Back to Journals » Clinical Optometry » Volume 10
Do different experimental tasks affect psychophysical measurements of motion perception in autism-spectrum disorder? An analysis
Authors Bakroon A , Lakshminarayanan V
Received 10 July 2018
Accepted for publication 29 October 2018
Published 12 December 2018 Volume 2018:10 Pages 131—143
DOI https://doi.org/10.2147/OPTO.S179336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Mr Simon Berry
Asmaa Bakroon,1 Vasudevan Lakshminarayanan1–3
1Theoretical and Experimental Epistemology Laboratory, School of Optometry and Vision Science, University of Waterloo, Waterloo, ON, Canada; 2Departments of Physics and Systems Design Engineering, University of Waterloo, Waterloo, ON, Canada; 3Department of Computer Engineering, University of Waterloo, Waterloo, ON, Canada
Abstract: There is a rapid increase in the number of individuals with high-functioning autism (HFA). Research on motion perception in HFA has shown deficits in processing motion information at the higher visual cortical areas (V5/middle temporal). Several hypotheses have been put forth to explain these deficits as being due to enhanced processing of small details at the expense of the global picture or as a global integration abnormality. However, there is a lot of variability in the results obtained from experiments designed to study motion in adults with autism. These could be due to the inherent diagnostic differences within even the same range of the autism spectrum and/or due to comparison of different experimental paradigms whose processing by the same visual neural areas could be different. In this review, we discuss the various results on motion processing in HFA, as well as the theories of motion perception in autism.
Keywords: autism-spectrum disorder, high-functioning autism, motion perception, biological motion, form perception, random-dot kinematogram, local motion, global motion
Background
Autism-spectrum disorder (ASD) is a developmental disability syndrome characterized by impairments in social communication and interaction defects. The prevalence of autism from the Centers for Disease Control and Prevention1 show one of 68 children is born with ASD, of which 43.9% are classified with high-functioning autism (HFA). The term HFA refers to average or above-average intellectual ability in the range of IQ >85, among other higher-functioning cognitive abilities, such as emotion recognition, expressions, social interaction, and executive function (EF), with a special emphasis on individual and group problem-solving.2,3 Individuals with HFA and Asperger’s syndrome (AS), which is within the autism spectrum, may have the ability for general societal interactions and have close-to-normal life activities, eg, studying, working, and driving. Most studies collect data from people with ASD who generally have average–high IQ and do not have severe abnormalities or other related development issues that may make data collection difficult. In this review, we will still denote individuals with HFA as within the general term ASD, which is the general convention in the literature.
Visual function, such as refractive error, strabismus, and color vision, is found to be normal in ASD; however, contrast sensitivity, motion perception, visual movement, and visual search may be more affected among the autism group when compared to a typical-development (TD) group.4 Studies of visual motion perception have shown that individuals with ASD have exceptional perceptual abilities for detecting small details in the environment, but are incapable of capturing the whole without giving full attention to the constituent parts.5–7 Indeed, Castelli et al8 showed that the ability of individuals with ASD to perform well on the standard Wechsler block-design task was due specifically to their advanced segmentation ability when compared to a normal-development group. Related research using the embedded-figure test9 suggested that children with autism have superior performance in detecting embedded figures than normal children and nonautistic children with intellectual disability. Other studies, however, have shown enhanced detection of local targets, with a typical global bias.10,11 Mottron et al12 used various local/global tasks, including traditional tasks of hierarchical processing, configural processing, and a disembedding task that contained letters presented either individually or in the pattern of the same letter. Target discrimination of the global scene using hierarchical or configural tasks showed no group differences; however, individuals with autism were faster than the TD group in processing local details within embedded pictures than isolated ones on the disembedding task.
Research findings vary in indicating a local processing bias or a global processing deficit, and often contradict one another. Several reviews have discussed these findings of differences in motion perception in ASD, and whether these differences are sensory symptom-related and/or due to social and perceptual knowledge latency in early childhood.13–15 Bias in local/global visual processing relevant to stimuli and task dependence has also been investigated.16 This suggests a reconsideration of the idea of impaired global (or rather, biased) processing between local/global information, with dominant intact or enhanced performance on tasks necessitating static spatial information processing and poor performance with dynamic information analysis.5,12,17,18
Our review here is selective, focusing on cases of adults with ASD and their sensitivity to various paradigms in processing coherent motion. The published results are often contradictory, eg, in experiments on “form from motion” (FFM), which includes detection of biological motion (BM), performance is found to be intact in form motion, with reduced sensitivity to BM,7,19 and cases where researchers divided the autism group into HFA and AS and found that atypical perception was HFA-related, not AS. Comparing the consequences of task relevance and autism-group subdivision allows conclusions about the abnormalities found in motion perception in autism. We conclude this article by addressing recent studies directly comparing different types of motion integration, and suggest a possible synthesis of the otherwise-contradictory and confusing results found in the literature.
Theories
Various investigators across multiple cognitive domains in autism have proposed different hypotheses falling between single domain-specific or domain-general mechanisms. Proponents of the theory of weak central coherence (WCC)20 explain that people with ASD have enhanced segmentation of local details and weak ability to discover the global meaning. For example, ASD shows superior performance on embedded-figure and block-design tasks (Figure 1; ie, static-target design within a complex large picture, including local details, where participants are required either to respond to what they only see or if they can see the global pictures).21
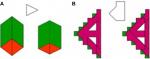  | Figure 1 Sample of embedded-figure task: (A) simple; (B) complex. Notes: Reproduced from Fonville L, Lao-Kaim NP, Giampietro V, et al. Evaluation of enhanced attention to local detail in anorexia nervosa using the embedded figures test; an fMRI study. PLoS ONE. 2013;8(5): e63964. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode”http://creativecommons.org/licenses/by/4.0/legalcode.108 |
However, WCC fails to explain the diversity of results in overall typical performance of individuals with ASD in extracting higher-level meaning included in different tasks. Happé et al proposed that global perception could be typical in autism, but autistic people have a biased “cognitive style” towered local details compared with the remaining population.22 However, other groups using specific tasks that require global integration of local details (eg, global dot motion or random-dot kinematograms [RDKs]) have reported impairments in global processing.23,24 Mottron et al25 proposed the enhanced perceptual function (EPF) theory, where they suggested that use of “high”- vs “low”-level information processing to qualify autistic performance may be misleading. They explained the superior involvement of perceptual regions in so-called high-level tasks by the significant superiority of “perceptual” processing that impacts social and behavioral abilities in ASD. This theory, however, argues that people with autism are able to process global information despite any qualitative or quantitative deficiency in local-level processing. Therefore, the perception of global picture is a relatively optional characteristic in ASD, while it is mandatory in the general population.26
Both theories (WCC and EPF) have been criticized as being either too narrow or too general to explain the full range of autistic symptoms, thereby giving rise to the theory of mind (ToM), which is currently the most dominant theory in ASD cognition. Corcoran27 explained the profound difficulties in social communication and stereotyped and even repetitive interests and activities on “false belief” tasks, where ASD participants showed less ability to “read” others’ minds or explain and solve or deal with social situations. The ToM does not explain the relationship between social integration processing and atypical visual processing, with the exception being difficulties in processing facial expressions.13 However, combining the ToM, or “mentalizing”, as Hill and Frith28 prefers to call it, and systemizing WCC and EPF should give rise to the core aspects of neurocognitive atypicalities in ASD.29 In other words, emphasizing the relationship between the ability of recognition and interoperation of all the details of a complex scene would reveal the core of cognitive functions in autism.
