Back to Journals » Journal of Pain Research » Volume 10
Duloxetine 60 mg for chronic low back pain: post hoc responder analysis of double-blind, placebo-controlled trials
Authors Alev L, Fujikoshi S, Yoshikawa A, Enomoto H , Ishida M , Tsuji T , Ogawa K, Konno S
Received 29 March 2017
Accepted for publication 21 June 2017
Published 24 July 2017 Volume 2017:10 Pages 1723—1731
DOI https://doi.org/10.2147/JPR.S138297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Levent Alev,1 Shinji Fujikoshi,2 Aki Yoshikawa,3 Hiroyuki Enomoto,1 Mitsuhiro Ishida,4 Toshinaga Tsuji,5 Kei Ogawa,1 Shinichi Konno6
1Bio-Medicine, 2Statistical Science, 3Scientific Communications, Medicines Development Unit, Eli Lilly Japan K.K., Kobe, 4Clinical Research Development, 5Medical Affairs Department, Shionogi & Co. Ltd, Osaka, 6Department of Orthopedic Surgery, Fukushima Medical University, Fukushima, Japan
Introduction: Duloxetine has demonstrated efficacy in chronic low back pain (CLBP). We examined the predictors of response to duloxetine for CLBP.
Patients and methods: This was a post hoc analysis of pooled data from 4 double-blind, randomized, placebo-controlled trials of duloxetine (60 mg/day for 12–14 weeks) in adult patients with CLBP. Primary outcome was proportion of patients with ≥30% reduction in Brief Pain Inventory (BPI) average pain (“pain reduction”) at 12–14 weeks. The proportion of patients with ≥30% and ≥50% (secondary outcome) pain reduction in duloxetine and placebo groups was compared. Variables for responder analyses were early improvement (≥15% pain reduction at Week 2), sex, age, baseline BPI average pain score, duration of CLBP, and number of painful body sites according to the Michigan Body Map (≥2 vs 1 [isolated CLBP]; 1 trial); relative risk (RR) and 95% confidence interval (CI) were calculated.
Results: Compared with placebo (n = 653), a greater proportion of duloxetine-treated patients (n = 642) achieved ≥30% (59.7% vs 47.8%; P < 0.001) and ≥50% pain reduction (48.6% vs 35.1%; P < 0.001). Among duloxetine-treated patients, early improvement was associated with greater likelihood of ≥30% (RR [95% CI], 2.91 [2.30–3.67]) or ≥50% (3.24 [2.44–4.31]) pain reduction. Women were slightly more likely than men to achieve ≥30% (RR [95% CI], 1.14 [1.00–1.30]) or ≥50% (1.17 [0.99–1.38]) pain reduction. Response rates were similar between age, CLBP duration, and baseline BPI average pain score subgroups. Patients with ≥2 painful sites were more likely to respond to duloxetine 60 mg relative to placebo than patients with isolated CLBP (RR, duloxetine vs placebo [95% CI]: ≥30% reduction, ≥2 painful sites 1.40 [1.18–1.66], isolated CLBP 1.07 [0.78–1.48]; ≥50% reduction, ≥2 painful sites 1.51 [1.20–1.89], isolated CLBP 1.23 [0.81–1.88]).
Conclusion: Early pain reduction was indicative of overall response. Patients with multiple painful sites had more benefit from duloxetine than patients with isolated CLBP.
Keywords: Brief Pain Inventory, chronic pain, SNRI, low back pain, Michigan Body Map, multiple painful sites
Introduction
Low back pain is a common disorder associated with high disability,1 high economic burden,2 and low quality of life,3,4 especially if chronic. Globally, the lifetime prevalence of low back pain is estimated at 38.9%.5 Low back pain that persists for >3 months is considered chronic low back pain (CLBP),6 which adversely affects both physical and mental health.3 Although CLBP may develop after a specific injury, in many cases, no precipitating event can be identified.6 CLBP may involve long-term changes to the central nervous system that affect the way pain is modulated and perceived.7 These changes include anatomical, biochemical, and functional abnormalities in the brain and spinal cord that result in increased pain perception and are collectively referred to as central sensitization.7,8 Although CLBP is defined clearly in terms of its minimum duration, patients with CLBP comprise a heterogeneous population with varying demographics, manifestations of pain, and radiological findings.8,9 Thus, clinicians struggle to identify the optimal treatment option for individual patients with CLBP.
