFR between early and late starters was only 1.8 mL/min/1.73 m2 with 6 months difference in dialysis start time, whereas we showed a difference in eGFR of 5.8 mL/min/1.73 m2 with 9.2–14.5 months of lead time. Our data, based on individual lead-time correction for eGFR data, support the conclusion of the IDEAL trial that early dialysis initiation was not associated with an improvement in survival.2 Besides, several observational studies have also investigated the effect of GFR at dialysis initiation on survival in CKD dialysis patients, with contradictory results. Some studies suggested better survival for patients who started dialysis early, whereas most studies suggested better survival for those who started late and most studies did not take into account lead-time bias.3–19 In the latter case, lead-time bias cannot explain their findings, because lead-time bias can only explain better survival for early starters. However, of these previous studies, only four took account of lead-time bias, but were never based on both eGFR and mGFR and had small study populations.5,6,10,18 One study was based on Kt/V measurements, which is beyond the scope of this article.5 Our eGFR results confirmed the findings of the two studies based on eGFR: survival benefit in favor of late starters.10,18 With a larger sample size, the present study extends these results by showing a stronger association between late start and survival benefit when accounting for lead-time bias. With regard to the mGFR results, only one other study also used mGFR and corrected for lead-time, showing a survival disadvantage for “late” starters.6 However, in this Hong Kong study, later starters were initial refusers, ie, no real late starters, compared to elective starters (baseline difference only 0.3 mL/min/1.73 m2), and they were in an initially worse condition upon starting dialysis. Therefore, these results were not comparable with our data. The relatively high percentage of patients with a low Khan score in this dialysis cohort, for both eGFR and mGFR, is in line with results of Khan et al.33 The pathophysiological mechanisms underlying the observed disadvantage of early starters remain unclear, but suggest harmful effects of the dialysis procedure itself.37–40
Our somewhat different findings between starting groups based on either eGFR or mGFR data could be explained by misclassification bias. Misclassification bias occurs when either outcome or exposure is misclassified, ie, the probability for early starters to be misclassified as late starter or vice versa. This type of bias is present with calculating eGFR based on the MDRD formula, and is almost completely eliminated using mGFR, which is not influenced by muscle mass.8,21,41 For instance, frail or elderly patients with muscle wasting have lower levels of plasma creatinine, resulting in falsely high eGFR levels compared to their true underlying kidney function. Therefore, they are prone to be misclassified as early starters; the opposite applies for late starters.42,43 In addition, eGFR overestimates kidney function in advanced CKD, as reflected by our higher values for the eGFR than mGFR starting groups.21,44 As a consequence, misclassification bias overestimates survival in the late-initiation group of eGFR and underestimates survival in early starters. Indeed, we demonstrated that the significant crude survival disadvantage for early versus late starters in the eGFR group without lead-time correction completely disappeared after adjustment for baseline confounders. Following this, misclassification bias could also explain the observed differences in adjusted mortality risks for early versus late starters when comparing mGFR and eGFR. In addition, plasma creatinine measurements in the present study were not always performed on standardized plasma creatinine assays, which theoretically could lead to imprecision of eGFR measurements, besides the introduced misclassification bias, due to the influence of muscle mass on eGFR measurements. mGFR seems more accurate in decision making on timing of dialysis initiation; when eGFR is used, a thorough realization of its weaknesses and pitfalls is needed.
The present study has potential limitations. First, we cannot rule out the presence of confounding by indication, resulting from clinical decision making at dialysis initiation. Although adjustment for a range of known confounders did not affect the results, we did not have information on uremic symptoms.17,45–48 Therefore, residual confounding could not be completely eliminated. Second, mean annual GFR decline was used, based on a selected group of patients with predialysis measurements from PREPARE-1. For both of these limitations, one might have concerns that early starters with or without uremic symptoms might have a faster decline in kidney function with worse prognosis than later starters. However, in the current study this was no limitation, since the opposite holds true for starting groups in PREPARE-1 and available data in NECOSAD. Furthermore, our results, ie, based on decline rates derived from PREPARE-1, fell within the observed range based on the available literature, which justified the use of the decline rates from PREPARE-1. Finally, we also used imputed individual GFR declines based on patients with available predialysis data in NECOSAD. Third, survivor bias (ie, immortal time bias) could be a potential limitation of addressing lead-time bias in this way, as individuals who died before starting dialysis were not included in our cohort. Only people who survived to dialysis initiation were analyzed, excluding those who died before starting dialysis. As a consequence, the individuals included in the present study will have better survival in general. Therefore, survival rates could have been overestimated in the present results, especially for late starters. The difference in survival rates between early and late starters could partially be explained by survivor bias. However, we corrected for health status by adjusting for several confounders, such as Khan’s score and age. Therefore, we consider the influence of survivor bias as minimal and will not alter the conclusion. However, predialysis dropout due to death was limited to 11% over the complete follow-up period in the PREPARE-1 study.25,26 Finally, mGFR values might not be completely accurate, since they were based on 24-hour urine collections. However, any errors were assumed to be randomly distributed over the study population and would dilute the effect.