Pellicano30 examined whether autism differences in cognitive skills at early developmental stages can change along with other emerging skills in various cognitive domains or whether these skills are developed independently. This study measured performance on several cognitive domains, including ToM, EF, and CC, in children 3–7 years old with autism, and then these were evaluated 3 years later in the same children performing the same experiments. The results showed that early EF and CC skills were longitudinally predictive of change in children’s ToM skills. On the other hand, cognitive performance between EF and CC was not linked over the 3-year period, even when variance due to age was taken into account. Verbal ability and nonverbal ability differences in cognitive performance remained stable as well. These results, however, agreed with earlier longitudinal studies on children with autism. Booth et al and Happè et al29,31 found temporal stability in individual differences within ToM, EF, and CC over a longer period. Again, there are few precise predictions about visual performance that come from these theories. Therefore, it is unclear if these visual abnormalities of local/global processing in autism are experienced due to abnormal neural connectivity and integration in the visual cortex or difficulties with visual attention and eye movements (especially for scene-exploration tasks).
Visual attention in ASD
Natural processing in visual perception is determined not only by the environment visual inputs, which refers to bottom-up processing, but also modulated by top-down processing by prior knowledge, such as facial expressions. Such knowledge develops through experience-dependent plasticity or during development, and includes contextual modulation of perception.32 Although our review selectively discusses findings from the viewpoint of local vs global processing in ASD, it is essential to understand top-down attention in autism. For example, Maekawa et al33 studied top-down and bottom-up visual information processing in adults with HFA using event-related potentials while running nonsocial spatial attentional stimuli composed of black–white windmill patterns. They found that HFA subjects were faster in detecting the target; however, there was no significant difference in target-stimulus-detection accuracy between the HFA and TD groups. Event-related-potential data, however, showed abnormal lower visual level processing evidenced by reduced P1 amplitude and P300 latency (300–500 ms) in HFA, which suggested that while bottom-up attention was relatively preserved in the autism group, the abnormal P300 finding indicated that top-down attentional processing was impaired in HFA. Typical behavioral and attentional perception to objects and nonsocial stimuli has also been found in related studies. For example, Loth et al34 suggested that the effect of prior knowledge on the conscious perception of degraded visual stimuli is intact for object stimuli, but not for face recognition. This pattern of results was even more pronounced in the results of eye tracking, which showed that the top-down effect on perception of faces was not only reduced but also virtually absent. However, research results on attention allocation to social and nonsocial stimuli have been mixed in ASD. Using eye tracking as an index of attention of main areas of gaze produced interesting results in ASD.35 Findings showed that autism had overall reduced social attention compared to TD and that diminished social attention may start as early as 6 months old and remain constant across ages in ASD.35 However, it is possible that unchanged social attention might be generated from accumulated deficits of long term atypical experiences in adults, whereas data from children represent a time in which symptomatology profiles are still emerging.36 However, Tegmark added that social attention differences in ASD appear to be modulated by the complexity of the social context.35 Visual attention gaze patterns for different dynamic and static social/nonsocial stimuli in children and adolescents with ASD have shown that ASD groups exhibit atypical gaze patterns associated with social stimuli, eg, they will gaze more at the body and give decreased attention to the eyes.37 All of this is correlated with the severity of social attention and hence social communication capabilities. Few studies have examined the factors of attention and gaze stability in adults with HFA, and results are controversial.38–40 That leaves the question open as to whether social attention abnormalities in ASD are due to specific difficulties with processing social information, are more related to visual processing abnormalities found in ASD, or a combination of both. However, there have not been studies link this reduction in attentional engagement to enhanced perception of local details and/or to decreased global perception or both, which is found in autism. Therefore, further investigation is required to define the ASD-specific attention profile across social and nonsocial dimensions, and its relationship to motion-perception processing.
