Back to Journals » International Medical Case Reports Journal » Volume 13
Duodenal Obstruction Due to Giant Gallstone: A Case Report
Authors Nguyen BH , Le Quan AT , Hai PM , Quang Hung V , Thai TT
Received 24 August 2020
Accepted for publication 20 October 2020
Published 17 November 2020 Volume 2020:13 Pages 651—656
DOI https://doi.org/10.2147/IMCRJ.S278058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Bac Hoang Nguyen,1 Anh Tuan Le Quan,2 Pham Minh Hai,2 Vu Quang Hung,3 Truc Thanh Thai4
1University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam; 2Department of Hepatobiliary and Pancreatic Surgery, University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam; 3Department of General Surgery, University of Medicine and Pharmacy Ho Chi Minh City, Ho Chi Minh City, Vietnam; 4University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
Correspondence: Truc Thanh Thai
University of Medicine and Pharmacy at Ho Chi Minh City, 217 Hong Bang Street, District 5, Ho Chi Minh City, Vietnam
Tel +84-908-381266
Email [email protected]
Background: Duodenal obstruction due to a gallstone, also known as Bouveret’s syndrome, is one type of gallstone ileus. This is a rare complication of cholelithiasis. Among gallstone ileus cases, duodenal obstruction is alsorare. Apart from rareness, diagnosis is challenging due to unspecific clinical manifestation. Treatment options have benefits and drawbacks with each as well. Therefore, setting an appropriate option in a certain patient is an important issue.
Case Report: An 85-year-old woman presented clinically with a gastric outlet obstruction. Upper gastrointestinal (GI) endoscopy was attempted but endoscopic exploration was limited because of duodenum filled by fluid. Rigler’s triad was detected on abdominal enhanced CT scan: duodenal obstruction, ectopic gallstone within duodenum lumen, pneumobilia. The stone was very large (9 cm long). The diagnosis was duodenal obstruction due to giant gallstone and cholecysto-duodenum fistula. The patient was treated with a radical one-stage procedure: fistulotomy for gallstone removal, cholecystectomy, side-to-side Roux-en-Y duodenojejunostomy. There were no complications related to surgery during the follow-up period.
Conclusion: Surgery plays an important role in management of duodenal obstruction due to a gallstone, especially a large and impacted stone. Radical one-stage surgery is one of the feasible, safe, and efficient procedures in selected patients.
Keywords: duodenal obstruction, giant gallstone, gallstone ileus, Bouveret syndrome, Bouveret’s syndrome
Introduction
Gallstone is one of the most common diseases worldwide with a wide range of clinical manifestations from no symptoms to severe symptoms with complications. Gallstones can cause acute or chronic cholecystitis, choledocholithiasis with or without acute cholangitis, gallstone pancreatitis, Mirrizzi's syndrome, gallstone ileus, and gallbladder cancer. Among these, acute cholecystitis is the most common complication while gallstone ileus is a rare complication.1 The latter accounts for 0.3% to 0.5% of cholelithiasis patients and 1% to 4% of the causes of mechanical intestinal obstruction.2,3 Bouveret's syndrome is an uncommon presentation of gallstone ileus, that represents 1% to 10% of causes of gallstone ileus.4–6 Duodenal obstruction due to gallstones happens when fistula between the gallbladder and duodenum or stomach exists. There are a wide range of clinical scenarios related to this issue. Most of these are vague and diagnosis of duodenal obstruction due to gallstones is usually based on medical imaging. For example, Chou et al reported two cases of gallstone ileus. The first was a 78-year old woman who had a two-day history of vomiting and epigastralgia. Plain abdominal film suggested small bowel obstruction and clinically attributed to adhesions. Later on, gallstone ileus was diagnosed by abdominal computed tomography (CT) based on the presence of pneumobilia, bowel obstruction, and an ectopic stone within the jejunum. The second case was a 76-year old man with a one-week history of epigastralgia. Plain abdominal film showed two round calcified stones in the right upper quadrant. Fistulography confirmed the presence of a cholecystoduodenal fistula and gallstone ileus was also diagnosed by abdominal CT.7 These examples show that clinical signs and symptoms are not specific for gallstone ileus. It is essential to use diagnostic imaging such as CT scan or MRI to confirm diagnosis of gallstone duodenal obstruction.
