Back to Journals » Drug Design, Development and Therapy » Volume 14
DPP-4 Inhibitor Linagliptin Ameliorates Oxidized LDL-Induced THP-1 Macrophage Foam Cell Formation and Inflammation
Authors Wang H, Li Y, Zhang X, Xu Z, Zhou J, Shang W
Received 21 March 2020
Accepted for publication 12 August 2020
Published 25 September 2020 Volume 2020:14 Pages 3929—3940
DOI https://doi.org/10.2147/DDDT.S249846
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Manfred Ogris
Haoran Wang,1 Yue Li,2 Xiaoliang Zhang,2 Zhonglin Xu,2 Jianzhong Zhou,3 Wei Shang2
1Department of Endocrinology, The Ninth People’s Hospital of Chongqing, Chongqing 400700, People’s Republic of China; 2Department of Cardiology, The Ninth People’s Hospital of Chongqing, Chongqing 400700, People’s Republic of China; 3Department of Cardiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400700, People’s Republic of China
Correspondence: Wei Shang
Department of Cardiology, The Ninth People’s Hospital of Chongqing, No. 69, Jia Ling Road, Bei Bei District, Chongqing 400700, People’s Republic of China
Tel +86-15823409229
Email [email protected]
Introduction: Atherosclerosis is one of the major causes of cardiovascular diseases. Lipid uptake and accumulation in macrophages play a major role in atherosclerotic plaque formation from its initiation to advanced atheroma formation. The dipeptidyl peptidase-4 (DPP-4) inhibitor Linagliptin is commonly used to lower blood glucose in type 2 diabetes patients. Recent studies report that Linagliptin has cardiovascular protective and anti-inflammatory effects.
Methods: THP-1 macrophage cells were treated with 100 nM PMA for 72 hour to induce foam cell formation. The differentiated cells were exposed to 100 μg/mL ox-LDL in the presence or absence of the DPP-4 inhibitor Linagliptin. The expression levels of DPP-4 and inflammatory cytokines were detected by RT-PCR, ELISA, and Western blot experiments. The cellular ROS level was measured by staining the cells with the fluorescent probe DCFH-DA. The separation of lipoprotein fractions was achieved by high-performance liquid chromatography (HPLC). The cells were labeled with fluorescent-labeled cholesterol to measure cholesterol efflux, and lipid droplets were revealed by Nile red staining.
Results: The presence of Linagliptin significantly reduced ox-LDL-induced cytokine production (IL-1β and IL-6) and ROS production. Linagliptin ameliorated ox-LDL-induced lipid accumulation and impaired cholesterol efflux in macrophages. Mechanistically, this study showed that Linagliptin mitigated ox-LDL-induced expression of the scavenger receptors CD36 and LOX-1, but not SRA. Furthermore, Linagliptin increased the expression of the cholesterol transporter ABCG1, but not ABCA1.
Conclusion: Linagliptin possesses a potent inhibitory effect on THP-1 macrophage-derived foam cell formation in response to ox-LDL. This effect could be mediated through a decrease in the expression of CD36 and LOX-1 on macrophages and an increase in the expression of the cholesterol transporter ABCG1. This study indicates that the DPP-4 inhibitor Linagliptin plays a critical role in preventing foam cell formation in vitro. However, future research using an atherosclerotic animal model is necessary to determine its effectiveness and to prove its potential implication in the prevention and treatment of atherosclerosis.
Keywords: atherosclerosis, foam cells, Linagliptin, CD36, LOX-1, ABCG-1, ATP binding cassette transporter G1
Introduction
Atherosclerosis has been recognized as one of the most dangerous threats to human health. The pathological dissection of atherosclerotic plaque features the deposition of cholesterol-rich lipids on the arterial wall. From the initial deposition of fatty streaks on the arterial intima to late-stage fibrous atheroma formation, macrophage lipid uptake and accumulation is the central mechanism of atherosclerosis development.1,2 In the early stages of atherosclerosis, circulating low-density lipoprotein (LDL) is retained on susceptible areas of the arterial wall where it undergoes oxidation to become oxidized LDL (ox-LDL). At this point, ox-LDL activates local endothelial cells to express adhesion molecules and release chemotactic chemokines, which attract circulating monocytes to adhere to the intimal wall and subsequently transpose into the subendothelial layers. The infiltrated monocytes further differentiate into macrophages, which can internalize ox-LDL through several major scavenger receptors including cluster of differentiation 36 (CD36), class A macrophage scavenger receptor (SRA) and ox-LDL receptor 1 (LOX-1).3 The deposited cholesterol can be removed from macrophages using reverse transport, termed as cholesterol efflux, which occur through passive diffusion or active transport.