Motion processing in adults and adolescents with ASD
Interoperation of global motion scenes often requires integration of both spatial and temporal information between low-order neurons with small directional receptive fields at V1 and high-order extended receptive fields, primarily in the middle temporal (MT)/medial superior temporal (MST) area.41 Researchers usually use a single moving point or contour to study local motion processing, eg, discriminating direction of a moving sine wave grating with spatiotemporal variations in luminance over time, which refer to simple, first-order motion (luminance-defined), which can be processed based on one point source, and this is enhanced for persons with autism.42 The ability to process second-order (texture-defined or non-Fourier) stimuli, which measures response to more than one point in space, has been found to be intact in autism.42
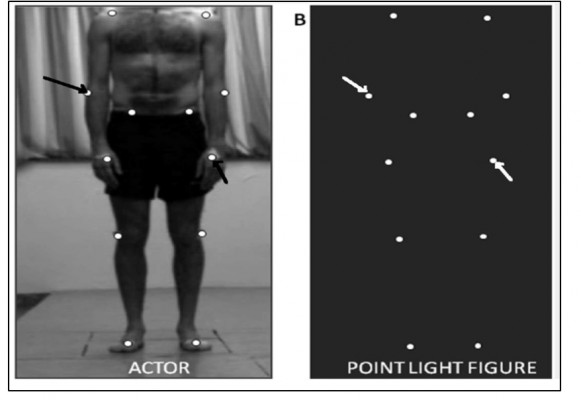
Global motion, on the other hand, can be studied using RDKs, where several dots or contours move relatively to one another, requiring the perceptual system to integrate individual local motions into a globally coherent motion (Figure 2).43 Usually, the RDK technique is used to measure the motion-coherence threshold, where a certain percentage of dots move together (coherent signal) in the same direction while the remaining moving dots run in random directions (noise). The threshold for coherent motion is then defined by the percentage of coherent dots required to detect the accurate direction of coherent motion at some predefined probability level. Performance on this task has been found to be directly related to activity in the MT/MST area, but not to activity in earlier visual areas.44 Another form of the RDK task is the measurement of perception of FFM. This is generated from a number of dots that move in a specific spatial relationship to generate a structure or shape, and can only be defined by the motion of the constituent dots. RDK is also used to study BM, which is the perception of human figures. These stimuli are generated by a few points of light that are attached to the joints of a moving human (Figure 2).45 FFM and BM perception – which is another FFM task – involve both the dorsal and ventral stream pathways.46 Functional magnetic resonance imaging (fMRI) studies suggest that deficits in BM perception could support a theory of dorsal stream dysfunction if MT/V5 reductions are associated with activity decrease in the right-hemisphere posterior superior temporal sulcus (STS), an area particularly sensitive to BM.47,48
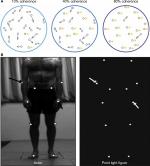  | Figure 2 Motion perception stimuli. Notes: (A) RDK stimuli with differing coherence levels. Adapted from Robertson CE, Martin A, Baker CI, Baron-Cohen S. Atypical integration of motion signals in autism spectrum conditions. PLoS One. 2012;7(11):e48173. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode”http://creativecommons.org/licenses/by/4.0/legalcode.43 (B) Point-light display generated by small lights attached to main points on the human body (actor), which create biological motion stimuli. Reproduced from Nackaerts E, Wagemans J, Helsen W, Swinnen SP, Wenderoth N, Alaerts K. Recognizing biological motion and emotions from point-light displays in autism spectrum disorders. PLoS One. 2012;7(9):e44473. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode”http://creativecommons.org/licenses/by/4.0/legalcode.76 |
Studies of visual motion perception in autism across different ages and using different tasks have proposed normal first-order motion perception5,42 and abnormal second-order motion perception.6,49 Results of coherent-motion tasks, however, show mixed sensitivity in local/global motion in autism. While several studies have reported decrease in performance in coherent global perception in autism,23 others have shown normal results when compared with a control group.12,50 A meta-analysis by van der Hallen et al16 suggested that it was not enhanced local visual processing nor a deficit in global visual processing, but a slow global processing that required longer time to respond. They also suggested that there were no direct effects of age, IQ, or sex on performance in autism. Long-duration stimuli presentation or/and long response time seems to impact enhanced performance of participants with autism.6,51–53 In addition, Koldewyn et al54 suggested an effect of task paradigms on perceptual performance in ASD.15 Of all of the divergent results of research on motion perception in autism, the issue of individual differences in visual motion sensitivity among individuals with ASD using RDK stimuli remains unresolved, and is a very important paradigm for research on motion perception.
RDK stimuli and autism
Our review here is selective, focusing on studies that use RDK stimuli to investigate global forms of motion perception in adults and adolescents with HFA, because of the following observations:
- evidence of fully mature motion processing occurs after the age of 11 years55
- aspects of early visual processing, such as crowding and visual attention, are relatively mature after age 10 years and are positively correlated with severity level in autism13,56
- Hadad et al15 addressed the parameters affecting global motion perception in individuals with abnormal early visual input, such as in ASD, from infant stage to adult
- RDK stimuli are particularly suited to assessing global motion processing; however, different paradigms of RDK (eg, signal/noise, FFM) can integrate different cues and thus subserve the perception of different types of movement.57
Moreover, we also discuss findings from studies on children with autism for comparative purposes, since studies that examine those specific paradigms in adults with autism are not available in the literature.
Local/global perception in ASD: study findings
The perception of global motion is of much interest. Table 1 summarizes some of the data that have been accumulated over the recent years in studies of motion perception in adults with ASD. However, measurements of the sensitivity to spatial and temporal factors of global motion are not always controlled across studies. Therefore, controversial results among comparable studies of motion perception in autism have been found and hence make it very difficult to draw firm conclusions. For example, Tsermentseli et al58 compared motion sensitivity between adults with autism and adults with dyslexia and a control group. They replicated Spencer and O’Brien’s59 motion paradigm on children with autism, which showed a high coherence threshold in this group. Tsermentseli et al also reported that adults with autism showed a high motion-coherence threshold, but only for individuals with HFA and not AS. On the other hand, Atkinson60 found increased thresholds for coherent motion in individuals with autism using an RDK stimulus. However, in this study the ASD group was composed mostly of adults with AS (n=12; HFA, n=1). In both studies,58,60 the AS group had similar full-scale IQ and mean age (FIQ 107.8, age 23.3 years and FIQ 106.2, age 30.9 years, respectively). Tsermentseli et al’s58 motion task consisted of a glass with a target area that formed circular patches defined by correlated dot triplets. The dots of the circular batch moved either to the right or to the left of the screen among randomly oriented dots (Figure 3).
  | Figure 3 Schematic of stimuli used by Tsermentseli et al58 for form coherence (left) and motion coherence (right). Notes: Reprinted by permission from Springer Nature: Springer. J Autism Dev Disord. Comparison of form and motion coherence processing in autistic spectrum disorders and dyslexia. Tsermentseli S, O’Brien JM, Spencer JV. 2008;38(7):1201–1210.58 |
On the other hand, Atkinson used white RDK dots that moved on a black background to the left or right of the screen. Parameter differences that affect global motion perception in adults with autism have been discussed elsewhere.15 For example, dot lifetime (limited or unlimited, which affects the ability to track individual dots) and speed have been found to elevate the threshold of coherent motion in autism (Table 1). However, in the study by Atkinson and Tsermentalisi, dot lifetime might not have created a difference, since the stimuli duration in both studies was very short (~250 ms).61 Also, the effects of speed may in fact increase the threshold for motion coherence if the dot spatial displacement is large, which was the case in Tsermentseli et al,58 even though no differences in AS were found. van der Hallen et al16 suggested that slow global processing in individuals with ASD was the cause of the different findings, and that ASD participants need more time to respond. However, in both studies, the time given to participants to respond was long (Table 1). One possible explanation for differences in findings between the studies of Atkinson and Tsermentalisi et al might be the modified psychophysical task used. Glass patterns are primarily random stimuli that generate perception of global motion. Glass patterns with concentric structure are considered to be easier to perceive than other types of correlated dot images (eg, radial and translational patterns).62 Evidence from fMRI63 shows that Glass patterns are processed in two stages: primary visual area V1 and higher-order areas of the MT area, yet the sensitivity to curvature and global form present in Glass patterns exist as early as the primary visual cortex. This could actually explain the high performance of AS patients in motion coherence formed by the Glass pattern, since the primary visual area is found to be intact in autism.51,64 Interestingly, Tsermentseli et al were the first to report impaired form processing in adults with AS. The authors suggested that more tightly integrated network among the dorsal and ventral streams in the visual system may cause abnormal response. Therefore, the evidence from this study does not support the “dorsal stream vulnerability” hypothesis, since most experiments have shown that ASD subjects had high motion-coherence thresholds, but intact performance on form-coherence tasks.49,52,59 Impaired motion perception in autism may result from diffuse, aspecific neural dysfunction of early neurointegrative mechanisms, which lead to deficits in the perception of complex stimuli.65 Robertson et al6 employed fMRI to verify this theory, and found slow responses to elemental visual information at V1, which presumably alters the rate at which those local details are integrated into a global percept. The results were significantly different with shorter stimulus duration in the ASD group when compared with the control group, indicating that integration of local signals into global percept is delayed in ASD.6 This agrees with previous results of Robertson et al,43 which showed short-duration stimuli (200 ms) decreased the performance of coherent motion in autism, but intact global processing was evidenced in ASD with longer stimuli presentation (eg., 400 and 1,500 ms).43 Typical functional brain areas have been reported in other fMRI studies between autism and control groups.50,51 Also, impairment in function or performance in one or more tasks is prevalent in autism studies. For example, limited dot lifetime increased threshold, as opposed to “infinite”-lifetime dots,66 and slow dot speed (1.5°/second) reduced coherent perception, as opposed to fast speed (6°/second; Table 1).