Major strengths of our study are that we were able to eliminate lead-time bias in an observational cohort study design and that we assessed the long-term effect of both eGFR and mGFR at dialysis initiation on survival (until 18 years of follow-up). Our results clearly indicate the importance of correcting for lead-time bias.
Our results could have an impact on the currently used KDIGO guideline for decision making on timing of dialysis initiation, which recommends to initiate dialysis in the presence of symptoms or signs attributable to kidney failure, often in the eGFR range of 5–10 mL/min/1.73 m2.1 However, considering this eGFR range, early initiation (ie, >7.9 mL/min/1.73 m2) showed a clear mortality disadvantage in the current study when lead time was accounted for. Furthermore, data on mGFR could be added in the guideline. In the context of misclassification of patients in eGFR early-starting groups, mGFR may be more reliable as a guide for timing of dialysis initiation.22 While the IDEAL study showed that the strategy to initiate dialysis with a mean eGFR <7.2 mL/min/1.73 m2 is safe, we show that based on solely kidney function, in some patients we can even go lower than an eGFR of 5.7 and an mGFR of 4.3 mL/min/1.73 m2.2 Further research is needed to examine this precise kidney function threshold and to implement these findings in the context of presence of uremic symptoms and quality of life.
Conclusion
We showed that lead-time bias is not only a methodological problem but also a clinical problem when assessing the optimal kidney function to start dialysis. Therefore, lead-time bias is extremely important to correct for. Taking account of lead-time bias, this controlled study showed that early dialysis initiation (ie, eGFR >7.9, mGFR >6.6 mL/min/1.73 m2) was not associated with an improvement in survival. Based solely on kidney function, this study suggests that in some patients, dialysis could be started even later than an eGFR <5.7 and mGFR <4.3 mL/min/1.73 m2. These results should naturally be interpreted in the context of clinical judgment and presence of any symptoms.
Acknowledgments
The nursing staff of the 38 different dialysis units, who collected most of the data, are gratefully acknowledged for their assistance. Moreover, we thank the staff of the NECOSAD trial office for assistance in the logistics of this study. NECOSAD study group: EW Boeschoten, RT Krediet, AJ Apperloo, JA Bijlsma, M Boekhout, WH Boer, PJM van der Boog, HR Büller, M van Buren, FT de Charro, CJ Doorenbos, MA van den Dorpel, A van Es, WJ Fagel, GW Feith, CWH de Fijter, LAM Frenken, JACA van Geelen, PGG Gerlag, W Grave, JPMC Gorgels, RM Huisman, KJ Jager, K Jie, WAH Koning-Mulder, MI Koolen, TK Kremer Hovinga, ATJ Lavrijssen, AJ Luik, J van der Meulen, KJ Parlevliet, MHM Raasveld, FM van der Sande, MJM Schonck, MMJ Schuurmans, CEH Siegert, CA Stegeman, P Stevens, JGP Thijssen, RM Valentijn, G H Vastenburg, CA Verburgh, HH Vincent, PF Vos.
Disclosure
The authors report no conflicts of interest in this work.