Treatment options for patients with CLBP include conventional pharmacologic (eg, nonsteroidal anti-inflammatory drugs [NSAIDs]) and nonpharmacologic (eg, spinal manipulation) therapies.10 However, for patients who do not respond to these therapies, treatment options are limited. Duloxetine is a unique analgesic that exhibits its efficacy through inhibiting serotonin and norepinephrine reuptake, with affinity for both serotonin and norepinephrine transporters.11,12 Duloxetine has been demonstrated to be efficacious in the treatment of chronic pain disorders,13 including CLBP, as shown in Phase III, double-blind, randomized, placebo-controlled trials.14–17
A better understanding of the response profile of duloxetine 60 mg (the approved dose for CLBP in most countries) would help physicians when making treatment decisions for patients with CLBP. At present, however, there are no confirmed predictors of response to duloxetine treatment for CLBP. Patients with CLBP report lower pain thresholds, greater pain responses, larger receptive fields, and longer pain duration in response to experimental stimuli, consistent with central sensitization.18–23 As a clinical consequence of central sensitization, patients with CLBP may also have chronic pain in body sites other than the low back.24 Given that duloxetine primarily acts on chronic pain, patients with a greater central sensitization component to their CLBP may respond better to duloxetine than patients with less central sensitization. Therefore, we hypothesized that patients with multiple painful sites would be more responsive to duloxetine than patients with isolated CLBP.
To examine if demographic and clinical factors, including early improvement with treatment, are associated with response to duloxetine, we conducted a post hoc analysis of pooled patient-level data from 4 double-blind, randomized, placebo-controlled trials of duloxetine 60 mg for CLBP.14–17 This analysis included an assessment of the relationship between the number of painful body sites (assessed using the Michigan Body Map25 [MBM]) and the response to duloxetine, assessed in the Japanese trial.14 The MBM is a graphic tool that allows patients to self-report where they experience pain in up to 35 distinct body sites, and its validity and reliability have been demonstrated in a series of 5 studies involving 402 patients with widespread pain due to fibromyalgia.25
Patients and methods
Study design
This was a post hoc analysis of pooled patient-level data from 4 trials with similar study designs (multicenter, double-blind, randomized, placebo-controlled trials of duloxetine for the treatment of CLBP) (ClinicalTrials.gov registration NCT00408876, United States [US];16 NCT00767806, US and Europe;17 NCT00424593, Europe, Brazil, and Mexico;15 and NCT01855919, Japan14) conducted between November 2006 and July 2014. Details of the individual trials are described in the primary publications.14–17 All trial protocols were approved by the institutional review boards of all sites and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent before any study-related procedures.
Inclusion and exclusion criteria
Inclusion criteria for all trials consisted of adult patients with a clinical diagnosis of nonspecific CLBP (ie, no known cause), with pain present on most days for ≥6 months and a Brief Pain Inventory (BPI) Severity26 24-hour average pain score (“BPI average pain”) of ≥4 at baseline. Major exclusion criteria included evidence of radicular compression or other vertebrae-related disorder (eg, spinal stenosis, spondylolisthesis), spinal fracture, history of >1 low back surgery or surgery within the past 12 months, invasive treatment for CLBP within the past month, and major depressive disorder. Specific inclusion/exclusion criteria for each trial are described in the primary publications.14–17
Patients were included in this post hoc analysis if they were randomized to duloxetine 60 mg (or placebo, for some analyses), received ≥1 dose of study drug, and had BPI average pain data available at baseline and at ≥1 time point after baseline. Patients with BPI average pain data at 2 weeks and ≥1 time point after 2 weeks were included in an analysis of early improvement.