Active transport involves several well described transporters including ATP binding cassette subfamily A member 1 (ABCA1), ABCG1 and HDL receptor scavenger receptor class B type I (SR-BI).4 When uptake and cellular esterification of cholesterol increases and export decreases (including cholesterol efflux), lipid-laden macrophages transform into foam cells and accumulate to form fatty streaks. The accumulation of ox-LDL-derived cholesterol often induces foam cell apoptosis, thereby leading to the formation of a necrotic, cholesterol-rich core. When the fibrous cap of an atheroma is washed away, the plaque becomes locally vulnerable.5 The rupture of vulnerable plaque causes occlusion or ischemic stenosis in the coronary or cerebrovascular arteries, which is commonly the major cause of sudden death.6
Gliptins are a class of anti-diabetic drugs that inhibit the activity of dipeptidyl peptidase-4 (DPP-4), thereby lowering blood glucose. Several different gliptins including Linagliptin are commonly used in clinics. Preclinical research has shown that 12 weeks of DPP-4 inhibition suppresses vascular inflammation in atherosclerotic experimental animals.7 Meanwhile, a randomized controlled clinical trial concluded that DPP-4 inhibition can suppress the initiation of atherosclerosis in type 2 diabetes patients.8 Overall, DPP-4 inhibition has demonstrated various benefits that may contribute to hindering the development or progression of atherosclerosis, such as reducing plasma lipid levels, suppressing inflammation, and promoting vascular relaxation.9 Linagliptin is one of the newest DPP-4 inhibitors on the market and is unique among gliptins in that it provides both cardiovascular protection and renal safety.10–12 Furthermore, several studies show that Linagliptin possesses an anti-inflammatory effect. One study shows that Linagliptin reduces inflammation and promotes wound healing in diabetic ob/ob mouse models.13 A most recent mouse model study has shown that Linagliptin exhibits a protective effect in high fat diet-induced atherosclerosis and promotes macrophages toward M2 polarization.14 In in vitro experiments, Linagliptin exhibits an anti-inflammatory effect by inhibiting monocyte adhesion to vascular endothelial cells.15 However, the underlying mechanism remains elusive. These facts indicate that Linagliptin could have a role in macrophage-mediated inflammation and cardiovascular outcome. In this study, the authors sought to investigate the molecular mechanism of Linagliptin in ox-LDL-elicited macrophages.
Materials and Methods
Cell Culture, Foam Cell Formation, and Treatment
Human monocytic cell line THP-1 was purchased from the American Type Culture Collection (ATCC, Rockville, USA). The cell lines were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum (FBS) and 1% compound of antibiotic regime including penicillin (100 U/mL) and streptomycin (100 mg/mL). All cultures were housed in a humidified 5% CO2 incubator at 37°C. To differentiate the macrophage cells, THP-1 cells were induced with 100 nM phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, St. Louis, USA) for 72 h to induce LDL uptake and foam cell formation. Ox-LDL was purchased from Thermo Scientific Inc. (Waltham, MA). THP-1 cells were left untreated or were treated for 18 h with 100 μg/mL ox-LDL15 to generate foam cells (FC), followed by stimulation with Linagliptin (50 nM and 100 nM)16 for 24 h.
Real-Time PCR Analysis
To isolate RNA from differentiated THP-1 macrophages or foam cells, a commercial High Pure RNA Isolation Kit (Roche, Basel, Switzerland) was used and the extraction procedures were performed in accordance with the manufacturer’s manual. Then, 1–2 µg total RNA was used for reverse transcription (RT) reaction with a iTaq Universal One-Step RT-qPCR Kit (Bio-Rad, Hercules, USA). The resulting cDNA mixture was diluted and used to detect the expression of target genes on a real-time LightCycler-96 instrument (Roche, Basel, Switzerland, USA).
