Back to Journals » Drug Design, Development and Therapy » Volume 13
Effect of dexmedetomidine on etomidate-induced myoclonus: a randomized, double-blind controlled trial
Authors Miao S , Zou L , Wang G, Wang X , Liu S, Shi M
Received 28 December 2018
Accepted for publication 25 March 2019
Published 27 May 2019 Volume 2019:13 Pages 1803—1808
DOI https://doi.org/10.2147/DDDT.S194456
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Shuai Miao,1,2 Lan Zou,1,2 Guanglei Wang,1,2 Xiuli Wang,1,2 Su Liu,1,2 Mengzhu Shi1,2
1Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, People’s Republic of China; 2Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, People’s Republic of China
Background: Etomidate used for the induction of general anesthesia can result in myoclonus. We tested the hypothesis that pretreatment with dexmedetomidine (Dex) reduces the incidence of etomidate-induced myoclonus during the induction of general anesthesia.
Materials and methods: One hundred patients who were scheduled for selective operations under general anesthesia were included in this randomized, double-blind controlled trial. Patients were randomized to receive either Dex 0.5 μg/kg in 20 mL of normal saline or the same volume of normal saline as pretreatment agents 15 mins before the injection of etomidate 0.3 mg/kg. The primary endpoint was the incidence of etomidate-induced myoclonus. Secondary endpoints were the severity of etomidate-induced myoclonus and the incidence of adverse effects from the onset of action of Dex or normal saline to the injection of etomidate, such as dizziness, respiratory depression, bradycardia, hypotension and nausea/vomiting.
Results: All of the 100 patients completed the trial. Dex resulted in a significant 38% reduction in the number of patients who experienced etomidate-induced myoclonus: 13 (26%) vs 32 (64%) (P=0.0001). Additionally, the severity of myoclonus was also reduced in the Dex group than that in the placebo group (P=0.02). Incidence of dizziness, respiratory depression, bradycardia, hypotension and nausea/vomiting was similar in both groups.
Conclusions: Pretreatment with Dex 0.5 μg/kg 15 mins before the induction of general anesthesia not only resulted in a 38% reduction in the incidence of etomidate-induced myoclonus, but also reduced the severity of myoclonus, without inducing any adverse effects.
Keywords: general anesthesia, adverse effects, myoclonus
Introduction
With a superior stable cardiovascular profile and minimal respiratory side effects compared with propofol, etomidate, a known induction agent, is used widely for general induction with different clinical features, such as the fast onset of action and a short half-life.1,2 Even though etomidate is considered to be a safe induction agent with low risk of hypotension,3 it has two common adverse effects, pain on injection and myoclonus,4 that have been widely studied in the literature. Pain on injection has been minimized by new fat emulsion of etomidate.5 However, the etomidate-induced myoclonus is still a clinical problem that has not been solved during the induction of anesthesia.6 Myoclonus, defined as sudden, brief, involuntary muscle jerks either irregular or rhythmic, has been reported to be as high as 85% of nonpremedicated patients after etomidate administration.7 Myoclonus can result in serious consequence in patients who had open-global injury, nonfasted emergency patients or those with limited cardiovascular reserves.8 Several drugs have been proved effective in preventing the incidence of etomidate-induced myoclonus, such as dezocine,9 lidocaine10 and midazolam;11 however, the mechanism of etomidate-induced myoclonus is still unclear.
Dexmedetomidine (Dex), a highly selective α2-adrenergic receptor agonist, provides some beneficial effects during the operations, such as sedation, antianxiety effects and analgesia. However, few studies have evaluated the effects of Dex on etomidate-induced myoclonus. Therefore, we conducted this randomized, double-blind controlled trial to investigate the hypothesis that pretreatment with Dex prevents the incidence of etomidate-induced myoclonus during the induction of anesthesia, and the primary endpoint is the incidence of etomidate-induced myoclonus.