The choice of treatment for Bouveret’s syndrome depends on the gallstone length, the site of gallstone impaction and the number of lithotripsy modes available to use.8 Endoscopy and surgery are the main procedures to treat this. Among these, endoscopy has presently been recommended as the first choice. However, the overall success rate is about 43%.8 Surgery for Bouveret's syndrome is usually classified into three categories belonging to radical or nonradical therapy. They are gastrolithotomy or enterolithotomy only (nonradical procedure), one-stage procedures and two-stage procedures (radical therapy). Radical therapy includes two parts. One is managing duodenal obstruction. Another one is cholecystectomy and repairing fistula between gallbladder and duodenum or stomach. Gastrolithotomy or enterolithotomy is simple and with low risk of complications. However, fistula-related and cholecystitis-related problems remain postoperatively. Although it is still controversial, it is recommended that one-stage or two-stage surgery or gastroenterolithotomy only should be chosen based on age, ASA and potentially difficult surgery.8
This paper aims to present a case of duodenal obstruction due to a giant gallstone which was treated by radical one-stage procedure.
Case Report
An 85-year-old woman presented with epigastric pain for four days and associated nausea and vomiting. A nonadherence to hypertension treatment was recorded. On the admission day, the patient experienced, epigastric pain but there was no fever and no tenderness in abdominal physical examination. Heart rate was 94 bpm while blood pressure was 190/70 mmHg. One litre of gastric contents was drained by a nasogastric tube. Blood count demonstrated slight increase of white blood cell (12.77 K/µL). The serum potassium concentration was 2.68 mmol/L, a significantly decreasing. Other blood results such as level of serum bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), amylase and renal function were normal.
Hypertension and electrolyte disturbance were adjusted by giving amlodipine 5 mg, captopril 25 mg and potassium chloride infusion. Abdominal CT scan was then performed. Rigler’s triad was detected on abdominal CT scan including duodenal obstruction with dilated stomach, ectopic gallstone within duodenum indicating cholecystoduodenal fistula, pneumobilia (Figure 1). Additionally, gastroduodenoscopy illustrated signs of duodenal obstruction such as gastroduodenal dilatation and filled fluid in duodenum. However, no stone was visible. She underwent an elective open surgery. She was classified into ASA III (American Society of Anesthesiologists III).
During the surgery, we found that there was chronic cholecystitis and tightly adhered to duodenum and hepatic hilum there was cholecystoduodenal fistula. A huge gallstone which was 9×6 cm in size had migrated to the lower second part of the duodenum (D2). This stone caused duodenal obstruction. With this site of impaction, we had a long distance to the stomach and jejunum. Another important issue was that the anterior duodenal wall had been widely impaired by the fistula and the healthy wall of D2 was much narrowed as a consequence. Therefore, we firstly performed fistulotomy to remove the gallstone. Figure 2 describes stone after being extracted. Then cholecystectomy, fistulectomy were done. After that duodenal defect was addressed. With the size defect of 7 cm in diameter, we performed a side-to-side Roux-en-Y duodenojejunostomy. A nasogastric tube was passed through the anastomosis and a surgical drain was placed into the right infrahepatic space.
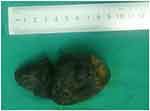 |
Figure 2 The gallstone causes cholecystoduodenal fistula. |
On the first postoperative day, the patient had bowel motility, nasogastric tube drained 50–100 mL gastric content per day. Right infrahepatic drain had little fluid. She was supplied with total parenteral nutrition for seven days. Then oral intake was increasingly applied from clean water, soup, milk, and finally solid foods. There was no enteric obstruction and intra-abdominal infection. Hypertension with systolic pressure ranged from 160–200 mmHg on the first postoperative day. This was controlled during a seven-day period with a therapy of Adalat LA 30 mg tid.
However, on the seventh postoperative day, she developed nosocomial pneumonia with purulent cough, inspiratory crackles, right upper lobe atelectasis on chest radiography, CRP of 82 ng/mL, WBC of 16 K/µL and %Neu of 86%. She was treated with meropenem, levofloxacine for a period of 10 days. The patient was discharged on the sixteenth postoperative day. After six months of follow-up, no complication was found.