Treatment protocol
Patients included in this analysis were treated with once-daily oral duloxetine 60 mg (Cymbalta®; Eli Lilly and Company, Indianapolis, IN, USA) or placebo for 12–14 weeks.14–17 In 3 trials, patients initiated duloxetine at 20–30 mg/day, which increased to 60 mg/day over the first 1–2 weeks.14–16 In 2 trials, patients in additional study arms received 20 mg/day or 120 mg/day duloxetine;15,16 patients in these study arms were not included in this analysis. In 1 trial,15 patients who did not respond (≥30% pain reduction) to duloxetine 60 mg/day by Week 7 had their dose increased to 120 mg/day (n = 27); those patients were included in this analysis and treated as nonresponders for ≥30% and ≥50% pain reduction at end point (Week 12) regardless of their actual BPI scores at that time.
Concomitant analgesics (NSAIDs, acetaminophen, opioids) were prohibited in 2 of the trials, other than as episodic rescue medication (short-acting analgesics only, ≤3 consecutive days, ≤20 total days).14,17 The other 2 trials allowed patients who were on stable doses of NSAIDs before study entry (total of 82 patients in the placebo arm, 86 patients in the duloxetine 60 mg/day arm) to continue these medications, but no changes to dose or frequency were allowed.15,16 These trials also allowed rescue use of short-acting analgesics, as mentioned earlier. Antidepressants were prohibited in all trials.
Outcome measures
The primary objective of all trials was the efficacy of duloxetine in the reduction of pain severity. The 24-hour average pain rating using the BPI Severity scale (ie, “BPI average pain”) was the primary outcome measure in all but 1 trial, where it was a secondary outcome measure (primary measure was the weekly mean of a 24-hour average pain score using a Likert scale).16 The BPI Severity scale is a patient-reported outcome in which pain during the past 24 hours is recorded on a scale of 0 (no pain) to 10 (pain as bad as you can imagine).26 The BPI Severity scale has been validated in patients with noncancer pain, including CLBP.26 In each of the 4 trials, duloxetine 60 mg/day was associated with a statistically greater reduction in BPI Severity average pain score at the end of treatment compared with placebo, and the magnitude of pain reduction was consistent across the trials.14,17
This post hoc analysis assessed response based on the improvement in BPI average pain from baseline to the end of treatment (12–14 weeks). The primary outcome was the proportion of patients with ≥30% reduction in BPI average pain, and the secondary outcome was the proportion of patients with ≥50% reduction in BPI average pain. We selected these pain reduction thresholds based on previous studies that concluded that a ≥30% reduction in pain severity score constituted a clinically significant improvement and corresponded to a patient-described category of “much improved”,27 whereas a ≥50% reduction in pain severity score corresponded to “very much improved”27 or a “successful treatment outcome”.28 Similarly, in a pooled analysis of data from trials of duloxetine for fibromyalgia and diabetic peripheral neuropathic pain, reductions in pain severity of 34% and 51% were associated with Patient Global Impression of Improvement ratings of “much better” and “very much better”, respectively.29 Therefore, ≥30% and ≥50% pain reduction thresholds represent clinically relevant end points in terms of pain relief for the patient.
Responder analysis
The association of the following variables with efficacy (≥30% pain reduction and ≥50% pain reduction at the end of treatment) was assessed for patients receiving duloxetine 60 mg: early improvement, defined as ≥15% reduction in BPI average pain at Week 2 (yes vs no); sex (female vs male); age (<65 years vs ≥65 years); baseline BPI average pain score (≥6 vs <6); CLBP duration (<5 years vs ≥5 years); and number of painful body sites during the past 3 months according to the MBM (≥2 vs 1 [isolated CLBP]; Japanese trial only14). We defined early improvement as ≥15% pain reduction at Week 2 based on previous studies of duloxetine for fibromyalgia30 and gabapentin for postherpetic neuralgia,31 which suggested that failure to achieve 10–20% reduction in pain during the first 2 weeks of treatment is predictive of failure to achieve 30% pain reduction at Week 10 or 12.
