Back to Journals » Clinical Ophthalmology » Volume 11
Early experiences of planning stereotactic radiosurgery using 3D printed models of eyes with uveal melanomas
Authors Furdová A , Sramka M, Thurzo A, Furdová A
Received 1 October 2016
Accepted for publication 17 November 2016
Published 31 January 2017 Volume 2017:11 Pages 267—271
DOI https://doi.org/10.2147/OPTH.S123640
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Furdová et al
Views: 473
Alena Furdová,1 Miron Sramka,2 Andrej Thurzo,3 Adriana Furdová3
1Department of Ophthalmology, Faculty of Medicine, Comenius University, 2Department of Stereotactic Radiosurgery, St Elisabeth Cancer Inst and St Elisabeth University College of Health and Social Work, 3Department of Simulation and Virtual Medical Education, Faculty of Medicine, Comenius University, Bratislava, Slovak Republic
Objective: The objective of this study was to determine the use of 3D printed model of an eye with intraocular tumor for linear accelerator-based stereotactic radiosurgery.
Methods: The software for segmentation (3D Slicer) created virtual 3D model of eye globe with tumorous mass based on tissue density from computed tomography and magnetic resonance imaging data. A virtual model was then processed in the slicing software (Simplify3D®) and printed on 3D printer using fused deposition modeling technology. The material that was used for printing was polylactic acid.
Results: In 2015, stereotactic planning scheme was optimized with the help of 3D printed model of the patient’s eye with intraocular tumor. In the period 2001–2015, a group of 150 patients with uveal melanoma (139 choroidal melanoma and 11 ciliary body melanoma) were treated. The median tumor volume was 0.5 cm3 (0.2–1.6 cm3). The radiation dose was 35.0 Gy by 99% of dose volume histogram.
Conclusion: The 3D printed model of eye with tumor was helpful in planning the process to achieve the optimal scheme for irradiation which requires high accuracy of defining the targeted tumor mass and critical structures.
Keywords: 3D printing, uveal melanoma, stereotactic radiosurgery, linear accelerator, intraocular tumor, stereotactic planning scheme
Introduction
The most aggressive type of intraocular tumor in adults is intraocular uveal melanoma. In the United States, its incidence is recorded in ~4.3 new cases per million people,1 in the Slovak National Cancer Registry, it is from 0.2 to 0.6/100,000 inhabitants of Slovakia.2
The current diagnostic tools such as ultrasound, optical coherence tomography, computed tomography (CT), and magnetic resonance imaging (MRI) with ophthalmological examination during the diagnosis of primary uveal melanoma has better results than in the past.
Radiotherapy (external beam, proton beam, charged particle, brachytherapy, Leksell Gamma Knife®, and stereotactic radiotherapy [SRT]) has become the preferred method of treatment in the vast majority of uveal melanoma patients in the past decades. This method uses different radiation modalities during uveal melanoma treatment. Another alternative treatment, applicable for small and middle stage of posterior uveal melanoma, is stereotactic radiosurgery (SRS). Typically, this category includes extracerebral lesions such as uveal melanoma, and SRS has been invented in treatment of intraocular tumors in the last two decades. This treatment ensures local control on good level, and the corresponding survival rates are comparable to other used treatments.3–5
The radiosurgery provided by one-fraction of linear accelerators (LINAC) is not a usual approach in the treatment of choroidal melanoma. This method uses the so-called image fusion of two sources: data from contrast-enhanced MRI and CT which are used for the calculation of planning coordinates. This treatment is similar to SRS; however, in this treatment, only one single fraction based on spatial accuracy of pre-calculated collimating system is administered.6
The eye with uveal melanoma must be immobilized before stereotactic irradiation. SRS sutures are placed under four extraocular direct muscles through the conjunctiva and through the lids. The stitches are fixed to the stereotactic Leibinger frame and the stereotactic frame is fixed to the head. On the same day, CT and MRI examinations with the system of immobilization of head and eye are performed.7
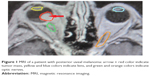
After the fusion of CT and MRI images, the stereotactic treatment planning procedure is optimized according to the critical structures – lenses, optic nerves, and chiasm (Figure 1). Calculation of the tumor volume is based on the region of interest (ROI) of the tumor. The therapeutic dose is 35.0 Gy by 99% of dose volume histogram (DVH).7
In the afternoon, the patient undergoes irradiation at linear accelerator. Sutures and frame are removed. The planned therapeutic dose in 1-day SRS session is 35.0 Gy (TDmin). Dose to the margin of the lesion varies from 35.0 to 38.0 Gy (TDmax 37.0–50.0 Gy). The doses to the critical structures are <8.0 Gy for the optic nerve and the optic disc and 10.0 Gy for the anterior segment of the eye.3,7