Materials and methods
This study was a prospective, randomized, double-blind controlled trial with two treatment arms and was registered at
Patients
100 American Society of Anesthesiologists (ASA) 1–2 patients scheduled for elective surgery under general anesthesia were included in the study. Exclusion criteria were: age <18, uncontrolled hypertension, history of epilepsy, bradycardia, heart blocks, heart failure, hepatic failure, psychiatric disease, sepsis or systemic infections, neurological disease, asthma, chronic cough, upper respiratory infection during the 2 weeks before surgery, allergy to Dex, and those who had received analgesics or sedatives within the previous 24 hrs.
Patients were blinded to the group allocation and were randomly assigned into 2 groups in a 1:1 group allocation to receive either Dex (Group D; n=50): Dex 0.5 µg/kg in 20 mL of normal saline or the same volume of normal saline (Group S; n=50). An independent statistician used a computer-generated random number list, and the allocation sequence was concealed in sequentially numbered, opaque, sealed envelopes. An independent research assistant not involved further in the study opened the sealed envelopes at the time of the study drug preparation and prepared the drug using 20-mL syringes labeled as the “study drug” outside the operation room.
Study protocol
Patients were kept fasting for 8 hrs preoperatively, and none of them received any premedication. On arrival at the operating room, baseline heart rate, respiratory rate, noninvasive blood pressure, electrocardiography and peripheral capillary oxygen saturation were recorded. A 20-gauge cannula was inserted into the dorsum of the patient’s hand, and subsequently ringer lactate was infused throughout the surgical procedure to deliver the intravenous anesthetics.
Anesthesia was induced with etomidate 0.3 mg/kg for a duration of 30 s by an anesthesiologist who was unaware of the group allocation after 15-min infusion of Dex or normal saline (defined as the time to the onset of action of Dex or normal saline). Two independent outcome assessors, who were also unaware of the group allocation, performed parallel observations of the etomidate-induced myoclonus in seconds for 3 mins after the injection of etomidate using the following grading:12 0 (none), 1 (mild: movement at the finger or wrist only), 2 (moderate: involving the face and leg), or 3 (severe: generalized response or fast abduction of a limb), and disagreements were solved by discussion. We recorded the highest severity of myoclonus for analysis during the 3-min observation period, and ventilation was assisted with 100% oxygen during the period. From the onset of action of Dex or normal saline to the injection of etomidate, adverse effects were recorded by one anesthesiologist who was blinded to the group allocation and not involved further in the study in order to avoid the bias of the outcome assessors who had observed myoclonus.
After the 3-min observation period, rocuronium bromide 0.6 mg/kg and sufentanil 0.3 µg/kg were given to both the groups to facilitate tracheal intubation. Anesthesia was maintained with propofol 4–6 mg/(kg·hr) and remifentanil 0.1–0.3 µg/(kg·min) until 10 mins before the completion of surgery. Mechanical ventilation was administered to maintain an end-expiratory carbon dioxide concentration of 35–40 mm Hg. Residual neuromuscular blockade was antagonized with neostigmine and atropine after completion of surgery. If the systolic blood pressure was <90 mm Hg or the diastolic blood pressure was <60 mm Hg, ephedrine 5–10 mg was administered. If the heart rate was <50 beats/min, atropine 0.5 mg was administered at once.
Measurements
The primary endpoint was the incidence of myoclonus during the first 3 mins following the injection of etomidate, defined as an involuntary, short contraction of some muscle fibers, of a whole muscle, or of different muscles of one group, leading to a short observable movement of the corresponding body part. The secondary endpoints were as follows: the severity of etomidate-induced myoclonus and the incidence of adverse effects from the onset of action of Dex or normal saline to the injection of etomidate, such as dizziness, respiratory depression, bradycardia, hypotension and nausea/vomiting.
Sample size
Sample size was estimated using STATA software 14.0 (STATA Corporation. College Station, TX, USA). The sample size calculation was based on the primary endpoint, the incidence of etomidate-induced myoclonus. Based on the results that were previously published, the incidence of myoclonus in Group S was expected to be around 0.7. Power analysis showed that a reduction in the incidence of myoclonus of 30% with α=0.05 and a 15% dropout rate within a 80% power could be detected with a sample size of at least 50 per group.
