Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 12
Dose Regimen Rationale for Panitumumab in Cancer Patients: To Be Based on Body Weight or Not
Authors Liao MZ, Berkhout M, Prenen H, Dutta S, Upreti VV
Received 15 May 2020
Accepted for publication 7 July 2020
Published 31 July 2020 Volume 2020:12 Pages 109—114
DOI https://doi.org/10.2147/CPAA.S262949
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Michael Z Liao, 1 Marloes Berkhout, 2 Hans Prenen, 3 Sandeep Dutta, 4 Vijay V Upreti 1
1Clinical Pharmacology, Modeling & Simulation, Amgen Inc., South San Francisco, CA, USA; 2Medical Development, Amgen B.V, Breda, the Netherlands; 3Department of Medical Oncology, University Hospital Antwerp, Edegem, Belgium; 4Clinical Pharmacology, Modeling & Simulation, Amgen Inc., Thousand Oaks, CA, USA
Correspondence: Vijay V Upreti
Clinical Pharmacology, Modeling & Simulation, Amgen Inc., 1120 Veterans Boulevard, South San Francisco, CA 94080, USA
Fax +1 650-837-9421
Email [email protected]
Introduction: Body weight can affect exposure, safety and efficacy of antibody-based therapies; sometimes these effects may not be clinically relevant. Panitumumab is approved for wild-type RAS metastatic colorectal cancer, using a body weight–based dosing regimen. Recently, a report cited fixed-dose usage of panitumumab, rather than approved body weight–based dosing. The current work evaluates optimal dosing regimen scientifically based on clinical data, modeling and simulation. Herein, we assessed the effect of fixed and body weight–based dosing on panitumumab pharmacokinetics to determine which approach resulted in the least interpatient pharmacokinetic variability.
Patients and Methods: From the Vectibix program, 352 patients enrolled in three studies were evaluated; they had received panitumumab (body weight–based dose: 6 mg/kg every 2 weeks) and had pharmacokinetic (maximum serum [Cmax] and trough [Cmin] concentrations) and body weight data available. Additionally, concentration-time profiles at fixed (480 mg) and body weight–based doses (6 mg/kg) were simulated using a population pharmacokinetics model developed from 1200 patients.
Results: After administration of panitumumab 6 mg/kg, Cmax and Cmin increased with increasing body weight; the mean Cmax and Cmin for patients weighing < 65 kg (lower quartile) were 23% and 30% lower, respectively, than for those weighing > 88 kg (upper quartile). The simulated area under the concentration–time curve (AUC) data also indicated that overall panitumumab exposure increased with increasing body weight for the body weight–based regimen. When AUC was simulated for a fixed dose (480 mg), the opposite effect was observed. Over the range of body weights, interpatient variability in simulated AUC was lower for the weight-based dose (29%) than for the fixed dose (34%).
Conclusion: Results demonstrate that the weight-based dose (6 mg/kg) reduced variability in panitumumab exposure across the range of body weights compared with the fixed-dose approach, indicating that a body weight–based approach is the recommended patient dosing strategy.
Keywords: panitumumab, pharmacokinetics, dose-exposure relationship, body weight, area under the curve, colorectal neoplasms
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
Ideal dosing strategies provide minimal interpatient variability, optimizing therapeutic outcomes.1 Body weight can have an effect on exposure, safety, and efficacy for antibody-based therapies. Dosages are often prescribed based on body weight when these effects are clinically relevant.1 Although there is no consensus for whether an investigational biologic dose should be fixed or body weight–based for first-in-human trials, it is recommended by the US Food and Drug Administration (FDA) that a full assessment of pharmacokinetics and pharmacodynamics be undertaken to reduce interpatient variability and assess optimal dosing to improve efficacy and safety.1
Panitumumab (Vectibix®; Amgen Inc., Thousand Oaks, CA, USA; www.amgen.com), a human monoclonal antibody targeting the epidermal growth factor receptor (EGFR), has demonstrated efficacy in wild-type RAS metastatic colorectal cancer (mCRC).2,3 The approved body weight–based dosing regimen of 6 mg/kg every 2 weeks (Q2W) was supported by scientific clinical study.4,5 Panitumumab pharmacokinetics are characterized by a 2-compartment model with linear and nonlinear clearance6 and a volume of distribution (Vd) of approximately 40 mL/kg, consistent with saturable binding to EGFR.7 Additionally, panitumumab exposure increased proportionally between 2 and 6 mg/kg.7
For some biologics, such as panitumumab, body weight–based dosing is necessary to achieve efficacy and safety after thorough dose optimization;8,9 however, it has been suggested that a fixed-dose approach may be used, rather than the label of body weight–based dosing.8,9 Hendrikx et al recommended panitumumab doses of 300 and 500 mg Q2W for patients weighing 40–80 and 80–140 kg, respectively, instead of the approved body weight–based regimen.9
The objective of this analysis was to assess the effect of body weight on panitumumab pharmacokinetics in order to evaluate whether a fixed dose would reduce interpatient variability compared with the FDA-approved body weight–based dosing. Data from 352 patients receiving panitumumab at body weight–based dosing (6 mg/kg Q2W) with pharmacokinetic data (maximum observed serum concentration [Cmax], minimum [trough] observed concentration [Cmin]), and recorded body weight available from three clinical trials (NCT00089635, NCT00083616, NCT00113763) were evaluated. Additionally, using the published pharmacokinetic model for panitumumab,6 simulation was performed to evaluate the variability of panitumumab area under the concentration versus time curve (AUC) over the dosing interval (AUCtau) using the fixed-dose approach versus the recommended body weight–based dose.