One could argue that the contrasting results can be attributed simply to spatial stimuli parameters. However, in all these studies (Table 1), a direct comparison to match age, sex, and IQ control groups revealed a decrease in coherent motion threshold in autism. A different factor, eg, diagnostic variance among ASD populations, can also be considered. Spencer and O’Brien59 divided their participants into those with HFA and those with AS, and found that motion-coherence thresholds differed significantly from controls for the HFA group, but not the AS group. Milne et al67 also found that only a subgroup of their ASD population (about 20%) had motion-coherence thresholds outside the typical range. This type of meta-data analysis would help in comparing among autism-syndrome subgroups and classifying the severity of coherent motion deficits among these groups.
Integration of FFM and BM in ASD
As noted previously, FFM and BM, which is a form of FFM, require spatiotemporal integration of local motion signals. Adding to its complexity, BM entails dynamic, hierarchically arranged pendular motions, which when viewed under optimal conditions group together to produce the global perception of biological activity. The processing mechanisms of BM and FFM are still being investigated, but evidence points to multiple visual brain areas being involved. It has been shown, for example, that the perception of BM activates occipital regions of the STS besides MT+, while coherent motion mainly activates the MT–MST complex.48 This suggests that BM relies on input from both dorsal and ventral areas of the extrastriate visual cortex.68 However, FFM using arranged patterns, such as concentric Glass patterns, activates a number of brain areas, such as fusiform/lingual gyri, middle occipital gyrus, and intraparietal sulcus.68 Interestingly, the fact that all three motion-processing mechanisms are beyond V1 suggests that V1 response is determined by local spatial elements.69 Comparison across these three types of display may thus be informative. Studies providing a direct comparison of performance across the different tasks in adults with autism, however, are minimal. Jones et al70 tested visual processing of CM, FFM, and BM on a group of adolescents with autism compared to a control group. All three motion stimuli were displayed with a random-dot (noise) background, which varied across the tasks. A psychophysical staircase method was used to determine the threshold. In this methodology, three noise dots were added after every two consecutive correct trials, and an incorrect response resulted in three noise dots being removed. In all three tasks, the dots ran at the same speed (Table 1) and had a limited lifetime (40 ms). The results showed no differences between the ASD group and the TD group in any of the three tasks. However, within-group differences were found among the ASD group. Autistic individuals with low IQ performed worse on all three visual processing tasks, but they were significantly worse in the BM task. This study suggests that these differences happened due to difficulties between the tasks and the stimuli, and also the diversity of symptoms defining the ASD disorder. On the other hand, Saygin et al52 found no group differences between ASD and TD groups or within the ASD group processing BM and FFM stimuli. Compared to the Jones et al study, the Saygin et al study was conducted on ASD adults (mean age 33.75 years) while the Jones et al age-group was younger (mean age 15.6 years). Although evidence of the developmental course for sensitivity to coherent motion is found by the age of 11 years, we could consider that ASD adolescents with low IQ may “catch up” with their peers without ASD at the adult stage. In addition, in Jones et al, the number of participants was larger, which allowed more diversity in the ASD syndrome among the group. This explanation may better predict the variable findings across both studies, but cannot necessarily indicate typical neural processing in ASD. Motion-perception deficits have been found in individuals with developmental disorders, eg, Williams syndrome71 and dyslexia.72 However, studies comparing perceptual difficulties between people with disorder development and autism have shown distinct differences in processing CM and FFM in autism, even in those with high functioning level.58 While several studies have found intact FFM in ASD, BM was more distinctly affected.12,49,50,60 Using fMRI, Koldewyn et al50 demonstrated that an autism group showed lower brain function during the BM task compared to a TD group. Brain activity in BM in the TD group was notable in a large area in the bilateral parietal cortex, primarily along the intraparietal sulcus, right dorsolateral prefrontal cortex, centered in the inferior frontal gyrus, a cluster in the anterior cingulate cortex, and a region in the right posterior STS. The autism group showed activity at an area in the bilateral inferior temporal cortex, including cortex in both the lateral occipital and fusiform gyrus (Figure 4). Interestingly, this activation increased when noise level was reduced in the BM stimulus and vice versa. The results agreed with a previous study of Koldewyn et al,49 where they used a psychophysical task in adolescent autistic subjects. Their results showed increased threshold in BM and intact FFM performance, as well as decreased sensitivity to CM. Impairments in processing both BM and CM have been shown in autism.60 This may be due to the neural mechanisms behind processing the BM, which include processing of local/global details and integration between multiple visual neural areas, particularly at the STS.73 The STS has an important role in processing body and facial movement,74 and may be involved in the interpretation of any social signal with a temporal component. As such, those with ASD may be fairly unaffected in their perceptual processing of BM per se, but exhibit specific impairments in emotion-related judgments and emotion processing of the point-light displays (PLDs) that are used as BM stimuli (Figure 3).60,73,75,76 Hubert et al77 found that those with ASD were able to detect BM given sufficient time, but they were not as good at emotional PLDs. Parron et al78 also found differences in PLD with respect to emotional displays. Parron et al’s study suggests that adolescents with ASD are able to group points of light related to inanimate objects as well as TD individuals suggesting that global processing is intact. On the other hand, when these points have an emotional content, the performance of ASD group is decreased.
  | Figure 4 Activated areas for biological and coherent motion in ASD compared to TD. Notes: Copyright © 2011 Blackwell Publishing Ltd. John Wiley and Sons. Reproduced with permission from Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Dev Sci. 2011;14(5):1075–1088.50 |
Recent studies have related genetic influences on BM perception in autism.79,80 It was found that sensitivity to local BM cues was negatively correlated with autistic traits through the dimension of social communication, with the covariation largely mediated by shared genetic effects. Therefore, to date the literature has provided a rather contradictory picture, due to the different paradigms, different variables assessed, and heterogeneity of participants, and thus more studies are needed to clarify these differences.