Statistical methods
Statistical analyses were performed on an intention-to-treat analysis using the Graphpad Prism 7.0 (GraphPad Software Inc, San Diego, CA, USA). The Kolmogorov–Smirnov test was used to show normal distribution of the continuous data. Normally distributed data were presented as mean (SD) and were compared using the Student’s t-test. Non-normally distributed data were presented as median (IQR) and were compared using the Mann–Whitney U test. Categorical variables, such as sex, ASA physical status, the incidence of myoclonus and adverse effects, were presented as frequencies (percentages) and were compared using Chi-square test or Fisher’s exact test. P<0.05 was considered as significant difference.
Results
Of the 135 consecutive patients assessed for eligibility, 100 met the inclusion criteria and were randomized into 2 groups of 50 patients each. There were no participants were excluded, and all participants’ data were analyzed (Figure 1). There were no significant differences in the demographic profile that we evaluated between the two groups (Table 1).
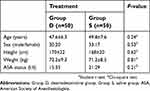 | Table 1 Patient baseline characteristics in the two treatment groups |
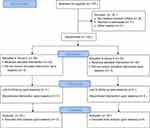 | Figure 1 Study flow. Abbreviations: Group D, dexmetomidine group; group S, saline group. |
Pretreatment with Dex allowed a significant 38% reduction in the incidence of etomidate-induced myoclonus (26% vs 64%, RR=0.40, 95% CI=0.239–0.659, P=0.0001). Additionally, pretreatment with Dex significantly reduced the severity level of myoclonus with the median intensity of myoclonus being 1 versus 2 in Group D versus Group S, respectively (P=0.02) (Table 2).
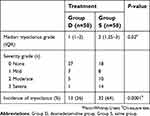 | Table 2 The incidence and severity of etomidate-induced myoclonus in the two treatment groups |
There were no differences in the incidence of dizziness, respiratory depression, bradycardia, hypotension or nausea/vomiting among the two groups (Table 3). Only two patients from Group D experienced bradycardia and one patient from Group D experienced hypotension. No participants from Group S experienced any of these adverse effects (Table 3).
 | Table 3 Adverse effects in the two treatment groups |
Discussion
The present study confirms that pretreatment with Dex 0.5 µg/kg 15 mins before the injection of etomidate can reduce the incidence and severity of etomidate-induced myoclonus. Additionally, no significant differences were found in the incidence of dizziness, respiratory depression, bradycardia, hypotension or nausea/vomiting between the two groups.
Different drugs have been proved effective in preventing the incidence of etomidate-induced myoclonus, such as midazolam,7 dezocine13,14 and lidocaine,10 and there were no obvious adverse effects. However, the exact mechanism of etomidate-induced myoclonus remains unclear. It may be explained by the following reasons for the occurrence of myoclonus. First, spontaneous nerve transmissions may occur when pathways associated with skeletal muscle control become more sensitive with the interruption of GABA neurons.15 Second, several studies have demonstrated that the etomidate-induced myoclonus might be associated with a seizure-like activity.16,17 Third, the inhibitory circuits are depressed earlier than excitatory neuronal circuits after injection of etomidate.18
Dex, a highly selective α2-adrenergic receptor agonist, can provide many beneficial effects, such as sedation and analgesia, without inducing significant respiratory depression.19,20 We found that the incidence of etomidate-induced myoclonus was significantly lower in the Dex group than in the control group, and this result was in concordance with other randomized controlled trials estimating Dex for etomidate-induced myoclonus.21–23 One published meta-analysis including 7 articles also demonstrated that Dex could reduce the incidence of etomidate-induced myoclonus.24 Even though all of the 7 studies adopted randomization methods, 3 of them did not illustrate the specific method. In addition, all of the 7 articles did not mention allocation concealment, and only 3 of them adopted a double-blind method. In our study, patients included the anesthesiologist who administered the etomidate, and outcome assessors were all unaware of the group allocation, which minimized the performance bias and detection bias. Accordingly, the results may be more reliable.