Western Blot Analysis
Differentiated THP-1 cells were lysed in RIPA buffer supplemented with a protease inhibitor tablet (Roche, Basel, Switzerland). Subsequent to centrifugation, the supernatants were collected, and the protein concentration was determined using a BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, USA). For Western blot analysis, a total of 20 μg protein samples were loaded into 10% SDS-PAGE gel and then transferred onto a PVDF membrane. The blots were blocked for 2 h at room temperature with 5% BSA-PBST buffer and incubated with primary antibodies overnight at 4 °C, followed by 1 h binding at room temperature with HRP-conjugated secondary antibodies. A Pierce ECL Western Blotting Substrate Kit (Thermo Fisher Scientific, Waltham, USA) was reacted to captured the protein signals. The optical density of the target protein was processed with an iBright Western Blot Imaging System (Thermo Fisher Scientific, Waltham, USA). The expression levels of the target proteins were normalized to β-actin. The following antibodies were used in this study: DPP-4 (1:1000, ABCAM, Cambridge, UK); LOX-1 (1:2000, R & D Systems, Minneapolis, USA); CD36 (R & D Systems, Minneapolis, USA); SRA (1:3000, Santa Cruz, Dallas, USA); ABCA1 (1:1000, ABCAM, Cambridge, UK), ABCG1 (1:1000, ABCAM, Cambridge, UK) and β-actin (1:5000, Santa Cruz, Dallas, USA); rabbit IgG HRP conjugated Antibody (1:2000, Thermo Fisher Scientific, Waltham, USA); mouse IgG HRP conjugated Antibody (1:2000, Thermo Fisher Scientific, Waltham, USA)
DCFH-DA Staining
To measure intracellular ROS production, differentiated THP-1 cells on a 96-well plate were reacted with the 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (DCFH-DA) probe for 15 min. Afterwards, the cells were washed with PBS, and the fluorescence intensity of the stained cells was measured at 485 nm (excitation) and 527 nm (emission). The data are presented as fold-changes with non-treated cells as baseline.
ELISA
To measure cellular levels of secreted IL-1β and IL-6, the culture media was collected and cleared to keep the supernatants. The cytokine levels were detected using two ELISA kits (R & D Systems, Minneapolis, USA) in accordance with the manufacturer’s instructions. Final concentrations were calculated from the absorbance values of each of the samples based on the plot from the standard curve.
HPLC
The high-performance liquid chromatography (HPLC) method was used to determine cellular total cholesterol (TC), free cholesterol (FC) and cholesterol esters (CE). In brief, the lipoprotein fractions were extracted using a lipid extraction kit (ABCAM, Cambridge, UK). Chromatographic separation was achieved with a column packed with Hitachi Gel 3057 (Hitachi, Tokyo, Japan). The mobile phase was acetonitrile-2-propanol (75:25, v/v). The concentration of lipid fractions was determined with a single chromatographic run with the extracted lipoproteins.
Cholesterol Efflux Measurement
The cholesterol efflux in differentiated THP-1 cells was measured using a commercial Cholesterol Efflux Assay Kit (Sigma-Aldridge, St. Louis, USA) by following the manufacturer’s protocol. In brief, THP-1 cells in a 96-well culture plate were washed with serum-free medium and reacted with labeling reagent for 16 h. Subsequent to incubation, the culture media were collected, and cell monolayers were lysed. The fluorescent intensity of the medium and cell lysates was read at 482 nm (excitation) and 515 nm (emission). The cholesterol efflux (%) was calculated based on the fluorescent intensity ratio of the media to the lysate.
Nile Red Staining
To analyze the lipid droplet content in differentiated THP-1 cells, the cells were stained with Nile red. Briefly, THP-1 cells were treated with ox-LDL for 18 h and then washed and stained with 1 µg/mL Nile red (Sigma, St. Louis, USA) in the dark for 10 min. An average of 10 random fields from each group were imaged by confocal laser scanning microscopy (Olympus, Tokyo, Japan). The Nile red and DAPI-labelled cells were counted and analyzed with Image J software (NIH).
Oil Red O Staining
After the indicated treatment, cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min. Cells were then stained with a saturated concentration of oil red O in 60% isopropanol for 1 h at 55 °C. After washing with PBS, slides were mounted with 4ʹ,6-diamidino-2-phenylindole (DAPI) and photographed.
Statistical Analysis
Experiments were repeated 3 times. The collected data are presented as means ± SD. ANOVA analysis was used to determine the differences in means between groups, with a p-value less than 0.05 representing statistical significance.