Statistical analysis
Patient-level data for this analysis were extracted from an integrated database containing data from all 4 trials. Baseline demographic characteristics are described as mean (SD) and/or median (minimum, maximum) for continuous variables and n (%) for categorical variables. The percentage of patients in each group who achieved ≥30% reduction or ≥50% reduction in BPI average pain from baseline to the end of treatment was calculated (“response rate”), and the duloxetine 60 mg and placebo groups were compared using a Cochran–Mantel–Haenszel test stratified by each trial. For the responder analysis, relative risk (RR) and 95% confidence intervals (95% CIs) of the response rate were calculated for each variable. For the MBM analysis (Japanese trial only14), the proportion of patients achieving ≥30% pain reduction and ≥50% pain reduction was assessed for patients with 1, ≥2, 2, 3, 4, and ≥5 painful body sites, compared between duloxetine and placebo groups using a Cochran–Mantel–Haenszel test, and the RR (duloxetine vs placebo) and 95% CI calculated according to the number of painful body sites. For the responder analyses, missing end-of-treatment data were imputed using a last observation carried forward method. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Study population
A total of 1,295 patients (placebo, n = 653; duloxetine 60 mg, n = 642) were included in this analysis. The baseline demographics and clinical characteristics were similar between the pooled placebo and pooled duloxetine groups (Table 1). The baseline BPI average pain score (mean 5.6, median 5.0) indicated moderate pain levels at study enrollment. Patients showed minimal depressive symptoms, as demonstrated by low scores on the Beck Depression Inventory-II scale in the Japanese and US trials, consistent with exclusion of patients with major depressive disorder.
  | Table 1 Demographic and clinical characteristics of patients with CLBP enrolled in 4 randomized, placebo-controlled trials of duloxetine treatment14–17 Notes: aData from Japanese Phase III trial.14 bData from Japanese Phase III and US trials.14,16 Abbreviations: BDI-II, Beck Depression Inventory-II; BPI, Brief Pain Inventory; CLBP, chronic low back pain; max., maximum; MBM, Michigan Body Map; min., minimum. |
Patient disposition
Completion rates exceeded 80% in all trials and were similar in the placebo and duloxetine groups (Table 2). A greater percentage of patients in the placebo group discontinued because of lack of efficacy compared with the duloxetine group. Conversely, a greater percentage of patients in the duloxetine group discontinued because of an adverse event compared with the placebo group.
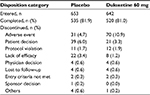  | Table 2 Disposition of patients with CLBP enrolled in 4 randomized, placebo-controlled trials of duloxetine treatment14-17 Abbreviation: CLBP, chronic low back pain. |
Overall response rate
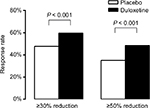
The percentage of patients with ≥30% pain reduction from baseline to the end of treatment was greater in patients treated with duloxetine 60 mg (59.7%) than in patients treated with placebo (47.8%; P < 0.001; Figure 1). Similar results were observed for ≥50% pain reduction (duloxetine 48.6% vs placebo 35.1%; P < 0.001; Figure 1).
Responder analysis by demographic and clinical factors
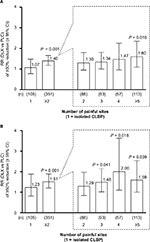
Among patients treated with duloxetine, female patients were slightly more likely to respond than male patients (RR [95% CI]: ≥30% pain reduction, 1.14 [1.00–1.30]; ≥50% pain reduction, 1.17 [0.99-1.38]; Figure 2). The response to duloxetine was similar regardless of other demographic and clinical factors (age, CLBP duration, baseline BPI average pain score; Figure 2).
  | Figure 2 (A, B) Responder analysis (A, ≥30% reduction in BPI average pain; B, ≥50% reduction in BPI average pain) for patients with CLBP treated with duloxetine 60 mg for 12–14 weeks. Notes: Response rates and RR (95% CI) are shown for the following factors: early improvement, defined as ≥15% reduction in BPI average pain at Week 2 (yes vs no); number of painful body sites according to the MBM (≥2 vs 1; Japanese trial only14); sex (F vs M); age (<65 y vs ≥65 y); CLBP duration (<5 years vs ≥5 years); and baseline BPI average pain score (≥6 vs <6). Abbreviations: BPI, Brief Pain Inventory; CI, confidence interval; CLBP, chronic low back pain; F, female; M, male; MBM, Michigan Body Map; RR, relative risk; y, years. |
Responder analysis by early improvement
Among patients treated with duloxetine, early improvement (≥15% pain reduction at Week 2) was associated with greater likelihood of achieving ≥30% (RR [95% CI], 2.91 [2.30–3.67]; Figure 2A) or ≥50% (RR [95% CI], 3.24 [2.44–4.31]; Figure 2B) pain reduction at the end of treatment.