Specific trajectories of motion integration
Role of neural noise
In processing complex spatiotemporal visual stimuli, neural “noise” often refers to the variation in neural responses that typically reduce the detection or discrimination of the signal and is parameterized by the signal:noise ratio. Sometimes, neural noise can enhance perceptual detection and discrimination via “stochastic resonance”, a property of nonlinear systems in which addition of noise can facilitate detection and discrimination of subthreshold signals.81 However, in autism, an emerging hypothesis postulates that excessive internal noise is a key factor influencing perceptual abilities.82 Reduced perceptual efficiency in ASD that is due to both increased internal noise and bad external noise filtering while highlighting internal noise has implications for perceptual, behavioral, and cognitive abnormalities. Perceptual learning often refers to the exclusion of environmental noise (external noise) and reduction of additive internal noise, thus effectively enhancing the stimulus and/or multiplicative internal noise reduction.83 In individuals with ASD, there is growing evidence that increased internal noise might play an important role in the reduction of their global visual perception.81,84,85 Recent results from a group of children and adolescents with ASD showed both elevated internal additive noise and reduced ability to filter out external noise from stimuli, accompanied by no evidence for abnormalities in internal multiplicative noise.82 Another observation was a link between internal additive noise to the severity of core behavioral symptoms of ASD. The experimenters considered the factors that can reduce the ability to extract task-relevant structures from visual inputs, such as signal:noise ratio and stimulus complexity, which can influence those with ASD. Zaidel et al84 suggested that heightened sensitivity to stimuli noise, rather than integration deficits, may characterize ASD. They referred to the finding that individuals with autism process the stimuli information per se, which will overload their visual integration. Adding to that, any visual noise will result in reduction of performance in ASD subjects more than a control group. Related studies reveal elevated internal noise in autism measured by blood-oxygen-level-dependent86 and electroencephalograpy87 responses to sensory stimuli. Contrary to these studies, other studies have suggested the opposite possibility of reduced internal noise in ASD, suggesting that reduced internal noise would enhance detection and discrimination of local details at the cost of global ones.81 Notably, a number of studies have challenged the noisy-brain hypothesis in ASD by demonstrating typical levels of variability in evoked electroencephalography88 responses to sensory stimulation, as well as in psychophysically estimated internal noise.89 At the core of this controversy lies the issue of whether this explanation applies to data reported in this review regarding the role of internal noise in directly affecting motion processing in ASD.
A related question is does internal neural noise reduce as a function of age in autism, thus improving visual processing? Research on age-related intraneural noise has shown increased neural noise as a function of age,90 which may result in reduced processing performance in the elderly. However, to date there have been no studies comparing adult internal neural noise in autism to younger age for autism or TD groups. The mechanism(s) underlying elevated internal noise in ASD is also under debate. A number of neural models suggest that there is a proliferation of neural connections in the sensory cortex of individuals with ASD,91–93 and thus misfiring synapses could easily result in noisy signals in the visual system.
While it is possible to argue that adding a theoretical hypothesis to the conflicting theories of visual processing in ASD could be “noisy”, we suggest that elevated internal noise and neural variability may explain some of the complex phenotypes in individuals with ASD.94 This is further complicated by the fact that estimates of neural variability are based on responses to noisy task stimuli, making it difficult to estimate the degree to which internal noise limits perceptual performance in ASD from external noise and/or both.
Role of excitatory/inhibitory neural responses
In typical populations, when stimuli size increases, a “spatial suppression” of high-contrast motion stimulus occurs to receive only the information within the limits of the classical receptive field. This is also affected by the contrast “gain control”, which is an inhibitory mechanism to prevent overresponse to high-contrast stimuli.95–97 These two visual responses are referred to as the excitatory/inhibitory (E/I) neurochemical balance in the context of visual motion perception. Abnormally weak spatial suppression, which is reflected in reducing the effect of increasing stimuli size, has been found in individuals with schizophrenia, as well as elderly people.98,99 Foss-Feig et al5 studied whether there were abnormalities in response-gain control in a group of children and adolescents with ASD compared to TD. They varied in size and contrast of drifting grating stimuli, using a two-alternative forced-choice method in a direction-discrimination task. The results showed that both groups exhibited increased threshold with increased stimulus size, and there was no overall group-performance difference for high-contrast levels. Interestingly, the autism group showed a twofold-enhanced performance for all stimuli sizes with high contrast than the TD group. For low-contrast stimuli, however, there were no group differences, and there was no correlation between contrast and size sensitivity with severity of autism syndrome for both contrast levels used in the experiment. This contrast-dependent enhancement of motion perception in ASD is qualitatively consistent with impairments in response-gain control, whereby inhibitory neural responses are atypically increased at high contrast. Notably, both response-gain control and receptive-field size are affected by the E/I balance in the brain.100 Schauder et al53 replicated the Foss-Feig et al study, but examined stimulus-size changes affecting gain control in autism. Their results revealed low sensitivity in participants with autism to small stimuli. This suggests large receptive fields in ASD and elevated excitation levels. These findings agree with the results of a previous fMRI study in adults with autism.101 Other studies ruled out E/I imbalance in the visual system of those with autism, but suggested that such an imbalance, if it exists, is likely to be small and thus does not explain the enhanced visual processing found in autism.102 In particular, contrast sensitivity and first-order visual processes have been found intact in ASD.64,103,104 Moreover, findings in E/I studies on autism do not really agree with the idea of E/I imbalance, which suggests that reduced center-surround inhibition affects weak spatial suppression that results in decreased effective stimulus contrast.105 A hypothetical model has linked the E/I balance to γ-band activation, which is found in many visual cortical areas that are induced by different stimuli or tasks. This model proposes a temporal synchronization of neural activity for integration of object features across different modalities.106 Based on this model, Peiker et al107 suggested that altered γ-band modulation may result in high excitatory and weak inhibitory interactions during brain processing of visual inputs, which is also supported by the evidence of epilepsy in ASD. Therefore, disturbance of neural modulation at center-surround antagonism in the high-order visual cortical (eg, MT/V5) might explain the enhanced response gained in ASD. However, the conclusion that this theory can fully explain the behavioral, cognitive, and perceptual differences observed in those with ASD is still weak.105
Conclusion
We have detailed experimental evidence of deficits in visual processing in high-functioning adults and adolescents with ASD. Although different studies suggest different deficits, some important conclusions about the critical role of several factors in determining abnormal visual processing in autism can be synthesized. One possible reconciliation of the mixed and often contradictory data is the diversity in neural brain mechanisms in processing motion perception for different paradigms of motion stimuli. As explained earlier, RDK stimuli are widely used to evaluate motion perception in different forms, eg, global or FFM. Each of these stimuli methods may be processed differently in the brain. In particular, those methods that are processed through the integration of the MT+ complex and other visual areas, such as the STS, will result in different performance. Adding to this, differences in stimuli parameters make it difficult to compare results of one study to another. For instance, two parameters defining speed–spatial offset of signal dots in an RDK and the temporal interval between sequential animation frames, as well as their interaction with density, have an impact on the threshold for coherent motion in HFA.15 These often-uncontrolled factors may also account for the inconsistent findings in adults with autism. This suggests that in future studies of motion perception in adults with ASD, one should consider these issues by taking into account the stimulus parameters that should be used for specific neural integration purposes, which activate particular visual neural areas in normal individuals and those with HFA.