Results
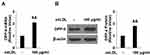
Ox-LDL Induces DPP-4 Expression in Macrophage-Derived Foam Cells
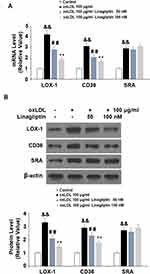
To differentiate monocytic THP-1 cells, the cells were induced with PMA for three days. The differentiated cells were then treated with ox-LDL to induce foam cell formation. Compared to untreated cells, exposure to ox-LDL induced about 2.1-fold DDP-4 mRNA expression (Figure 1A) and 1.8-fold protein expression (Figure 1B).
Molecular Structure of Linagliptin
To elucidate the pharmacological effect of DPP-4 inactivation in macrophage form cells, the DPP-4 inhibitor Linagliptin was used. Linagliptin is a xanthine-based compound with the chemical formula C25H28N8O2, commonly described as 7H-xanthine bearing (4-methylquinazolin-2-yl) methyl, methyl, but-2-yn-1-yl and 3-aminopiperidin-1-yl substituents at positions 1, 3, 7 and 8, respectively (Figure 2).
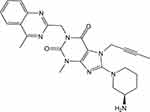 |
Figure 2 The molecular structure of Linagliptin. |
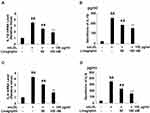
Linagliptin Alleviates ox-LDL-Induced Production of IL-1β and IL-6
Subsequently, we treated the differentiated THP-1 cells with ox-LDL in the presence or absence of Linagliptin and surveyed the production of the cytokines IL-1β and IL-6. Compared to the non-treated cells, ox-LDL alone induced about 3.5-fold mRNA of IL-1β, however it only induced about 2.5- and 1.9-fold IL-1β mRNA when 50 nM and 100 nM Linagliptin were present, respectively (Figure 3A). At the protein level, ox-LDL alone induced more than 10.6-fold IL-1β secretion, yet it only induced 7.8- and 6.4-fold IL-1β production in the presence of the two respective doses of Linagliptin (Figure 3B). Linagliptin exhibited a similar effect on IL-6 expression. At the mRNA level, ox-LDL alone induced about 4.3-fold mRNA of IL-6, though it only induced about 3.1- and 1.8-fold IL-6 in the presence of the two doses of Linagliptin, respectively (Figure 3C). As for protein expression, ox-LDL alone induced about 9.6-fold production of IL-6, while it only induced approximately 6.4- and 4.3-fold IL-6 secretion in the presence of the two doses of Linagliptin (Figure 3D).
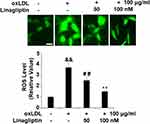
Linagliptin Mitigates ox-LDL-Induced Oxidative Stress
To measure the cellular ROS level, we stained the cells with the probe DCFH-DA. Quantitative analysis showed that ox-LDL alone increased cellular ROS to 3.7-fold, however, the presence of the two doses of Linagliptin mitigated cellular ROS production, which resulted in ox-LDL inducing only about 2.5- and 1.5-fold ROS, respectively (Figure 4).
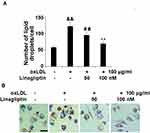
Linagliptin Mitigates ox-LDL-Increased Cholesterol Accumulation
Based on the protective effect of Linagliptin observed in the above experiments, we examined its effect on ox-LDL-induced lipid droplet accumulation in foam cells. We found that treatment with Linagliptin reduced ox-LDL-induced lipid droplet accumulation (Figure 5A). Oil red O staining of foam cells is shown in Figure 5B. Through HPLC analysis, we found that ox-LDL alone induced about 3.4-fold total cholesterol (TC), 1.9-fold free cholesterol (FC), and almost 6-fold cholesterol ester (CE). However, Linagliptin was capable of mitigating the cellular accumulation of lipid droplets in a dose-dependent manner, with the high dose of Linagliptin reducing TC content to 1.3-fold, FC content to 1.2-fold, and CE content to 1.6-fold (Table 1). When the CE/TC ratio was calculated, non-treated cells were at 37.08%, and exposure to ox-LDL increased this ratio to 63.57%. However, the two doses Linagliptin reduced the ratio to 49.65% and 42.13%, respectively (Table 1).