Discussion
In 1986, Leon Bouveret first described gastric outlet obstruction syndrome caused by an impacted gallstone in the duodenum.9 Pathophysiologically, the recurrent inflammation and pressure necrosis caused by large offending gallstone lead to erosion through the gallbladder wall and GI wall. As a result, fistula between the gallbladder and the adhered portion of the gastrointestinal tract is formed.10,11 Cholecystoduodenal fistula is the most common biliary-enteric fistula in 32.5% to 96.5%.12 The majority of gallstones smaller than 2.5 cm may pass spontaneously through a normal gastrointestinal tract and are excreted uneventfully in the stools.10,11,13 Gallstones larger than 2.5 cm are more likely to become impacted stones, resulting in gallstone ileus. The most frequent location is terminal ileum (89.5%) while there are only 1% to 10% of cases in duodenum.4,5,12
Clinical diagnosis is mainly based on Bouveret’s syndrome which is defined as gastroduodenal obstruction caused by an impacted gallstone. Main and frequent symptoms are nausea, vomiting and epigastric pain.6 Ultrasonography plays a limited role in accuracy. Rigler’s triad in abdominal CT scan demonstrating duodenal obstruction with dilated stomach, ectopic gallstone within duodenum lumen and pneumobilia helps to improve diagnosis accuracy.12 Fortunately, our case was a typically clinical scenario of Bouveret’s syndrome. In addition, endoscopy showed Bouveret’s syndrome with a dilated stomach and fluid fulfilled duodenum. These clinical and endoscopic symptoms were indicators of Bouveret’s syndrome as described in Cappell and Davis’s comprehensive review.6 Therefore, an abdominal CT scan was indicated early. CT scan revealed typical Rigler’s triad which has a sensitivity up to 93%.14 In a retrospective study by Lassandro et al, Rigler’s triad was observed in 77.8% of cases, which is much higher compared to 14.8% and 11.1% in radiographs and ultrasonography, respectively.15
As a rare condition, there is no consensus on the treatment of duodenal obstruction due to a gallstone. The therapeutic strategy is often chosen based on several aspects including the patient’s age, comorbidities, the effect of obstruction on the general condition, local inflammatory changes, the fistula’s size, the size and number of gallstones.4,16 The primary goal is to address the obstruction by removing the impacted stone. This can be achieved by endoscopic or surgical therapy.
According to a systematic review of Bouveret's syndrome, published in 2020, failure of endoscopic intervention has been shown to be associated with increasing gallstone length and impaction in distal duodenum (D3 and D4). The cut point of gallstone length that predicted failing was >4 cm with an AUC of 0.83, p<0.001 (95%CI: 0.74–0.90).8 The authors also revealed that the use of multimodal of endoscopic lithotripsy contributed significantly to the increased success rate. Although the gallstone in our case was impacted in the second part of duodenum, not distal parts, the length of gallstone was up to 9 cm. Besides that, in this systematic review, Ong et al suggested the novel predictive tool to predict the probability of success of endoscopic therapy. According to this tool, the score of our case was −6.96 with a probability of success ranging from 0 to 10%. In our condition, we only have mechanical lithotripsy modality. We chose surgery as the first line of treatment. We did not attempt endoscopic intervention. Attempting this can cause prolonging the time interval to release mechanical obstruction and late complications of gastroduodenal obstruction will happen as a result of late effective management. In addition, trying to do endoscopic retrieval of large stone can lead to severe complications including perforation of duodenum, distal small bowel obstruction, esophageal injury, migration of gallstone fragments into mediastinum, cardiac arrhythmias. Therefore, the choice of the initial interventional procedure is very important.
In terms of stone removal, when endoscopic procedure is not possible, surgery is the next option including open or laparoscopic surgery. During the operation, the whole intestine should be examined, since concomitant gallstone ileus is present at another location in the digestive tract in about 16% of the cases.3 If it is possible, the stone should be relocated into the stomach and extracted by gastrotomy. If this is not feasible, stone extraction through enterotomy should be attempted. The explanation is that incising at a healthy wall area will help wound healing better. This can decrease the risk of postoperative complications. When having high risk of failure or failing with both maneuvers, stone extraction with duodenotomy should be done in the anterior surface of duodenum. Then, the parietal duodenal defect could be simply sutured or a duodenojejunostomy performed.17 So far, this step, duodenal obstruction is overcome. It means the primary goal of treatment is achieved. Nevertheless, cholecystitis and fistula still remain. One question is whether we can leave cholecystitis and fistula. This is still a controversial problem. On the one hand, supporters suggest a simple management which only solves duodenal obstruction. On the other hand, others argue that cholecystitis and fistula should be addressed (radical surgery). Radical surgery can be performed with one stage or two stage. With respect to duodenal fistula repair, there are two techniques. One is simple closure of defect by suturing. Another one is duodenojejunostomy.