Patients and Methods
The study protocols for the three clinical trials assessed were approved by the appropriate institutional review boards and independent ethics committees at participating study centers and were conducted in accordance with the Declaration of Helsinki (Supplemental Table S1). All patients in these studies provided written informed consent prior to the initiation of the respective studies. All studies included in our analysis were published, multicenter, open-label trials evaluating panitumumab 6 mg/kg Q2W in patients with mCRC.10,11 Patients with available Cmax and Cmin and a recorded body weight were included in the analysis; demographic data were also collected. For patients with pharmacokinetic data available and a recorded body weight at baseline, concentration-time profiles were determined on the basis of actual patient data.
In addition, concentration-time profiles were simulated using a fixed dose and the approved body weight–based dose from a previously published population pharmacokinetics model developed based on approximately 1200 patients.6 The fixed dose used for the simulation was selected based on median baseline body weight across the clinical studies and the approved body weight–based dose (6 mg/kg).7 Based on a median body weight of 80 kg and the approved panitumumab dose of 6 mg/kg, a fixed dose of 480 mg was used for the simulation.
The population pharmacokinetic model used for the simulation was a 2-compartment model with parallel linear and nonlinear clearance. The error model included both proportional and additive error, which improved the model substantially versus models with only proportional or additive error. The final model estimated that for a typical 60-year-old male patient with mCRC weighing 80 kg (the approximate median body weight of patients included the model), estimates for the linear clearance, the maximum nonlinear clearance (maximum elimination rate [Vmax]/Michaelis-Menten constant [Km]), the central volume of distribution (V1), peripheral volume of distribution (V2) and the Km were 0.273 L/d, 28.4 L/d, 3.95 L, 2.59 L and 0.426 µg/mL, respectively. Final model covariates included body weight, sex, and cancer type on clearance, body weight and age on Vmax, and body weight and sex on central V1. Using the final model, panitumumab pharmacokinetic profiles were generated for 1000 virtual patients by Monte Carlo simulation of fixed dose (480 mg) Q2W and body weight–based dose (6 mg/kg) Q2W, using body weights reflective of body weights across the patient population in the three clinical trials.10,11 AUCtau was calculated using noncompartmental analysis from the simulated pharmacokinetic profiles, using linear trapezoidal integration as a method of estimation. Interpatient variability was determined for all pharmacokinetic parameters.
Results
Of the 619 patients enrolled in the three studies and assigned to panitumumab, a total of 352 patients received panitumumab at body weight–based dosing of 6 mg/kg Q2W and had pharmacokinetic and body weight data available. Demographic data for all 619 patients are shown in Table 1. After administration of panitumumab, Cmax and Cmin increased with increasing body weight (Figure 1A and B). The mean Cmax and Cmin for patients weighing <65 kg (lower quartile) were 23% and 30% lower, respectively, than for those weighing >88 kg (upper quartile). For lower versus upper body weight quartiles, mean Cmax was 143 versus 187 µg/mL; mean Cmin was 27 versus 38 µg/mL. The variability contributed from body weight was lower than observed total variability (37–59%).