 |
Table 1 Effects of Linagliptin on Free Cholesterol and Cholesterol Esters in THP-1 Macrophage-Derived Foam Cells (Measured by HPLC) |
Linagliptin Ameliorates ox-LDL-Induced Expression of LOX-1 and CD36
To explore the mechanism whereby Linagliptin prevented the formation of foam cells, we assessed the expression of ox-LDL scavenger receptors on the cell surface. Treatment with ox-LDL induced 4.2-, 3.1- and 2.9-fold LOX-1, CD36, and SRA mRNA expression, respectively. However, Linagliptin showed an ameliorative effect on the expression of LOX-1 and CD36, but not SRA. Linagliptin reduced the mRNA of LOX-1 and CD36 in a dose-dependent manner, with the high dose reducing these expression levels to 1.9- and 1.7-fold, respectively (Figure 6A). In regard to the protein level, ox-LDL alone increased the expression of LOX-1, CD36, and SRA expression to 3.3-, 2.9-, and 2.7-fold, respectively. However, Linagliptin evinced lesser amelioration of LOX-1 and CD36 expression, but not that of SRA, with the high dose of Linagliptin reducing the expression of LOX-1 and CD36 to 1.5- and 1.8-fold, respectively (Figure 6B).
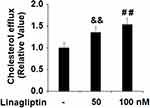
Linagliptin Promotes Cholesterol Efflux in Foam Cells
In this study, we also determined cholesterol efflux in the presence or absence of Linagliptin in our treatment experiment. Compared to its absence and under the same ox-LDL treatment conditions, 50 nM and 100 nM Linagliptin increased cholesterol efflux to 1.35- and 1.53-fold, respectively (Figure 7).
Linagliptin Increases Cholesterol Transporter ABCG1 Expression
Lastly, we profiled the mRNA expression levels of the cholesterol transporters ABCG1 and ABCA1. Linagliptin treatment dose-responsively induced ABCG1 but not ABCA1 expression as compared with its absence. The two doses of Linagliptin increased the level of ABCG1 to 1.7- and 2.2-fold, respectively (Figure 8A). In regard to protein expression, Linagliptin exhibited similar induction of ABCG1 but failed to show any impact on ABCA1, with the two doses of Linagliptin increasing ABCG1 expression to1.6- and 2.1-fold (Figure 8B).
Discussion
Uptake of ox-LDL by macrophages plays a key role in foam cell formation and the pathogenesis of atherosclerosis. The accumulation of macrophage-derived foam cells on the arterial intima is a hallmark of the early stages of atherosclerotic lesion formation.2 Foam cells release various pro-inflammatory mediators and promote lesion growth. In the advanced stage of atherosclerosis, dead macrophage foam cells often crystallize to form an unstable atheroma.1 In atherosclerotic conditions, the formation of the foam cells primarily results from the excess uptake of ox-LDL or impaired cholesterol efflux in macrophages.17 The targeted inhibition of foam cell formation has been considered as a promising approach to prevent and treat atherosclerosis.18 Additionally, diabetes-associated cardiovascular complications often involve atherosclerotic formation.19
Recent progress has shown that DPP-4 inhibitors have anti-atherogenic effects independent of their glucose-lowering capacity.20,21 Several different types of DPP-4 inhibitors have shown ameliorative effects on residential macrophages and foam cell formation in preclinical and clinical studies. Linagliptin therapy reduces M1-polarized macrophage migration but induces M2-dominant adipose tissue macrophages.22 Both Linagliptin and Sitagliptin have been found to suppress the expression of pro-inflammatory proteins and promote the expression of anti-inflammatory markers in liver-resident macrophages.23 The most recent study indicates that Linagliptin increases M2 macrophage polarization by inhibiting DPP-4 expression and activity.14 Similarly, Alogliptin inhibits visceral adipose tissue macrophage content in atherosclerotic mice.7 Vildagliptin suppresses macrophage foam cell formation in both non-diabetic and diabetic mice.24 Sitagliptin suppresses foam cell formation by inhibiting the expression of CD36 and LOX-1.25 The suppression of Teneligliptin on macrophage foam cell formation was reported in both diabetic rodents and human subjects.26 These facts indicate that DPP-4 inhibition may have a ubiquitous suppressive effect on macrophage foam cell formation. A meta-analysis suggested a possible beneficial effect of DPP-4 inhibitors on cholesterol, which could contribute to a reduction in cardiovascular risk.27 These data suggest that DPP-4 inhibitors might potentially reduce atherogenesis, primarily by inhibiting the inflammatory response and cholesterol homeostasis. However, the molecular mechanism driving this modulation of macrophage and foam cell formation remains unclear.