References
Kidney Disease - Improving Global Outcomes (KDIGO). KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Chapter 5: Referral to specialists and model of care. Kidney Int Suppl. 2013;3:5–14. | ||
Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619. | ||
Bonomini V, Feletti C, Scolari MP, Stefoni S. Benefits of early initiation of dialysis. Kidney Int Suppl. 1985;17:S57–S59. | ||
Bonomini V, Vangelista A, Stefoni S. Early dialysis in renal substitutive programs. Kidney Int Suppl. 1978(8):S112–S116. | ||
Korevaar JC, Jansen MA, Dekker FW, et al. When to initiate dialysis: effect of proposed US guidelines on survival. Lancet. 2001;358(9287):1046–1050. | ||
Tang SC, Ho YW, Tang AW, et al. Delaying initiation of dialysis till symptomatic uraemia: is it too late? Nephrol Dial Transplant. 2007;22(7):1926–1932. | ||
Tattersall J, Greenwood R, Farrington K. Urea kinetics and when to commence dialysis. Am J Nephrol. 1995;15(4):283–289. | ||
Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14(9):2305–2312. | ||
Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47–53. | ||
Crews DC, Scialla JJ, Boulware LE, et al. Comparative effectiveness of early versus conventional timing of dialysis initiation in advanced CKD. Am J Kidney Dis. 2014;63(5):806–815. | ||
Evans M, Tettamanti G, Nyrén O, Bellocco R, Fored CM, Elinder CG. No survival benefit from early-start dialysis in a population-based, inception cohort study of Swedish patients with chronic kidney disease. J Intern Med. 2011;269(3):289–298. | ||
Fink JC, Burdick RA, Kurth SJ, et al. Significance of serum creatinine values in new end-stage renal disease patients. Am J Kidney Dis. 1999;34(4):694–701. | ||
Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant. 2010;25(8):2616–2624. | ||
Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887–896. | ||
Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77(8):700–707. | ||
Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A. Survival and dialysis initiation: comparing British Columbia and Scotland registries. Nephrol Dial Transplant. 2009;24(10):3186–3192. | ||
Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24(10):3175–3182. | ||
Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13(8):2125–2132. | ||
Wright S, Klausner D, Baird B, et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010;5(10):1828–1835. | ||
Tripepi G, Jager KJ, Dekker FW, Wanner C, Zoccali C. Bias in clinical research. Kidney Int. 2008;73(2):148–153. | ||
Grootendorst DC, Michels WM, Richardson JD, et al. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant. 2011;26(6):1932–1937. | ||
Tattersall J, Dekker F, Heimbürger O, et al. When to start dialysis: updated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) study. Nephrol Dial Transplant. 2011;26(7):2082–2086. | ||
de Jager DJ, Halbesma N, Krediet RT, et al. Is the decline of renal function different before and after the start of dialysis? Nephrol Dial Transplant. 2013;28(3):698–705. | ||
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. | ||
de Goeij MC, de Jager DJ, Grootendorst DC, et al. Association of blood pressure with the start of renal replacement therapy in elderly compared with young patients receiving predialysis care. Am J Hypertens. 2012;25(11):1175–1181. | ||
de Goeij MC, Liem M, de Jager DJ, et al. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract. 2012;121(1–2):c73–c82. | ||
de Goeij MC, Voormolen N, Halbesma N, et al. Association of blood pressure with decline in renal function and time until the start of renal replacement therapy in pre-dialysis patients: a cohort study. BMC Nephrol. 2011;12:38. | ||
de Goeij MC, van Diepen M, Jager KJ, Tripepi G, Zoccali C, Dekker FW. Multiple imputation: dealing with missing data. Nephrol Dial Transplant. 2013;28(10):2415–2420. | ||
Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. | ||
van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. | ||
Kenward MG, Carpenter J. Multiple imputation: current perspectives. Stat Methods Med Res. 2007;16(3):199–218. | ||
Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. | ||
Khan IH, Catto GR, Edward N, Fleming LW, Henderson IS, MacLeod AM. Influence of coexisting disease on survival on renal-replacement therapy. Lancet. 1993;341(8842):415–418. | ||
European Renal Association–European Dialysis and Transplantation Association. ERA-EDTA Registry Annual Report 2009. ERA/EDTA Registry. Amsterdam: Academic Medical Center; 2009. | ||
Balafa O, Vlahu C, Sampimon D, Coester AM, Struijk DG, Krediet RT. Lack of correlation between baseline peritoneal membrane status and pre-dialytic characteristics. Adv Perit Dial. 2010;26:16–20. | ||
Bhan V, Soroka S, Constantine C, Kiberd BA. Barriers to access before initiation of hemodialysis: a single-center review. Hemodial Int. 2007;11(3):349–353. | ||
Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int. 2009;75(11):1223–1229. | ||
Hackett AS, Watnick SG. Withdrawal from dialysis in end-stage renal disease: medical, social, and psychological issues. Semin Dial. 2007;20(1):86–90. | ||
Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061–1070. | ||
Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis. 2003;41(6):1293–1302. | ||
Ohkawa S, Odamaki M, Yoneyama T, Hibi I, Miyaji K, Kumagai H. Standardized thigh muscle area measured by computed axial tomography as an alternate muscle mass index for nutritional assessment of hemodialysis patients. Am J Clin Nutr. 2000;71(2):485–490. | ||
Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171(5):396–403. | ||
Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Pappas LM, Cheung AK. Creatinine production, nutrition, and glomerular filtration rate estimation. J Am Soc Nephrol. 2003;14(4):1000–1005. | ||
Botev R, Mallié JP, Couchoud C, et al. Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4(5):899–906. | ||
Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–183. | ||
Ledebo I, Kessler M, van Biesen W, et al. Initiation of dialysis-opinions from an international survey: report on the Dialysis Opinion Symposium at the ERA-EDTA Congress, 18 September 2000, Nice. Nephrol Dial Transplant. 2001;16(6):1132–1138. | ||
Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009;76(3):257–261. | ||
Termorshuizen F, Korevaar JC, Dekker FW, et al. Time trends in initiation and dose of dialysis in end-stage renal disease patients in the Netherlands. Nephrol Dial Transplant. 2003;18(3):552–558. |
Supplementary material
Hospitals participating in the NECOSAD study
Maasstad Hospital Rotterdam, Deventer Hospital Deventer, Sint Lucas Andreas Hospital Amsterdam, Academic Medical Center Amsterdam, Maxima Medical Center Veldhoven, Catharina Hospital Eindhoven, Medical Center Haaglanden Den Haag, University Medical Center Groningen, Kennemer Gasthuis Haarlem, Atrium Medical Center Heerlen, Medical Center Leeuwarden, Leiden University Medical Center Leiden, Elisabeth Hospital Tilburg, University Medical Center Utrecht, Antonius Ziekenhuis Nieuwegein, Hospital Gelderse Vallei Ede, Haga Hospital Leyenburg Den Haag, Academic Hospital Maastricht, Jeroen Bosch Hospital Den Bosch, Medisch Spectrum Twente Enschede, Albert Schweitzer Hospital Dordrecht, Alysis Zorggroep Rijnstate Hospital Arnhem, Dianet Dialysis Center Lunetten Utrecht, Canisius Wilhelmina Hospital Nijmegen, Vie Curi Medical Center Venlo, Leveste Scheper Hospital Emmen, Dianet Dialysis Center Holendrecht Amsterdam, Haga Hospital Rode Kruis Den Haag, Rijnland Hospital Leiderdorp, Admiraal de Ruyter ziekenhuis Goes, Medical Center Alkmaar, Laurentius Ziekenhuis Roermond, Dialysis Center ‘t Gooi Hilversum, Groene Hart Hospital Gouda, Westfries Gasthuis Hoorn, TergooiHospitals Hilversum, Martini Ziekenhuis Groningen, Zaans Medical Center Zaandam.
Formulae
To calculate estimated glomerular filtration rate (eGFR), we used the Modification of Diet in Renal Disease formula:
|
|
To calculate measured GFR (mGFR) based on 24-hour urine samples, we used:
|
|
PREPARE-1
  | Table S2 Effect of GFR at dialysis initiation on survival and length of lead time Notes: aAdjusted HR for model with mean GFR decline from PREPARE-11–3; badjusted HR for the model with individual GFR declines from NECOSAD4. Adjusted for age, sex, ethnicity, Khan comorbidity score, primary kidney diseases, systolic and diastolic blood pressure, smoking, and antihypertensive use. Abbreviations: GFR, glomerular filtration rate; HR, hazard ratio; CI, confidence interval; mGFR, measured glomerular filtration rate; eGFR, estimated glomerular filtration rate; PREPARE-1, PREdialysis PAtient REcord-1; NECOSAD, Netherlands Cooperative on the Adequacy of Dialysis-2. |
PREPARE-11–3 was a retrospective follow-up study of 500 consecutive incident predialysis patients with chronic kidney disease stages 4–5. These patients were treated in one of the outpatient clinics of eight Dutch hospitals between 1999 and 2001. Patients had been referred to these outpatient clinics when creatinine clearance was below 20 mL/min. In addition, these patients were at least 18 years of age, had not had prior renal replacement therapy, and need for renal replacement therapy was expected within 1 year. The clinical course of predialysis patients was followed through medical charts until the start of dialysis, transplantation, death, loss to follow-up, or January 1, 2008, whichever came first.
References
de Goeij MC, de Jager DJ, Grootendorst DC, et al. Association of blood pressure with the start of renal replacement therapy in elderly compared with young patients receiving predialysis care. Am J Hypertens. 2012;25(11):1175–1181. | ||
de Goeij MC, Liem M, de Jager DJ, et al. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract. 2012;121(1–2):c73–c82. | ||
de Goeij MC, Voormolen N, Halbesma N, et al. Association of blood pressure with decline in renal function and time until the start of renal replacement therapy in pre-dialysis patients: a cohort study. BMC Nephrol. 2011;12:38. | ||
de Jager DJ, Halbesma N, Krediet RT, et al. Is the decline of renal function different before and after the start of dialysis? Nephrol Dial Transplant. 2013;28(3):698–705. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.