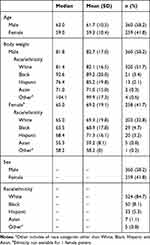 |
Table 1 Demographics and Baseline Characteristics of All Enrolled Patients Assigned to Panitumumab from Three Clinical Trials |
Using population pharmacokinetic modeling and simulation, the simulated AUCtau data indicated that, overall, panitumumab exposure increased with increasing body weight with weight-based dosing (Figure 2A). For the fixed dose (480 mg), the opposite effect was observed; overall, panitumumab exposure decreased with increasing body weight (Figure 2B).
 |
Figure 2 Simulated AUCtau values for 1000 patients modeled to receive panitumumab at (A) body weight–based dose (6 mg/kg) and (B) fixed dose (480 mg) based on the published population pharmacokinetic model.6 Abbreviation: AUCtau, area under the concentration versus time curve over the dosing interval. |
Over the wide range of patient body weights, the total variability in simulated AUCtau was lower for the weight-based dose (29%) than for the fixed dose (34%; Table 2), as expected. Additionally, the difference in median AUCtau between patients with body weight in upper and lower quartiles was smaller for weight-based (40%) than fixed (80%) dosing.
 |
Table 2 Simulated AUCtau Values for Patients Modeled to Receive Panitumumab Q2W as a Fixed or Body Weight–Based Dose |
Discussion
The most appropriate dosing regimen for individual monoclonal antibodies is variable and dependent on the effect of factors such as body weight on the pharmacokinetics of the monoclonal antibody.12 The FDA recommends that a full assessment of pharmacokinetics be undertaken to reduce interpatient variability in drug exposure with a view to optimizing dosing and improving efficacy and safety.1 Based on the results of Phase 3 studies, panitumumab was approved as a body weight–based dose regimen for the treatment of mCRC.4 However, it is suggested that a fixed-dose approach has practical advantages in clinical practice;12 for example, a fixed dose may simplify dose administration, streamline compounding and reduce medication error.8,9 Fixed doses of panitumumab are not FDA approved, but have been suggested;9 therefore, we assessed the body weight–based variability in exposure to the body weight–adjusted dose (6 mg/kg) across three clinical trials, and compared the FDA-approved body weight–based dosing with a fixed-dose approach in a simulated model.
In our analysis of pharmacokinetic data from clinical trials using body weight–based dosing, Cmax and Cmin increased with increasing body weight. In the simulation, the weight-based dose (6 mg/kg) led to substantially less variability in panitumumab exposure across the range of body weights than did fixed dosing (480 mg), supporting the premise that a body weight–based approach leads to less variability in drug exposure across patients.8
A population pharmacokinetic model of panitumumab was developed using serum concentration data from 1200 patients and 14 clinical studies. This analysis found that body weight, age, sex, and cancer type contributed to interindividual variability in panitumumab pharmacokinetics. However, age, sex, and cancer type were found to contribute only minimally to the total variability in panitumumab exposure (using AUCss as the indicator);6 therefore, dose adjustments based on these covariates are considered unnecessary.7 In contrast, body weight made the greatest contribution to interpatient variability; when expressed as the log-transformed AUCss, the body weight–based dose regimen reduced the total variance in exposure by 69.2%.6 Moreover, when considering pharmacokinetic variability in this analysis, 97% of all patients treated with panitumumab 6 mg/kg Q2W had steady-state Cmin >3.83 μg/mL, indicating panitumumab remained above pharmacologically meaningful concentrations.7 This provides further support that dosing based on body weight is reasonable and adequate.
Body mass index, body surface area, lean body mass, or ideal body weight have been proposed as alternative parameters for body size–adjusted dosing. Although lean body mass accounts for less variability in AUCss than body weight,6 body weight is preferable because it is more conveniently obtained clinically.6 Other body size measurements would require imputation,6 and have the potential to contribute to dosing error.
Data from clinical trials and from pharmacokinetic simulations indicate that the approved body weight–based dosing (6 mg/kg Q2W) results in less variability in panitumumab exposure across patient body weights than does a fixed dose. Exposure-response analysis may also be used to support body weight–based dosing; however, in the three studies supporting the efficacy of monotherapy, the first blood sample to assess panitumumab serum concentration was not collected until week 7. Approximately one-quarter of patients had discontinued panitumumab before week 7 of the study and did not have any pharmacokinetic data available. The lack of pharmacokinetic data available for patients who discontinue early may lead to bias in any traditional attempt to model effectiveness as a function of pharmacokinetics. Thus, there appears to be no feasible way to conduct an analysis of these data without introducing survivor bias. Nonetheless, it is expected that less variability in panitumumab exposure would be beneficial in terms of clinical outcomes. Importantly, body weight–based dosing was approved by the FDA based on the efficacy and safety associated with panitumumab in clinical trials;4 any proposal to change the dosing approach would need a full assessment of efficacy and safety, including an assessment of practical benefits resulting from the simplified approach to dose administration.