This study used sequentially differentiated and induced THP-1 macrophage-derived foam cells and investigated the effect of Linagliptin in foam cells. PMA differentiated THP-1 macrophages have very similar morphology, surface markers, and cytokine production profiles.28,29 Consequent to ox-LDL treatment, differentiated THP-1 macrophages are also used to study foam cell formation.30,31 This study showed that PMA differentiated macrophage THP-1 cells express DPP-4, which is induced in response to ox-LDL stimulation, indicating that DPP-4 inhibition could have a regulatory effect on macrophage function. When Linagliptin was used to inhibit macrophage DPP-4, it had a significant effect on ox-LDL-induced cellular inflammation and cholesterol metabolism, both of which are important mechanisms of atherosclerosis. The particular effect on inflammation shows that Linagliptin treatment reduced ox-LDL-induced IL-1β and IL-6 expression as well as cellular ROS production. The regulatory effect on cholesterol observed in this study indicates that Linagliptin improved ox-LDL-induced cholesterol accumulation in macrophages.
Mechanistically, this study established that Linagliptin suppressed cholesterol influx by suppressing scavenge receptor CD36 and LOX-1 expression but improved cholesterol efflux by upregulating ABCG1. Notably, our data indicate that ox-LDL induced all three major scavenger receptors, but it appears that Linagliptin only reduced CD36 and LOX-1 expression, but not that of SRA. Similarly, Linagliptin only increased ABCG1 but not AGCA1, suggesting that the effect of Linagliptin on these cholesterol modulators is selective. Macrophage CD36 binds to ox-LDL and facilitates cholesterol uptake, so its expression is critical to macrophage trapping and foam cell formation.32 LOX-1 was originally identified as the receptor for ox-LDL on endothelial cells but also has low basal expression on macrophages, which could be induced by ox-LDL.33
ABCG1 and ABCA1 are two major players involved in exporting cholesterol from macrophages. AGCA1 and ABCG1 synergistically remove cholesterol and reduce lipid accumulation.34 Macrophage-specific ABCG1 and AGCA1 conditional double knockout mice show resistance to atherosclerosis. However, overexpression of ABCG1 alone is able to promote lesion development,35 suggesting that macrophage ABCG1 could have a dominant role in cellular cholesterol accumulation and lesion development. Both CD36 as well as ABCG1 have been suggested as novel therapeutic targets for atherosclerosis.3
Based on these evidences, we conclude that the protective effect of Linagliptin on foam cell formation is closely associated with its selective suppression of the expression of the scavenger receptors CD36, LOX-1 and selective promotion of the cholesterol transporter AGCG1. The reduction in cholesterol content and increase in cholesterol efflux induced by Linagliptin indicates that macrophage DPP-4 inhibition could prevent ox-LDL uptake in macrophages. Furthermore, Linagliptin exhibits an anti-inflammatory effect by reducing the release of key cytokines and cellular ROS production. A graphical representation of the underlying molecular mechanism is shown in Figure 9. Finally, the limitations of this study have to be mentioned. We demonstrated that Linagliptin treatment mitigated lipid accumulation by preventing ox-LDL from binding to its receptors CD36 and LOX-1 and promoted cholesterol efflux by increasing the expression of the transporter ABCG1. However the connection between DPP-4 inhibition and its pharmacological effect is still puzzling. Currently, we do not understand how the inhibition of DPP-4 in macrophages reduces the activity of these receptors and transporters. As mentioned before, Linagliptin shows the capacity to modulate macrophages toward M2 polarization,14,22 but its action in the process of foam cell formation could be different. One feasible hypothesis is that DPP-4 agonists can be considered as a class of competitive inhibitors to determine whether the effect of Linagliptin is solely dependent on DPP-4 activity in foam cells. Another limitation of the current study is that all of the experiments performed in this study were conducted using cultured THP-1 cells. THP-1 cells have been used as a convenient source for studying macrophage and foam cell formation in vitro, but we have to address the important differences between the immortalized cells and their physiological counterparts. Future in vivo investigation would be critical to confirm the potential effect of Linagliptin on macrophage foam cell formation.