Responder analysis by number of painful body sites
In the Japanese trial, most patients (77%) had pain in ≥2 body sites (mean 3.5 painful sites; Table 1). The most commonly reported pain sites, other than the low back, were shoulders (right, 28.7% of all patients; left, 24.6%), knees (right, 21.1%; left, 23.5%), and neck (23.7%).
Responder analysis of MBM data suggested that patients with ≥2 painful sites were more likely to respond to duloxetine than patients with only 1 painful site, ie, isolated CLBP (RR [95% CI]: ≥30% pain reduction, 1.22 [0.95–1.56]; ≥50% pain reduction, 1.20 [0.88–1.62]; Figure 2). In addition, the RR (duloxetine vs placebo) of achieving ≥30% pain reduction at the end of treatment was greater among patients with ≥2 painful sites than in those with only 1 painful site (P < 0.001) and increased with increasing number of painful sites (Figure 3A). Similar results were observed for patients achieving ≥50% pain reduction (Figure 3B).
Discussion
This is the first analysis to examine the effect of demographic and clinical factors, including early improvement and number of painful body sites, on the response to duloxetine 60 mg in patients with CLBP. The analysis included data from 4 randomized controlled trials of duloxetine 60 mg for the treatment of CLBP. The level of improvement with duloxetine within the first 2 weeks was indicative of the likelihood of achieving a clinically significant response at 12–14 weeks. Importantly, patients with ≥2 painful body sites were more likely to obtain greater benefit from duloxetine than patients with isolated CLBP, and the proportion of patients obtaining benefit increased with increasing number of painful body sites. The likelihood of response to duloxetine was not affected strongly by other baseline factors examined. Our results could assist clinicians in predicting and assessing the efficacy of duloxetine for patients with CLBP, depending on the number of painful body sites and early improvement.
In this post hoc responder analysis, early improvement with duloxetine 60 mg was associated with more frequently achieving a clinically significant reduction in pain at the end of the study period. This result is consistent with other responder analyses of duloxetine in chronic pain conditions, although these analyses focused on early nonresponders. In a post hoc analysis of duloxetine 60 mg or 120 mg for CLBP or osteoarthritis, only 35%–39% of the patients with <10% pain reduction at Week 2 achieved ≥30% pain reduction and only 17%–19% achieved ≥50% pain reduction at Week 12.32 These results are very similar to those in our analysis, in which 28% of early nonresponders achieved ≥30% pain reduction and 21% achieved ≥50% pain reduction. This similarity is not surprising given that Williamson et al32 included patients from 3 of the 4 trials that we included.15–17 However, we restricted our analysis to duloxetine 60 mg to focus on the recommended dose for CLBP. In another post hoc analysis of duloxetine 60 mg or 120 mg for fibromyalgia, 86% of patients with <15% pain reduction at Week 2 failed to achieve ≥30% pain reduction at Week 12.30 In our analysis, 72% of patients with <15% pain reduction at Week 2 failed to achieve ≥30% pain reduction at Weeks 12–14. Differences between these results may be related to the different diseases studied, the multiple doses of duloxetine included by Wang et al, or differences in patient population. Early improvement with treatment has also been shown to be indicative of overall response in studies of other drugs (opioids, gabapentin) in chronic pain conditions.31,33 In clinical practice, a lack of improvement during the first 2 weeks of pharmacological treatment may signal that the patient is unlikely to benefit from persisting with treatment and that a change in management strategy should be considered.