In the second part of this review, we addressed studies that tested specific trajectories that may impact integration of motion perception in individuals with autism. Recent research allows the definition of neural noise sensitivity in ASD and offers some insight into the mechanism of integration of motion perception. Studies demonstrate a worse outcome after increasing internal neural noise that regulate hyper/hyposensitivity within the same visual modality. We also discussed these differences in the context of gain-control modulation, which might also account for enhanced or decreased activation to different impairments presented in ASD.
In summary, studying motion perception using psychophysical methods opens a new vista in autism studies. However, it is important to take into account all the factors mentioned herein, such as matching stimuli methods that account for similar specialized neural pathways, in order to understand better the mechanisms by which different areas of visual input are recruited to mediate motion skills.
Disclosure
The authors report no conflicts of interest in this work.
References
Time C, Methodolo a SD, Lotter A, Wing N, Ishii NR, Bohman NR. Summary of Autism Spectrum Disorder (ASD) Prevalence Studies. 1986;2015. Available from: www.cdc.gov/ncbddd/autism/data.html. Accessed November 22, 2018. | ||
Frith U. Autism: Explaining the Enigma. 2nd ed. Malden, MA: Wiley Blackwell; 2003. | ||
McPartland J, Klin A, Volkmar F. Asperger Syndrome. Assessing and Treating High Functioning Autism Spectrum Disorders. 2nd ed. New York: The Guilford Press; 2014. | ||
Bakroon A, Lakshminarayanan V. Visual function in autism spectrum disorders: a critical review. Clin Exp Optom. 2016;99(4):297–308. | ||
Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J Neurosci. 2013;33(19):8243–8249. | ||
Robertson CE, Thomas C, Kravitz DJ, et al. Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain. 2014;137(Pt 9):2588–2599. | ||
del Viva MM, Igliozzi R, Tancredi R, Brizzolara D. Spatial and motion integration in children with autism. Vision Res. 2006;46(8–9):1242–1252. | ||
Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–1849. | ||
Shah A, Frith U. An islet of ability in autistic children: a research note. J Child Psychol Psychiatry. 1983;24(4):613–620. | ||
Vandenbroucke MW Scholte HS, van Engeland H, Lamme VA, Kemner C. Coherent versus component motion perception in autism spectrum disorder. J Autism Dev Disord. 2008;38(5):941–949. | ||
Plaisted K, Swettenham J, Rees L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. J Child Psychol Psychiatry. 1999;40(5):733–742. | ||
Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms. J Child Psychol Psychiatry. 2003;44(6):904–913. | ||
Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49(22):2705–2739. | ||
Kaiser MD, Shiffrar M. The visual perception of motion by observers with autism spectrum disorders: a review and synthesis. Psychon Bull Rev. 2009;16(5):761–777. | ||
Hadad B, Schwartz S, Maurer D, Lewis TL. Motion perception: a review of developmental changes and the role of early visual experience. Front Integr Neurosci. 2015;9:49. | ||
Van der Hallen R, Evers K, Brewaeys K, Van den Noortgate W, Wagemans J. Global processing takes time: a meta-analysis on local–global visual processing in ASD. Psychol Bull. 2015;141(3):549–573. | ||
Mottron L, Mineau S, Martel G, et al. Lateral glances toward moving stimuli among young children with autism: early regulation of locally oriented perception? Dev Psychopathol. 2007;19(1):23–36. | ||
Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: a “complex” issue. J Cogn Neurosci. 2003;15(2):218–225. | ||
Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychol Sci. 2003;14(2):151–157. | ||
Frith U. Why we need cognitive explanations of autism. Q J Exp Psychol (Hove). 2012;65(11):2073–2092. | ||
Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? J Child Psychol Psychiatry. 1993;34(8):1351–1364. | ||
Happé FG. Studying weak central coherence at low levels: children with autism do not succumb to visual illusions. a research note. J Child Psychol Psychiatry. 1996;37(7):873–877. | ||
Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43(7):1044–1053. | ||
Spencer J, O’Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport. 2000;11(12):2765–2767. | ||
Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. In: Journal of Autism and Developmental Disorders. 2006(36):27–43. | ||
Mottron L, Bouvet L, Bonnel A, et al. Veridical mapping in the development of exceptional autistic abilities. Neurosci Biobehav Rev. 2013;37(2):209–228. | ||
Corcoran R. Book reviews. J psychophysiology. Boston: Hogrefe; 1999;13:57–58. | ||
Hill EL, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):281–289. | ||
Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9(10):1218–1220. | ||
Pellicano E. Individual differences in executive function and central coherence predict developmental changes in theory of mind in autism. Dev Psychol. 2010;46(2):530–544. | ||
Booth R, Charlton R, Hughes C, Happé F. Disentangling weak coherence and executive dysfunction: planning drawing in autism and attention-deficit/hyperactivity disorder. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):387–392. | ||
Baluch F, Itti L. Mechanisms of top-down attention. Trends Neurosci. 2011;34(4):210–224. | ||
Maekawa T, Tobimatsu S, Inada N, et al. Top-down and bottom-up visual information processing of non-social stimuli in high-functioning autism spectrum disorder. Res Autism Spectr Disord. 2011;5(1):201–209. | ||
Loth E, Gómez JC, Happé F. When seeing depends on knowing: adults with Autism Spectrum Conditions show diminished top-down processes in the visual perception of degraded faces but not degraded objects. Neuropsychologia. 2010;48(5):1227–1236. | ||
Chita-Tegmark M. Attention Allocation in ASD: a review and meta-analysis of eye-tracking studies. Rev J Autism Dev Disord. 2016;3(3):209–223. | ||