A team of specialists comprising ophthalmologist, neurosurgeon, and medical physicist are responsible for SRS planning. The process of this planning is based on CT and MRI. Fusion of data from both the imaging techniques accurately specifies the anatomical structures, differentiation of targeted volume, tumor mass from healthy tissue, and the most critical structures (chiasma opticum, brain stem, skin of the head, bilateral optic nerves, and lenses). Precise planning is important for determining the stereotactic coordinates of radiation beams that will be applied into targeted tumor mass. Irradiation of critical structures by inappropriate doses can lead to loss of vision or other complications and can reduce the quality of patient’s life after the therapy.8,9
SRS planning in a computer system can be made either automatically or manually. Defining the head surface is important in the calculation of the penetrating depth of each radiation beam. Medical physicist can manually draw borders of targeted tumor and also define borders of critical structures in each CT/MRI scanned slice. The whole team of specialists are responsible for correctly defining the structures. Current development of 3D printing enables us to create models of objects, shapes, and structures that seemed almost impossible to be printed earlier. It is important to note that two-dimensional (2D) radiographic images, such as X-rays, MRI, or CT scans, can be converted to digital 3D print files, allowing the creation of customized anatomical and medical structures.10 Planning process requires high accuracy in defining the targeted tumor mass and critical structures. In this study, the shape and borders of an intraocular tumor were created for the first time successfully by using 3D printing technique in patients indicated for SRS. It helped to obtain an idea of real-shaped eye with tumor during stereotactic planning process, for which the 3D printed eye model with tumor mass is required. Influence of subjectivity and the possibility of an error during the process of determining the tumor borders are eliminated in 3D model. Understanding of bizarre shapes of a tumor in real 3D model can increase the accuracy of planning.
This study consists of the assessment of uveal melanoma treatment, provided by one-day session of LINAC based on SRS method. In 2015, optimization of the planning scheme with 3D printed individual model of the eye with intraocular tumor in five patients was invented, and this model was used by optimizing the planning scheme of each patient.
Methods
The aim of this study was to develop a new modality for the visualization of intraocular tumors in 3D space for planning stereotactic radiosurgical procedure on linear accelerator. The Ethics Committee of the University Teaching Hospital Bratislava deemed written informed consent and ethics approval were not needed as the data of the patients were not used for direct therapy, only as a support for model situation – 3D model.
By the 1-day session surgery, the patient undergoes CT and MRI examinations with the condition that the target eye is immobilized by the stereotactic frame. The fusion of the CT and MRI data is used in the planning for the stereotactic treatment and is optimized against not only the critical structures such as optic nerve and lens of the target eye but also the lens and optic nerve at the contralateral side and the chiasm too. SRS is executed in a 1-day session. Model LINAC C 600 C/D Varian with 6 MeV X is used as linear accelerator.
The tumor volume (size) is calculated on the basis of the ROI. 3D reconstruction proves that the calculation was done correctly. The pre-calculated therapeutic dose for each tumor mass is 35.0 Gy in 99% of DVH. At the same day after pre-calculation, usually afternoon, patients undergo targeted irradiation with linear accelerator. SRS planning scheme ensures that the doses for the critical structures are <8.0 Gy in the area of optic nerve and optic disc and <10.0 Gy in the area of anterior segment of the eye. Sutures and stereotactic frame are removed under local anesthesia.
Five models of the eye globe with intraocular tumor were printed into 3D form based on radiographic 2D data from CT. 3D Slicer, a free, open source software version 4.5.0+ (https://www.slicer.org/) for data segmentation was used to generate the virtual 3D model of the targeted eye. Imported data set from CT was with an accuracy of 1 mm scan thickness. The created model consisted of scleral globe, corneal segment, lens, optic nerve, and mass of a tumor. Muscles and corpus vitreum were not included. Lens and optic nerve that are very important anatomical structures for orientation are visible points at the small printed 3D model. Virtual 3D model was sliced, additional support was calculated, and the 3D model was prepared for printing in 3D printing slicing software Simplify3D® (Cincinnati, OH, USA). Printing technology fused with deposition modeling was used. Thickness of one layer in vertical line was 100 μm which provides an ideal proportion between accuracy and velocity of 3D printing process. The estimated time for the printing process in 3D printer was from 15 to 30 min per model. The 3D models of this study were printed in different colors. One 3D model was printed as tripled size to compare structural visibility and localization of the tumor.