Conclusions
In conclusion, the approved body weight–based dosing for panitumumab of 6 mg/kg Q2W regimen was supported by scientific clinical study data and pharmacokinetic modeling and simulations to provide optimal panitumumab exposure over the range of body weights of patients receiving the drug in clinical trials, suggesting this dose regimen would provide optimal clinical benefit.
Abbreviations
AUC, area under the concentration–time curve; AUCSS, area under the concentration–time curve at steady-state; AUCtau, area under the concentration–time curve at steady-state over the dosing interval; BW, body weight; CI, confidence interval; Cmax, maximal (peak) observed serum concentration; Cmin, minimum (trough) observed serum concentration; CV, coefficient of variation; EGFR, epidermal growth factor receptor; FDA, US Food and Drug Administration; Km, Michaelis Menten constant; mCRC, metastatic colorectal cancer; Q2W, every 2 weeks; V1, central volume of distribution; V2, peripheral volume of distribution; Vd, volume of distribution; Vmax, maximum velocity.
Data Sharing Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
Acknowledgments
The authors thank Lee B. Hohaia, PharmD, CMPP (ICON, North Wales, PA), whose work was funded by Amgen Inc., for medical writing assistance in the preparation of this manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The study was funded by Amgen Inc. Michael Z. Liao is an employee of and stockholder in Amgen. Marloes Berkhout is an employee of Amgen B.V. and has restricted shares in Amgen. Hans Prenen has received honoraria and/or travel grants from Amgen, Bayer, Ipsen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, Terumo, and Vifor pharma. Sandeep Dutta is an employee of and stockholder in Amgen. Vijay V. Upreti is an employee of and stockholder in Amgen. The authors report no other conflicts of interest in this work.
References
1. Zhao L, Ren TH, Wang DD. Clinical pharmacology considerations in biologics development. Acta Pharmacol Sin. 2012;33(11):1339–1347. doi:10.1038/aps.2012.51
2. Douillard J-Y, Siena S, Tabernero J, et al. Overall survival (OS) analysis from PRIME: randomized phase III study of panitumumab (pmab) with FOLFOX4 for first-line metastatic colorectal cancer (mCRC). J Clin Oncol. 2013;31:abstract3620. doi:10.1200/jco.2013.31.15_suppl.3620
3. Kim TW, Elme A, Kusic Z, et al. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer. 2016;115(10):1206–1214. doi:10.1038/bjc.2016.309
4. Vectibix® (panitumumab). Full prescribing information. Thousand Oaks, CA: Amgen Inc; 2017.
5. Vectibix (panitumumab). Summary of product characteristics. Breda, Netherlands: Amgen Europe B.V; 2018.
6. Ma P, Yang BB, Wang YM, et al. Population pharmacokinetic analysis of panitumumab in patients with advanced solid tumors. J Clin Pharmacol. 2009;49(10):1142–1156. doi:10.1177/0091270009344989
7. Yang BB, Lum P, Chen A, et al. Pharmacokinetic and pharmacodynamic perspectives on the clinical drug development of panitumumab. Clin Pharmacokinet. 2010;49(11):729–740. doi:10.2165/11535970-000000000-00000
8. Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49(9):1012–1024. doi:10.1177/0091270009337512
9. Hendrikx J, Haanen J, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22(10):1212–1221. doi:10.1634/theoncologist.2017-0167
10. Hecht JR, Mitchell E, Neubauer MA, et al. Lack of correlation between epidermal growth factor receptor status and response to panitumumab monotherapy in metastatic colorectal cancer. Clin Cancer Res. 2010;16(7):2205–2213. doi:10.1158/1078-0432.CCR-09-2017
11. Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–1664. doi:10.1200/JCO.2006.08.1620
12. Bai S, Jorga K, Xin Y, et al. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51(2):119–135. doi:10.2165/11596370-000000000-00000
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