Rice K, Moriuchi JM, Jones W, Klin A. Parsing heterogeneity in autism spectrum disorders: visual scanning of dynamic social scenes in school-aged children. J Am Acad Child Adolesc Psychiatry. 2012;51(3):238–248. | ||
Speer LL, Cook AE, McMahon WM, Clark E. Face processing in children with autism. Autism. 2007;11(3):265–277. | ||
Johnson KA, Robertson IH, Kelly SP, et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45(10):2234–2245. | ||
Yerys BE, Wallace GL, Sokoloff JL. Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2009;2(6):322–333. | ||
Grubb MA, Behrmann M, Egan R, Minshew NJ, Heeger DJ, Carrasco M. Exogenous spatial attention: evidence for intact functioning in adults with autism spectrum disorder. J Vis. 2013;13(14):9. | ||
Aaen-Stockdale C, Thompson B. Visual motion: from cortex to percept. In: Molotchnikoff S, Rouaot G, editors. IntechOpen;2012. 2012:111–138. | ||
Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128(Pt 10):2430–2441. | ||
Robertson CE, Martin A, Baker CI, Baron-Cohen S. Atypical integration of motion signals in autism spectrum conditions. PLoS One. 2012;7(11):e48173 | ||
Ilg UJ. The role of areas MT and MST in coding of visual motion underlying the execution of smooth pursuit. Vision Res. 2008;48(20):2062–2069. | ||
Johansson G. Visual perception of biological motion and a model for its analysis. Percept Psychophys. 1973;14(2):201–211. | ||
Goodale MA, Westwood DA. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol. 2004;14(2):203–211. | ||
Pelphrey KA, Michelich CR, Viola RJ, Mack PB, Allison T, Mccarthy G. Neurofunctional organization of biological motion perception: an fMRI study of eye, hand, and mouth movements. Cereb Cortex. 2003;156(12):5286. | ||
Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35(6):1167–1175. | ||
Koldewyn K, Whitney D, Rivera SM. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133(Pt 2):599–610. | ||
Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Dev Sci. 2011;14(5):1075–1088. | ||
Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): an fMRI study. Neuropsychologia. 2010;48(6):1644–1651. | ||
Saygin AP, Cook J, Blakemore SJ. Unaffected perceptual thresholds for biological and non-biological form-from-motion perception in autism spectrum conditions. PLoS One. 2010;5(10):e13491–13497. | ||
Schauder KB, Park WJ, Tadin D, Bennetto L. Larger receptive field size as a mechanism underlying atypical motion perception in autism spectrum disorder. Clin Psychol Sci. 2017;5(5):827–842. | ||
Koldewyn K, Jiang YV, Weigelt S, Kanwisher N. Global/local visual processing in autism: not a disability, but a disinclination. J Autism Dev Disord. 2013;43(10):2329–2340. | ||
Hadad BS, Maurer D, Lewis TL. Long trajectory for the development of sensitivity to global and biological motion. Dev Sci. 2011;14(6):1330–1339. | ||
Jaworski JL, Eigsti IM. Low-level visual attention and its relation to joint attention in autism spectrum disorder. Child Neuropsychol. 2017;23(3):316–331. | ||
Gallagher HL, Frith CD. Dissociable neural pathways for the perception and recognition of expressive and instrumental gestures. Neuropsychologia. 2004;42(13):1725–1736. | ||
Tsermentseli S, O’Brien JM, Spencer JV. Comparison of form and motion coherence processing in autistic spectrum disorders and dyslexia. J Autism Dev Disord. 2008;38(7):1201–1210. | ||
Spencer JV, O’Brien JM. Visual form-processing deficits in autism. Perception. 2006;35(8):1047–1055. | ||
Atkinson AP. Impaired recognition of emotions from body movements is associated with elevated motion coherence thresholds in autism spectrum disorders. Neuropsychologia. 2009;47(13):3023–3029. | ||
Manning C, Charman T, Pellicano E. Brief report: coherent motion processing in autism: is dot lifetime an important parameter? J Autism Dev Disord. 2015;45(7):2252–2258. | ||
Wilson HR, Wilkinson F, Asaad W. Concentric orientation summation in human form vision. Vision Res. 1997;37(17):2325–2330. | ||
Yamasaki T, Fujita T, Ogata K, et al. Electrophysiological evidence for selective impairment of optic flow perception in autism spectrum disorder. Res Autism Spectr Disord. 2011;5(1):400–407 | ||
Chakraborty A, Anstice NS, Jacobs RJ, et al. Global motion perception is independent from contrast sensitivity for coherent motion direction discrimination and visual acuity in 4.5-year-old children. Vision Res. 2015;115:83–91. | ||
Bertone A, Faubert J. Demonstrations of decreased sensitivity to complex motion information not enough to propose an autism-specific neural etiology. J Autism Dev Disord. 2006;36(1):55–64. | ||
Sutherland A, Crewther DP. Magnocellular visual evoked potential delay with high autism spectrum quotient yields a neural mechanism for altered perception. Brain. 2010;133(Pt 7):2089–2097. | ||
Milne E, White S, Campbell R, Swettenham J, Hansen P, Ramus F. Motion and form coherence detection in autistic spectrum disorder: relationship to motor control and 2:4 digit ratio. J Autism Dev Disord. 2006;36(2):225–237. | ||
Braddick OJ, O’Brien JMD, Wattam-Bell J, Atkinson J, Turner R. Form and motion coherence activate independent, but not dorsal/ventral segregated, networks in the human brain. Current Biology. 2000;10(12):731–734. | ||
Ajina S, Kennard C, Rees G, Bridge H. Motion area V5/MT+ response to global motion in the absence of V1 resembles early visual cortex. Brain. 2015;138(Pt 1):164–178. | ||
Jones CR, Swettenham J, Charman T, et al. No evidence for a fundamental visual motion processing deficit in adolescents with autism spectrum disorders. Autism Res. 2011;4(5):347–357. | ||
Bellugi U, Sabo H, Vaid J. Spatial Deficits in Children with Williams Syndrome. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. | ||
Talcott JB, Hansen PC, Willis-Owen C, Mckinnell IW, Richardson AJ, Stein JF. Visual magnocellular impairment in adult developmental dyslexics. Neuroophthalmology. 1998;20(4):187–201. | ||
Alaerts K, Swinnen SP, Wenderoth N. Neural processing of biological motion in autism: an investigation of brain activity and effective connectivity. Sci Rep. 2017;7:5612. | ||
Behrmann M, Avidan G, Leonard GL, et al. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44(1):110–129. | ||