Regarding simple treatment, reports support that simple stone extraction is reasonable.3,5,18 This technique does not only manage the patient’s main problems, but it has also been associated with a lower rate of complication and mortality. The percentage of morbidity and mortality was 12% while this figure in radical surgery was 20% to 30%.4,17,19,20 In addition, a cholecystoduodenal fistula may be considered as a biliodigestive anastomosis. This is the reason why most patients remained asymptomatic after this procedure.4,17 Besides that, one report demonstrated some fistulas closed spontaneously within 30–60 days. Therefore, there were some further asymptomatic periods in the presence of a permeable biliary tract.17
Regarding radical treatment, some authors argue that remaining fistulous orifice has a high risk of recurrent cholecystitis and cholangitis.21,22 In 1966, Warshaw and Bartlett reported that among 18 cases of enterolithotomy without cholecystectomy and fistula repair, there were six recurrent cholecystitis cases.23 Additionally, a dysfunctional scleroatrophic gallbladder can develop cancer.23,24 There was a significant difference of gallbladder carcinoma incidence between two groups with and without biliary fistula at 15% and 0.8%, respectively.25 According to current literature, cholecystectomy and fistula repair may prevent risks of cancer and complication.12,23,26,27 As mentioned above, the complication rate of radical treatment still high. This disadvantage plays an important role when choosing treatment method, especially for patients with poor general physical status. However, in this condition, we can separate procedure into two stages.4,17 One stage should be performed in selected patients (patient’s condition good, local inflammatory changes for safer cholecystectomy, gangrenous cholecystitis, or residual gallstones).2,4,12,17
Generally, both simple and radical treatment have benefits and drawbacks. Most authors agree that simple stone extraction is enough for patients in bad general condition as septic shock, peritonitis. Patients with advanced age are also suitable for this procedure. Moreover, this indication should be considered for cases with suspected high risk of intraoperative and postoperative complications.4,12,17 Recently, Ong et al provided a flow chart of management strategy for Bouveret's syndrome with detailed criteria.8 This strategy guides how to get an ideal option for a certain patient.
According to Ong et al’s strategy, our patient had a high risk of surgery because she was more than 83 years old with ASA more than II and potentially difficult surgery due to tightly adhesive inflammation to hepatic hilum and duodenum. With this high risk, we should have performed only surgical stone extraction. Actually, our case had a different scenario. After surgical exploration, we performed fistulotomy to remove the stone. This meant that we had to do radical surgery in one stage instead of only stone removal as discussed above. We did not use gastrotomy or jejunotomy to extract the stone because of high risk of failure. The site of incision was far away from the site of impaction (lower D2) while the stone was huge and tightly impacted. In addition, the likelihood of complications was high and operating time would be longer. We did not perform duodenotomy to remove stone because repair of duodenotomy in this case was assessed to be unsafe. The patient’s duodenal wall was much narrowed due to erosion of big fistula. Moreover, chronic inflammation made it unhealthy. Therefore, we had to separate the gallbladder from the duodenum by transecting fistula for stone removal through the hole and then duodenal fistulectomy was done. Fistulectomy created a duodenal parietal defect with 7-cm diameter. This defect took up over a half of circumference of the second part of duodenum. With large tissue loss, we had to do a side-to-side Roux-en-Y duodenojejunostomy. In 2008, Iancu et al reported a case of Bouveret’s syndrome with 5×9 cm stone. Due to an 11-cm antroduodenal parietal defect, he had to perform side-to-side Roux-en-Y antroduodenojejunal anastomosis. The outcome showed no leaking, no stenosis.4 Our patient also had a good outcome with radical surgery. Due to the difficult technique, this procedure requires experienced surgeons on hepatobiliary surgery and should be only done in selected patients. However, in some special situations like our case, we must perform one-stage radical surgery. We had to open the fistula for stone removal aiming to avoid without unsafely duodenotomy. We thought that there was no different and better option.
Conclusion
Duodenal obstruction due to gallstone is rare. Diagnosis is mainly based on Bouveret’s syndrome and Rigler’s triad on CT scan. Radical one-stage surgery may be an ideal option in case of a huge, tightly impacted stone in lower D2 and extreme tissue loss. It is feasible, safe and efficient in selected patients.
Ethical Approval
The need for institutional ethics approval for this case report was waived. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Funding
This study received no funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165(4):399–404. doi:10.1016/S0002-9610(05)80930-4
2. Clavien P-A, Richon J, Burgan S, Rohner A. Gallstone ileus. BJS. 1990;77(7):737–742.
3. O’Neill C, Colquhoun P, Schlachta CM, Etemad-Rezai R, Jayaraman S. Gastric outlet obstruction secondary to biliary calculi: 2 cases of Bouveret syndrome. Can J Surg. 2009;52(1):E16–18.
4. Iancu C, Bodea R, Al Hajjar N, Todea-Iancu D, Bala O, Acalovschi I. Bouveret syndrome associated with acute gangrenous cholecystitis. J Gastrointestinal Liver Dis. 2008;17(1):87–90.