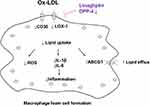 |
Figure 9 Graphical representation of the underlying molecular mechanism. |
In conclusion, our study suggests that DPP4 inhibition in macrophages may have an impact on macrophage foam cell formation and lipid metabolism, which highlights another layer of the pleiotropic effects of the type 2 diabetes agent Linagliptin in myeloid cells.
Highlights
- Macrophage cholesterol homeostasis plays a key role in foam cell formation;
- Linagliptin ameliorates ox-LDL-induced impaired lipid accumulation and cholesterol efflux in macrophage foam cells;
- Linagliptin suppresses ox-LDL-induced production of IL-1β, IL-6, and cellular ROS;
- Linagliptin decreases ox-LDL receptor CD36 and LOX-1 expression but selectively increases the cholesterol transporter AGCG1.
Ethics Statement
Human monocytic cell line THP-1, was purchased from the American Type Culture Collection (ATCC, Rockville, USA). Experiments were approved by the ethics committee of the Ninth People’s Hospital of Chongqing.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Linton MRF, Yancey PG, Davies SS, et al. The Role of Lipids and Lipoproteins in Atherosclerosis. Dartmouth (MA): MDText.com, Inc.; 2000.
2. Kavurma MM, Rayner KJ, Karunakaran D. The walking dead: macrophage inflammation and death in atherosclerosis. Curr Opin Lipidol. 2017;28(2):91–98. doi:10.1097/MOL.0000000000000394
3. Shen WJ, Azhar S, Kraemer FB. SR-B1: a unique multifunctional receptor for cholesterol influx and efflux. Annu Rev Physiol. 2018;80:95–116. doi:10.1146/annurev-physiol-021317-121550
4. Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20(1):17–28. doi:10.1111/jcmm.12689
5. Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Mechanisms of foam cell formation in atherosclerosis. J Mol Med (Berl). 2017;95(11):1153–1165. doi:10.1007/s00109-017-1575-8
6. Insull W
7. Shah Z, Kampfrath T, Deiuliis JA, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi:10.1161/CIRCULATIONAHA.111.041418
8. Mita T, Katakami N, Shiraiwa T, et al.; Collaborators on the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE) Trial. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE): a randomized controlled trial. Diabetes Care. 2016;39:455–464. doi:10.2337/dc15-2145
9. Duan L, Rao X, Xia C, Rajagopalan S, Zhong J. The regulatory role of DPP4 in atherosclerotic disease. Cardiovasc Diabetol. 2017;16(1):76. doi:10.1186/s12933-017-0558-y
10. Koibuchi N, Hasegawa Y, Katayama T, et al. DPP-4 inhibitor linagliptin ameliorates cardiovascular injury in salt-sensitive hypertensive rats independently of blood glucose and blood pressure. Cardiovasc Diabetol. 2014;13:157. doi:10.1186/s12933-014-0157-0
11. Aroor AR, Manrique-Acevedo C, DeMarco VG. The role of dipeptidylpeptidase-4 inhibitors in management of cardiovascular disease in diabetes; focus on linagliptin. Cardiovasc Diabetol. 2018;17(1):59.
12. Schürmann C, Linke A, Engelmann-Pilger K, et al. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J Pharmacol Exp Ther. 2012;342(1):71–80. doi:10.1124/jpet.111.191098
13. Nishida S, Matsumura T, Senokuchi T, et al. Inhibition of inflammation-mediated DPP-4 expression by linagliptin increases M2 macrophages in atherosclerotic lesions. Biochem Biophys Res Commun. 2020;524(1):8–15. doi:10.1016/j.bbrc.2020.01.027
14. Yamadera S, Nakamura Y, Inagaki M, et al. Linagliptininhibits lipopolysaccharide-induced inflammation in human U937 monocytes. Inflamm Regen. 2018;38:13. doi:10.1186/s41232-018-0071-z
15. Lara-Guzmán OJ, Gil-Izquierdo Á, Medina S, et al. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol. 2018;15:1–11. doi:10.1016/j.redox.2017.11.017
16. Zhang YJ, Huang XH, Yuan YH. Linagliptin protects human chondrogenic ATDC5 cells against advanced glycation end products (AGEs)-induced apoptosis via a mitochondria-dependent pathway. Chem Biol Interact. 2020;315:108901. doi:10.1016/j.cbi.2019.108901
17. Pennings M, Meurs I, Ye D, et al. Regulation of cholesterol homeostasis in macrophages and consequences for atherosclerotic lesion development. FEBS Lett. 2006;580(23):5588–5596. doi:10.1016/j.febslet.2006.08.022
18. Wang D, Yang Y, Lei Y, et al. Targeting foam cell formation in atherosclerosis: therapeutic potential of natural products. Pharmacol Rev. 2019;71(4):596–670.