Among the dose-dependent hemodynamic changes caused by Dex, hypotension and bradycardia are the two common adverse effects. The study by Luan et al21 suggested that compared with a dose of 0.5 µg/kg administered, a dose of Dex 1 µg/kg was associated with the higher incidence of hypotension and bradycardia. Therefore, we chose the pretreatment with Dex 0.5 µg/kg in the present study. In our study, one patient experienced hypotension and two experienced bradycardia in the Dex group, respectively. However, these hemodynamic changes were not significantly different between the two groups. Besides, no patient was reported to experience dizziness, respiratory depression or nausea/vomiting. Accordingly, the pretreatment with Dex 0.5 µg/kg was safe and did not increase the incidence of adverse effects.
Several limitations should be taken into consideration in the present study. First, it was powered and designed to detect the efficacy of Dex on preventing the etomidate-induced myoclonus, but not to ascertain the potential benefits of this reduction on specific patients, such as the elder. Second, we only recorded the incidence and severity of etomidate-induced myoclonus except the duration of myoclonus that might help detect differences in the severity of myoclonus. Finally, we only applied Dex 0.5 µg/kg in this study. Therefore, further studies demonstrating the dose–response in specific patients are needed to ascertain the benefits of Dex in preventing the etomidate-induced myoclonus. Third, in the present study, we only compared the effect of Dex on the myoclonus with a placebo, and it is more appropriate for further studies to compare two or more drugs with a known effect on the etomidate-induced myoclonus, such as midazolam.23
In conclusion, pretreatment with Dex 0.5 µg/kg allows for a 38% reduction in the incidence of etomidate-induced myoclonus, as well as reducing the severity of myoclonus without inducing any adverse effects.
Data sharing statement
The individual participant's data underlying published results reported in this study can be accessed with approval from the corresponding author after 6 months of publication of the main results. The study protocol, statistical analysis plan and clinical study report will also be available.
Acknowledgments
This work was financially supported by grants from the Nature Science Foundation of Jiangsu Province (BK20161175) and the “Six One” Project of Jiangsu Province (LGY2016039).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Song J-C, Lu Z-J, Jiao Y-F, et al. Etomidate anesthesia during ERCP caused more stable haemodynamic responses compared with propofol: a randomized clinical trial. Int J Med Sci. 2015;12:559–565. doi:10.7150/ijms.11521
2. Morel J, Salard M, Castelain C, et al. Haemodynamic consequences of etomidate administration in elective cardiac surgery: a randomized double-blinded study. Br J Anaesth. 2011;107:503–509. doi:10.1093/bja/aer169
3. Möller Petrun A, Kamenik M. Bispectral index-guided induction of general anaesthesia in patients undergoing major abdominal surgery using propofol or etomidate: a double-blind, randomized, clinical trial. Br J Anaesth. 2013;110:388–396. doi:10.1093/bja/aes416
4. Nyman Y, von Hofsten K, Ritzmo C, Eksborg S, Lönnqvist PA. Effect of a small priming dose on myoclonic movements after intravenous anaesthesia induction with Etomidate-Lipuro in children. Br J Anaesth. 2011;107:225–228. doi:10.1093/bja/aer129
5. Doenicke A, Roizen MF, Nebauer AE, Kugler A, Hoernecke R, Beger-Hintzen H. A comparison of two formulations for etomidate, 2-hydroxypropyl-beta-cyclodextrin (HPCD) and propyleneglycol. Anesth Analg. 1994;79:933–939.