Results
In 2015, 3D printing model of an eye with uveal melanoma to improve the SRS planning scheme was invented (Figures 1 and 2). By using the software for segmentation (3D Slicer), a virtual 3D model of eye globe with tumor based on tissue density was created from CT and MRI data (Figure 3). A sharp cut of the model was made to uncover the inner surface of the globe, preserving important structures. Virtual model was then processed in the slicing software (Simplify3D) and printed on 3D printer using fused deposition modeling technology (Figures 4 and 5). The material used for printing was polylactic acid.
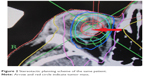  | Figure 2 Stereotactic planning scheme of the same patient. |
  | Figure 3 Virtual model of the eye, outer view; arrow indicates optic nerve. |
  | Figure 4 Printed 3D model of the eye with intraocular uveal melanoma, middle-stage T2; arrow indicates tumor mass. |
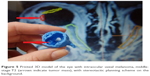  | Figure 5 Printed 3D model of the eye with intraocular uveal melanoma, middle-stage T2 (arrows indicate tumor mass), with stereotactic planning scheme on the background. |
Discussion
The management of uveal melanoma has changed in the last decades, focusing toward the techniques sparing the eye globe. The alternatives to radical enucleation vary from basic observation up to external beam radiotherapy, transpupillary thermotherapy, endoresection with pars plana vitrectomy, block-excision, charged particles, Leksell Gamma Knife®, brachytherapy using various radioisotopes, and SRS.11,12
More than 15 years this stereotactic irradiation is used in clinical practice to treat patients with uveal melanoma. Over the past few years, the therapeutic single dose was reduced up to 35.0 Gy without reduction in tumor control rate. Possible advantage of hypofractionated treatment, as many radiobiological studies indicate, is that a very large single fraction is used to sterilize uveal melanoma cell lines. It has obtained additional interest in fractionated SRT. The advantage of a feasible fractionation is used by LINAC. Hypofractionated scheme of four or five fractions with total doses from 50.0 to 70.0 Gy is employed by most of the LINAC studies. Different studies report that efficacy of SRT in uveal melanoma treatment has been proven on local tumor with control rates >90%. In the treatment of juxtapapillary choroidal melanoma, the SRT offers another noninvasive alternative for enucleation and brachytherapy with high tumor control rate.13,14
If the tumor is too large for conventional brachytherapy, then the gamma knife radiosurgery and SRS are used as the alternative in therapy for uveal melanoma. Few published results support SRS therapy in specific cases of uveal melanoma.15,16
Compared to other forms of radiotherapy, typical radiogenic side effects after SRT are reported in radiation retinopathy, cataract development, neovascular glaucoma, and opticopathy. It can lead to visual acuity loss and in worst cases also to secondary enucleation. In general, SRS and SRT are considered as effective treatment modalities for uveal melanoma. SRS is one of the new methods; hence, the need for multicenter trial, in order to compare the outcomes against other methods, is recommended. Stereotactic photon therapy for uveal melanoma, using CT and MRI images, is very safe and is a precise treatment option with excellent local control. Visual reduction was noticed in a high number of patients because of unfavorable tumor size and location in the vicinity of critical structures (eg, optic nerve and macula). Stereotactic irradiation for uveal melanoma by using LINAC is feasible and well tolerated. This eye-preserving treatment can be offered to patients who have medium-sized and unfavorably located uveal melanoma.
Although rare, optic neuropathy can occur after radiosurgical treatment for lesions that are localized close to the visual pathways. One-step LINAC-based SRS with single dose of 35.0 Gy in conjunction with a mechanical immobilization system with four sutures is a highly effective method to treat small- and middle-stage uveal melanomas.7
The 3D printing has its new role in ophthalmology by validating and demonstrating how the real models of tumors look like. Nowadays, precise device modeling allows the material to be printed at a resolution of 600 μm. This approach is used also in other type of ophthalmological surgery, cataract surgery, which enabled the development of Cana’s ring (CR) and special 3D pupil expansion device.17
Another practical use of 3D printing in ophthalmology is for the examination of eye fundus. The procedure depends on the correct position between smartphone and spherical Volk lens. The lens adapter coupled with the smartphone has the connecting device developed and 3D printed from plastic materials.18
Different studies evaluated the efficacy of SRS for uveal melanoma. Previous study reported that tumor control rates 5 and 10 years after therapy were >90%.19 Radiogenic side effects are similar to other forms of radiotherapy. Neovascular glaucoma is the reason of visual acuity loss and is the necessity of secondary enucleation. Nowadays, stereotactic photon beam radiotherapies are considered effective treatment for intraocular melanoma. Future studies and follow-up are necessary to find the optimal treatment modalities.19
The 3D printed model of eye globe with tumor is helpful in stereotactic planning process. Planning of SRS is based on CT and MRI data. Accuracy of defining all the structures in the planning software depends on the understanding of the collocation. By creating a new 3D modality for tumor visualization, real model of the eye was provided for the specialists, which enabled them more effective planning for the SRS. Virtual model can be seen on computer only, but real 3D model can be held by the physician and physicist in their hand and can give them a better imagination of the real situation inside the eye globe, for example, distance of the tumor to the lens and distance to the optic disc.