An important and novel finding is that the number of painful body sites may predict the likelihood of response to duloxetine. To our knowledge, this is the first study to compare treatment response in patients with multiple painful sites with the response in patients with isolated CLBP. We used the MBM, a recently validated graphic tool that allows patients to identify up to 35 different painful sites.25 The MBM has been used primarily in studies of fibromyalgia,25 and the Japanese trial included in this analysis was among the first to use the MBM in patients with CLBP.14 Approximately three-quarters (77%) of patients in the Japanese trial reported 2 or more painful body sites. Indeed, in an analysis of the US National Health and Aging Trends Survey, >85% of older adults who had back pain during the past month (not necessarily CLBP) reported pain in ≥1 other site and >50% had pain in ≥3 other sites.34 In our study, patients with ≥2 painful body sites were more likely to gain greater benefit from duloxetine 60 mg than patients with isolated CLBP. Importantly, the extent of benefit increased with increasing number of painful sites. A greater response among patients with multiple painful sites is consistent with duloxetine acting on chronic pain mechanisms to modulate descending pain pathways.13 The presence of multiple painful sites, including the low back, is thought to result from changes in the central nervous system, particularly reduced activity of descending inhibitory pathways, which amplify pain perception.8,24 Although limited, our analysis suggests that the number of painful body sites may be a predictor of response to duloxetine. If confirmed in subsequent studies, the MBM may be useful for predicting the likelihood that individual patients with CLBP will respond to duloxetine.
The strengths of our analysis include the use of pooled patient-level data from 4 randomized, placebo-controlled trials conducted in multiple countries with similar study designs and methods, the large sample size for most responder variables, and the novel use of the MBM. One limitation, however, is that data on the number of painful body sites were only available for Japanese patients from a single trial, resulting in a lower sample size for this variable, especially for patients with isolated CLBP (placebo, n = 54; duloxetine, n = 51), which limited the statistical power for this subgroup. Other potential limitations of the analysis include its post hoc nature, the limited number of possible predictors assessed, and the single dose of duloxetine analyzed. However, we focused on the 60 mg dose as this is the recommended dose for CLBP in most countries.35
Conclusion
In this post hoc responder analysis of 4 trials, the level of pain relief provided by duloxetine 60 mg in the first 2 weeks of treatment was indicative of whether a clinically significant response was achieved after 12–14 weeks of treatment. Further, results from 1 trial suggested that patients with multiple sites of pain may be more likely to derive greater benefit from duloxetine than patients with isolated CLBP. Aside from these differences, duloxetine 60 mg was efficacious in the treatment of patients with CLBP, regardless of age, sex, pain severity, or duration of CLBP.
Acknowledgments
The authors would like to thank all study participants and Dr Chad Brummet (University of Michigan), who provided the Michigan Body Map. Medical writing assistance was provided by Rebecca Lew, PhD, CMPP, and Mark Snape, MB BS, CMPP, of ProScribe – Envision Pharma Group, and was funded by Eli Lilly Japan K.K. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3). This study was sponsored by Eli Lilly Japan K.K., manufacturer and licensee of Cymbalta® in Japan. The study NCT01855919 was conducted by Shionogi & Co. Ltd. and funded by Eli Lilly Japan K.K. and Shionogi & Co. Ltd.
Disclosure
SK has received consulting fees and honoraria from Eli Lilly Japan K.K. and Shionogi & Co. Ltd and has received research funding from Shionogi & Co. Ltd. LA, HE, SF, KO, and AY are employees of Eli Lilly Japan K.K. MI and TT are employees and minor stock holders of Shionogi & Co. Ltd. LA and SF hold shares in Eli Lilly and Company. The authors report no other conflicts of interest in this work.