6. Komatsu R, You J, Mascha EJ, Sessler DI, Kasuya Y, Turan A. Anesthetic induction with etomidate, rather than propofol, is associated with increased 30-day mortality and cardiovascular morbidity after noncardiac surgery. Anesth Analg. 2013;117(6):1329–1337. doi:10.1213/ANE.0b013e318299a516
7. Isitemiz I, Uzman S, Toptaş M, et al. Prevention of etomidate-induced myoclonus: which is superior: fentanyl, midazolam, or a combination? A retrospective comparative study. Med Sci Monit. 2014;20:262–267. doi:10.12659/MSM.889833
8. Hueter L, Schwarzkopf K, Simon M, Bredle D, Fritz H. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiol Scand. 2003;47:482–484.
9. Zhu Y, Yang Y, Zhou C, Bao Z. Using dezocine to prevent etomidate-induced myoclonus: a meta-analysis of randomized trials. Drug Des Devel Ther. 2017;11:2163–2170. doi:10.2147/DDDT.S137464
10. Gultop F, Akkaya T, Bedirli N, Gumus H. Lidocaine pretreatment reduces the frequency and severity of myoclonus induced by etomidate. J Anesth. 2010;24:300–302. doi:10.1007/s00540-010-0869-6
11. Zhou C, Zhu Y, Liu Z, Ruan L. Effect of pretreatment with midazolam on etomidate-induced myoclonus: a meta-analysis. J Int Med Res. 2017;45:399–406. doi:10.1177/0300060516682882
12. Doenicke AW, Roizen MF, Kugler J, Kroll H, Foss J, Ostwald P. Reducing myoclonus after etomidate. Anesthesiology. 1999;90:113–119. doi:10.1097/00000542-199901000-00017
13. Lv Z, Lu Z, Fang J, et al. Intravenous dezocine pretreatment reduces the incidence and intensity of myoclonus induced by etomidate. J Anesth. 2014;28:944–947. doi:10.1007/s00540-014-1842-6
14. He L, Ding Y, Chen H, Qian Y, Li Z. Dezocine pretreatment prevents myoclonus induced by etomidate: a randomized, double-blinded controlled trial. J Anesth. 2015;29:143–145. doi:10.1007/s00540-014-1854-2
15. Gancher S, Laxer KD, Krieger W. Activation of epileptogenic activity by etomidate. Anesthesiology. 1984;61:616–617. doi:10.1097/00000542-198411000-00029
16. Grant IS, Hutchison G. Epileptiform seizures during prolonged etomidate sedation. Lancet (Lond, Engl). 1983;2:511–512.
17. Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: seizures and general anesthetic drugs. Anesth Analg. 2008;107:1689–1703. doi:10.1213/ane.0b013e3181852595
18. Kugler J, Doenicke A, Laub M. The EEG after etomidate. Anaesthesiol Resusc. 1977;106:31–48.
19. Mantz J, Josser J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28:3–6. doi:10.1097/EJA.0b013e32833e266d
20. Wagner DS, Brummet CM. Dexmedetomidine: as safe as safe can be. Semin Anesth Perioper Med Pain. 2006;25:77–83. doi:10.1053/j.sane.2006.02.003
21. Luan HF, Zhao ZB, Feng JY, et al. Prevention of etomidate-induced myoclonus during anesthetic induction by pretreatment with dexmedetomidine. Braz J Med Biol Res. 2015;48:186–190. doi:10.1590/1414-431X20144100
22. Mizrak A, Koruk S, Bilgi M, et al. Pretreatment with dexmedetomidine or thiopental decreases myoclonus after etomidate: a randomized, double-blind controlled trial. J Surg Res. 2010;159:e11–e16. doi:10.1016/j.jss.2009.07.031
23. Dey S, Kumar M. Comparison of pretreatment with dexmedetomidine with midazolam for prevention of etomidate-induced myoclonus and attenuation of stress response at intubation: a randomized controlled study. J Anaesthesiol Clin Pharmacol. 2018;34(1):94–98. doi:10.4103/joacp.JOACP_297_16
24. Du X, Zhou C, Pan L, Li C. Effect of dexmedetomidine in preventing etomidate-induced myoclonus: a meta-analysis. Drug Des Devel Ther. 2017;11:365–370. doi:10.2147/DDDT.S121979
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