Conclusion
Possibility to hold the real 3D model of the affected eye helps specialists to understand and imagine the size and localization of the tumor compared to 2D radiographic imaging. Developing the 3D model of an eye with intraocular tumor at the time of preparing the planning scheme for SRS is a new step for the team of specialists to perform individualized plan for the patient with uveal melanoma.
Disclosure
The authors report no conflicts of interest in this work.
References
Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110(5):956–961. | ||
Furdová A, Oláh Z, Svetlošáková Z, Kusenda P. The current state of the evidence of malignant tumors of the eye and its adnexa (dg. C69) in the Slovak Republic and in the Czech Republic. Cesk Slov Oftalmol. 2012;68(5):195–201. | ||
Furdova A, Sramka M. Uveal Malignant Melanoma and Stereotactic Radiosurgery. Saarbrücken: LAP LAMBERT Academic Publishing; 2014. | ||
Cohen VML, Carter MJ, Kemeny A, Radatz M, Rennie IG. Metastasis-free survival following treatment for uveal melanoma with either stereotactic radiosurgery or enucleation. Acta Ophthalmol Scand. 2003;6:383–388. | ||
Damato B, Kacperek A, Chopra M, Campbell IR, Errington RD. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62(5):1405–1411. | ||
Furdova A, Slezak P, Chorvath M, Waczulikova I, Sramka M, Kralik G. Radical surgical treatment (enucleation) or stereotactic radiosurgery in patients with posterior uveal melanoma – no difference in treatment outcome. Neoplasma. 2010;57(4):377–381. | ||
Furdova A, Sramka M, Chorvath M, Kralik G. Linear accelerator stereotactic radiosurgery in intraocular malignant melanoma. Austin J Radiat Oncol Cancer. 2015;1(2):1–5. | ||
Dieckmann K, Dietmar G, Bogner J, et al. Optimizing LINAC-based stereotactic radiotherapy of uveal melanomas: 7 years clinical experience. Int J Radiat Oncol Biol Phys. 2006;66(Suppl):S47–S52. | ||
Furdova A, Strmen P, Waczulikova I, Chorvath M, Sramka M, Slezak P. One-day session LINAC-based stereotactic radiosurgery of posterior uveal melanoma. Eur J Ophthalmol. 2012;22(2):226–235. | ||
Hong SC. 3D printing and ophthalmology for the community. J Cytol Histol. 2015;6(4):116. | ||
Mosci C, Mosci S, Barla A, Squarcia S, Chauvel P, Iborra N. Proton beam radiotherapy of uveal melanoma: Italian patients treated in Nice, France. Eur J Ophthalmol. 2009;19:654–660. | ||
Mueller AJ, Talies S, Schaller UC, Horstmann G, Wowra B, Kampik A. Stereotactic radiosurgery of large uveal melanomas with the gamma knife. Ophthalmology. 2000;107:1381–1387. | ||
Henderson MA, Shirazi H, Lo SS, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy in the treatment of uveal melanoma. Technol Cancer Res Treat. 2006;5(4):411–419. | ||
Krema H, Somani S, Sahgal A, et al. Stereotactic radiotherapy for treatment of juxtapapillary choroidal melanoma: 3-year follow-up. Br J Ophthalmol. 2009;93:1172–1176. | ||
Dieckmann K, Dietmar G, Zehetmayer M, Bogner J, Georgopoulos M, Potter R. LINAC based stereotactic radiotherapy of uveal melanoma: 4 years clinical experience. Radiother Oncol. 2003;67:199–206. | ||
Furdova A, Sramka M, Chorvath M, et al. Stereotactic radiosurgery in intraocular malignant melanoma – retrospective study. Neuro Endocrinol Lett. 2014;35(1):28–36. | ||
Canabrava S, Diniz-Filho A, Schor P, Fagundes DF, Lopes A, Batista WD. Production of an intraocular device using 3D printing: an innovative technology for ophthalmology. Arq Bras Oftalmol. 2015;78(6):393–394. | ||
Myung D, Jais A, He L, Blumenkraz ML, Chang RT. 3D printed smartphone indirect lens adapter for rapid, high quality retinal imaging. J Mobile Technol Med. 2014;3:9–15. | ||
Zehetmayer M. Stereotactic photon beam irradiation of uveal melanoma. Dev Ophthalmol. 2012;49:58–65. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.