Vaina LM, Lemay M, Bienfang DC, Choi AY, Nakayama K. Intact “biological motion” and “structure from motion” perception in a patient with impaired motion mechanisms: a case study. Vis Neurosci. 1990;5(4):353–369. | ||
Nackaerts E, Wagemans J, Helsen W, Swinnen SP, Wenderoth N, Alaerts K. Recognizing biological motion and emotions from point-light displays in autism spectrum disorders. PLoS One. 2012;7(9):e44473. | ||
Hubert B, Wicker B, Moore DG, et al. Brief report: recognition of emotional and non-emotional biological motion in individuals with autistic spectrum disorders. J Autism Dev Disord. 2007;37(7):1386–1392. | ||
Parron C, Da Fonseca D, Santos A, Moore DG, Monfardini E, Deruelle C. Recognition of biological motion in children with autistic spectrum disorders. Autism. 2008;12(3):261–274. | ||
Pavlova MA. Biological motion processing as a hallmark of social cognition. Cereb Cortex. 2012;22(5):981–995. | ||
Centelles L, Assaiante C, Etchegoyhen K, Bouvard M, Schmitz C. Understanding social interaction in children with autism spectrum disorders: does whole-body motion mean anything to them? Encephale. 2012;38(3):232–240. | ||
Davis G, Plaisted-Grant K. Low endogenous neural noise in autism. Autism. 2015;19(3):351–362. | ||
Park WJ, Schauder KB, Zhang R, Bennetto L, Tadin D. High internal noise and poor external noise filtering characterize perception in autism spectrum disorder. Sci Rep. 2017;7(1):17584. | ||
Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc Natl Acad Sci USA. 1998;95(23):13988–13993. | ||
Zaidel A, Goin-Kochel RP, Angelaki DE. Self-motion perception in autism is compromised by visual noise but integrated optimally across multiple senses. Proc Natl Acad Sci USA. 2015;112(20):6461–6466. | ||
Simmons DR, McKay L, McAleer P, Toal E, Robertson A, Pollick FE. Neural noise and autism spectrum disorders. Perception. 2007;61(36):119–120. | ||
Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable evoked responses in autism. Neuron. 2012;75(6):981–991. | ||
Milne E. Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Front Psychol. 2011;2:51. | ||
Butler JS, Molholm S, Andrade GN, Foxe JJ. An examination of the neural unreliability thesis of autism. Cereb Cortex. 2017;27(1):185–200. | ||
Manning C, Tibber MS, Charman T, Dakin SC, Pellicano E. Enhanced integration of motion information in children with autism. J Neurosci. 2015;35(18):6979–6986. | ||
Pardhan S, Gilchrist J, Elliott DB, Beh GK. A comparison of sampling efficiency and internal noise level in young and old subjects. Vision Res. 1996;36(11):1641–1648. | ||
Hernandez LM, Rudie JD, Green SA, Bookheimer S, Dapretto M. Neural signatures of autism spectrum disorders: insights into brain network dynamics. Neuropsychopharmacology. 2015;40(1):171–189. | ||
Ha S, Sohn I-J, Kim N, Sim HJ, Cheon K-A. Characteristics of brains in autism spectrum disorder: structure, function and connectivity across the lifespan. Exp Neurobiol. 2015;24(4):273–284. | ||
Khan S, Michmizos K, Tommerdahl M, et al. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain. 2015;138(Pt 5):1394–1409. | ||
Vilidaite G, Yu M, Baker DH. Internal noise estimates correlate with autistic traits. Autism Res. 2017;10(8):1384–1391. | ||
Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature. 2003;424(6946):312–315. | ||
Tadin D, Silvanto J, Pascual-Leone A, Battelli L. Improved motion perception and impaired spatial suppression following disruption of cortical area MT/V5. J Neurosci. 2011;31(4):1279–1283. | ||
Moutsiana C, Field DT, Harris JP. The neural basis of Centre-Surround interactions in visual motion processing. PLoS One. 2011;6(7):e22902. | ||
Enoch JM, Lakshminarayanan V, Itzhaki A, Schechter G, Marmor M. Layer-by-layer perimetry and haloperidol: implications for schizophrenia and other diseases. In: Belmaker RH, Sandler M, and Dahlstrom A, editors. Progress in Catecholamine Research., Part C: Clinical Aspects. New York: Alan Liss Publishers; 1988:131–136. | ||
Enoch JM, Savage GL, Lakshminarayanan V. Anomalous visual response in Tourette’s syndrome. In: Seventh International Visual Field Symposium, Amsterdam, September 1986. Dordrecht: Martinus Nijhoff Publishers / Dr W. Junk, Publishers; 1987;49:667–672. | ||
Katzner S, Busse L, Carandini M. GABAA inhibition controls response gain in visual cortex. J Neurosci. 2011;31(16):5931–5941. | ||
Schwarzkopf DS, Anderson EJ, de Haas B, White SJ, Rees G. Larger extrastriate population receptive fields in autism spectrum disorders. J Neurosci. 2014;34(7):2713–2724. | ||
Said CP, Egan RD, Minshew NJ, Behrmann M, Heeger DJ. Normal binocular rivalry in autism: implications for the excitation/inhibition imbalance hypothesis. Vision Res. 2013;77:59–66. | ||
Milne E, Buckley D. Contrast sensitivity thresholds in children with autistic spectrum disorder. Br Ir Orthopt J. 2010;7:62–65. | ||
Koh HC, Milne E, Dobkins K. Spatial contrast sensitivity in adolescents with autism spectrum disorders. J Autism Dev Disord. 2010;40(8):978–987. | ||
Dickinson A, Jones M, Milne E. Measuring neural excitation and inhibition in autism: different approaches, different findings and different interpretations. Brain Res. 2016;1648 (Pt A):277–289. | ||
Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32(1):209–224. | ||
Peiker I, Schneider TR, Milne E, et al. Stronger neural modulation by visual motion intensity in autism spectrum disorders. PLoS One. 2015;10(7):e0132531. | ||
Fonville L, Lao-Kaim NP, Giampietro V, et al. Evaluation of enhanced attention to local detail in anorexia nervosa using the embedded figures test; an fMRI study. PLoS ONE. 2013;8(5): e63964. | ||
Freitag CM, Konrad C, Häberlen M, et al. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46(5):1480–1494. | ||
Chen Y, Norton DJ, McBain R, Gold J, Frazier JA, Coyle JT. Enhanced local processing of dynamic visual information in autism: evidence from speed discrimination. Neuropsychologia. 2012;50(5):733–739. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