5. Brennan GB, Rosenberg RD, Arora S. Bouveret syndrome. Radiographics. 2004;24(4):1171–1175.
6. Cappell MS, Davis M. Characterization of Bouveret’s syndrome: a comprehensive review of 128 cases. Am J Gastroenterol. 2006;101(9):2139–2146.
7. Chou J-W, Hsu C-H, Liao K-F, et al. Gallstone ileus: report of two cases and review of the literature. World J Gastroenterol. 2007;13(8):1295–1298.
8. Ong J, Swift C, Stokell BG, et al. Bouveret Syndrome: A Systematic Review of Endoscopic Therapy and a Novel Predictive Tool to Aid in Management. J Clin Gastroenterol. 2020;54(9):758–768.
9. Bouveret L. Stenose du pylore, adherent a la vesicule calculeuse. Rev Med. 1896;16:1–16.
10. Fox PF. Planning the operation for cholecystoenteric fistula with gallstone ileus. Surg Clin North Am. 1970;50(1):93–102. doi:10.1016/S0039-6109(16)39035-1
11. VanLandingham SB, Broders CW. Gallstone ileus. Surg Clin North Am. 1982;62(2):241–247. doi:10.1016/S0039-6109(16)42683-6
12. Nuno-Guzman CM. Gallstone ileus, clinical presentation, diagnostic and treatment approach. World J Gastrointest Surg. 2016;8(1):65–76. doi:10.4240/wjgs.v8.i1.65
13. Abou-Saif A, Al-Kawas FH. Complications of gallstone disease: mirizzi syndrome, cholecystocholedochal fistula, and gallstone ileus. Am J Gastroenterol. 2002;97(2):249–254. doi:10.1111/j.1572-0241.2002.05451.x
14. Yu C-Y. Value of CT in the diagnosis and management of gallstone ileus. World J Gastroenterol. 2005;11(14):2142–2147. doi:10.3748/wjg.v11.i14.2142
15. Lassandro F, Gagliardi N, Scuderi M, Pinto A, Gatta G, Mazzeo R. Gallstone ileus analysis of radiological findings in 27 patients. Eur J Radiol. 2004;50(1):23–29. doi:10.1016/j.ejrad.2003.11.011
16. Liew V, Layani L, Speakman D. Bouveret’s syndrome in Melbourne. ANZ J Surg. 2002;72(2):161–163. doi:10.1046/j.1445-2197.2002.02319.x
17. Mavroeidis VK, Matthioudakis DI, Economou NK, Karanikas ID. Bouveret Syndrome—The Rarest Variant of Gallstone Ileus: A Case Report and Literature Review. Case Rep Surg. 2013;2013:839370. doi:10.1155/2013/839370
18. Thompson RJ, Gidwani A, Caddy G, McKenna E, McCallion K. Endoscopically assisted minimally invasive surgery for gallstones. Ir J Med Sci. 2009;178(1):85–87. doi:10.1007/s11845-007-0096-9
19. Zuegel N, Hehl A, Lindemann F, Witte J. Advantages of one-stage repair in case of gallstone ileus. Hepato-Gastroenterology. 1997;44(13):59–62.
20. Reisner RM, Cohen JR. Gallstone ileus: a review of 1001 reported cases. Am Surg. 1994;60(6):441–446.
21. Redding ME, Anagnostopoulos CE, Wright HK. Cholecystopyloric fistula with gastric outlet obstruction: a rare form of gallstone ileus and its management. Ann Surg. 1972;176(2):210–212. doi:10.1097/00000658-197208000-00015
22. Piedad OH, Wels PB. Spontaneous internal biliary fistula, obstructive and nonobstructive types: twenty-year review of 55 cases. Ann Surg. 1972;175(1):75–80. doi:10.1097/00000658-197201000-00013
23. Warshaw AL, Bartlett MK. Choice of operation for gallstone intestinal obstruction. Ann Surg. 1966;164(6):1051–1055.
24. Cooperman AM, Dickson ER, ReMine WH. Changing concepts in the surgical treatment of gallstone ileus: a review of 15 cases with emphasis on diagnosis and treatment. Ann Surg. 1968;167(3):377–383.
25. Bossart PA, Patterson AH, Zintel HA. Carcinoma of the gallbladder. A report of seventy-six cases. Am J Surg. 1962;103:366–369.
26. Fraser WJ. Intestinal obstruction by gall-stone. Br J Surg. 1954;42(172):210–212.
27. Welch JS, Huizenga KA, Roberts SE. Recurrent intestinal obstruction due to gallstones. Proc Staff Meet Mayo Clin. 1957;32(22):628–632.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