19. Chait A, Bornfeldt KE. Diabetes and atherosclerosis: is there a role for hyperglycemia? J Lipid Res. 2009;50(Supplement):S335–S339. doi:10.1194/jlr.R800059-JLR200
20. Rizzo M, Rizvi AA, Spinas GA, Rini GB, Berneis K. Glucose lowering and anti-atherogenic effects of incretin-based therapies: GLP-1 analogues and DPP-4-inhibitors. Expert Opin Investig Drugs. 2009;18(10):1495–1503. doi:10.1517/14728220903241633
21. Singh TP, Vangaveti VN, Malabu UH. Dipeptidyl peptidase-4 inhibitors and their potential role in the management of atherosclerosis–A review. Diabetes Metab Syndr. 2015;9(4):223–229. doi:10.1016/j.dsx.2015.04.005
22. Zhuge F, Ni Y, Nagashimada M, et al. DPP-4 inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes. 2016;65(10):2966–2979. doi:10.2337/db16-0317
23. Wang X, Hausding M, Weng SY, et al. Gliptins suppress inflammatory macrophage activation to mitigate inflammation, fibrosis, oxidative stress, and vascular dysfunction in models of nonalcoholic steatohepatitis and liver fibrosis. Antioxid Redox Signal. 2018;28(2):87–109. doi:10.1089/ars.2016.6953
24. Terasaki M, Nagashima M, Nohtomi K, et al. Preventive effect of dipeptidyl peptidase-4 inhibitor on atherosclerosis is mainly attributable to incretin’s actions in nondiabetic and diabetic apolipoprotein E-null mice. PLoS One. 2013;8(8):e70933. doi:10.1371/journal.pone.0070933
25. Dai Y, Wang X, Ding Z, Dai D, Mehta JL. DPP-4 inhibitors repress foam cell formation by inhibiting scavenger receptors through protein kinase C pathway. Acta Diabetol. 2014;51(3):471–478. doi:10.1007/s00592-013-0541-3
26. Terasaki M, Hiromura M, Mori Y, et al. A dipeptidyl peptidase-4 inhibitor suppresses macrophage foam cell formation in diabetic db/db mice and type 2 diabetes patients. Int J Endocrinol. 2018;2018:8458304. doi:10.1155/2018/8458304
27. Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther. 2012;29(1):14–25. doi:10.1007/s12325-011-0088-z
28. Daigneault M, Preston JA, Marriott HM, Whyte MKB, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5:e8668–e8668. doi:10.1371/journal.pone.0008668
29. Kohro T, Tanaka T, Murakami T, et al. A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J Atheroscler Thromb. 2004;11:88–97. doi:10.5551/jat.11.88
30. Chiurchiù V, Lanuti M, Catanzaro G, Fezza F, Rapino C, Maccarrone M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis. 2014;233(1):55–63. doi:10.1016/j.atherosclerosis.2013.12.042
31. Chiurchiù V, Izzi V, D’Aquilio F, et al. Endomorphin-1 prevents lipid accumulation via CD36 down-regulation and modulates cytokines release from human lipid-laden macrophages. Peptides. 2011;32(1):80–85. doi:10.1016/j.peptides.2010.09.024
32. Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med. 2014;46:e99. doi:10.1038/emm.2014.38
33. Crucet M, Wu¨st SJ, Spielmann P, Lu¨scher TF, Wenger RH, Matter CM. Hypoxia enhances lipid uptake in macrophages: role of the scavenger receptors Lox1, SRA, and CD36. Atherosclerosis. 2013;229:110–117. doi:10.1016/j.atherosclerosis.2013.04.034
34. Gelissen IC, Harris M, Rye KA, et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi:10.1161/01.ATV.0000200082.58536.e1
35. Westerterp M, Murphy AJ, Wang M, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112(11):1456–1465. doi:10.1161/CIRCRESAHA.113.301086
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.