References
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. | ||
Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. | ||
Nakamura M, Nishiwaki Y, Ushida T, Toyama Y. Prevalence and characteristics of chronic musculoskeletal pain in Japan. J Orthop Sci. 2011;16(4):424–432. | ||
Suka M, Yoshida K. Low back pain deprives the Japanese adult population of their quality of life: a questionnaire survey at five healthcare facilities in Japan. Environ Health Prev Med. 2008;13(2):109–115. | ||
Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. | ||
Webster LR, Markman J. Medical management of chronic low back pain: efficacy and outcomes. Neuromodulation. 2014;17(suppl 2):18–23. | ||
Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29(1):6–19. | ||
Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states – maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25(2):141–154. | ||
Airaksinen O, Brox JI, Cedraschi C, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(suppl 2):S192–S300. | ||
Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–491. | ||
Bymaster FP, Beedle EE, Findlay J, et al. Duloxetine (Cymbalta), a dual inhibitor of serotonin and norepinephrine reuptake. Bioorg Med Chem Lett. 2003;13(24):4477–4480. | ||
Karpa KD, Cavanaugh JE, Lakoski JM. Duloxetine pharmacology: profile of a dual monoamine modulator. CNS Drug Rev. 2002;8(4):361–376. | ||
Smith HS, Smith EJ, Smith BR. Duloxetine in the management of chronic musculoskeletal pain. Ther Clin Risk Manag. 2012;8:267–277. | ||
Konno S, Oda N, Ochiai T, Alev L. A randomized, double-blind, placebo-controlled phase III trial of duloxetine monotherapy in Japanese patients with chronic low back pain. Spine (Phila Pa 1976). 2016;41(22):1709–1717. | ||
Skljarevski V, Desaiah D, Liu-Seifert H, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine (Phila Pa 1976). 2010;35(13):E578–E585. | ||
Skljarevski V, Ossanna M, Liu-Seifert H, et al. A double-blind, randomized trial of duloxetine versus placebo in the management of chronic low back pain. Eur J Neurol. 2009;16(9):1041–1048. | ||
Skljarevski V, Zhang S, Desaiah D, et al. Duloxetine versus placebo in patients with chronic low back pain: a 12-week, fixed-dose, randomized, double-blind trial. J Pain. 2010;11(12):1282–1290. | ||
Farasyn A, Meeusen R. The influence of non-specific low back pain on pressure pain thresholds and disability. Eur J Pain. 2005;9(4):375–381. | ||
Giesbrecht RJ, Battie MC. A comparison of pressure pain detection thresholds in people with chronic low back pain and volunteers without pain. Phys Ther. 2005;85(10):1085–1092. | ||
Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50(2):613–623. | ||
Imamura M, Chen J, Matsubayashi SR, et al. Changes in pressure pain threshold in patients with chronic nonspecific low back pain. Spine (Phila Pa 1976). 2013;38(24):2098–2107. | ||
Puta C, Schulz B, Schoeler S, et al. Enhanced sensitivity to punctate painful stimuli in female patients with chronic low back pain. BMC Neurol. 2012;12:98. | ||
Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep. 2011;13(6):513–520. | ||
Hartvigsen J, Natvig B, Ferreira M. Is it all about a pain in the back? Best Pract Res Clin Rheumatol. 2013;27(5):613–623. | ||
Brummett CM, Bakshi RR, Goesling J, et al. Preliminary validation of the Michigan Body Map (MBM). Pain. 2016;157(6):1205–1212. | ||
Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. | ||
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. | ||
Robinson ME, Brown JL, George SZ, et al. Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med. 2005;6(5):336–345. | ||
Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain. 2010;11(2):109–118. | ||
Wang F, Ruberg SJ, Gaynor PJ, Heinloth AN, Arnold LM. Early improvement in pain predicts pain response at endpoint in patients with fibromyalgia. J Pain. 2011;12(10):1088–1094. | ||
Jensen MP, Hsu PH, Vanhove GF. Early pain reduction can predict treatment response: results of integrated efficacy analyses of a once-daily gastroretentive formulation of gabapentin in patients with postherpetic neuralgia. Pain Med. 2012;13(8):1059–1066. | ||
Williamson OD, Schroer M, Ruff DD, et al. Onset of response with duloxetine treatment in patients with osteoarthritis knee pain and chronic low back pain: a post hoc analysis of placebo-controlled trials. Clin Ther. 2014;36(4):544–551. | ||
Kalso E, Simpson KH, Slappendel R, Dejonckheere J, Richarz U. Predicting long-term response to strong opioids in patients with low back pain: findings from a randomized, controlled trial of transdermal fentanyl and morphine. BMC Med. 2007;5:39. | ||
Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154(12):2649–2657. | ||
Cymbalta® (duloxetine hydrochloride) [prescribing information]. Indianapolis, IN, USA: Eli Lilly and Company; 2016. Available from: http://pi.lilly.com/us/cymbalta-pi.pdf. Accessed February 2, 2017. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.