Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Distribution of Vernix Caseosa and Associated Factors Among Newborns Delivered at Adama Comprehensive Specialized Hospital Medical College, Ethiopia, in 2022: Cross-Sectional Study
Authors Mesfin S , Afework M , Bikila D , Tessema A, Sento M
Received 28 September 2022
Accepted for publication 9 December 2022
Published 28 December 2022 Volume 2022:15 Pages 2903—2914
DOI https://doi.org/10.2147/CCID.S387720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Seble Mesfin,1 Mekbeb Afework,2 Dereje Bikila,3 Alemayehu Tessema,4 Midekso Sento1
1Department of Biomedical Science, Adama Comprehensive Specialized Hospital Medical College, Adama, Eastern Ethiopia; 2Department of Anatomy, School of Medicine, College of Health science, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Nursing, Arsi University College of Health Science, Assela, Ethiopia; 4Department of Pediatrics, Adama Comprehensive specialized Hospital Medical College, Adama, Eastern Ethiopia
Correspondence: Seble Mesfin; Mekbeb Afework, Tel +983501666 ; +911411285, Email [email protected]; [email protected]
Background: Vernix caseosa is a complex proteolipid material synthesized partly by fetal sebaceous glands during the last trimester of pregnancy. Understanding the structure and function of newborn skin is crucial for determining optimal thermal support, infection control, and skin moisturization. So far, in Ethiopia, there is no research done related to the distribution of vernix caseosa and associated factors on newborn skin. Doing such research could give awareness about factors associated with the distribution of vernix caseosa on newborns’ skin and to take necessary protective measures for those that may be affected.
Objective: The objective of this study is to assess the distribution of vernix caseosa and associated factors among newborns delivered at Adama Comprehensive Specialized Hospital Medical College from November to December 1, 2021.
Methodology: Hospital-based cross-sectional study design was conducted from November to December 1, 2021 at Adama Comprehensive Specialized Hospital Medical College (ACSHMC). Four hundred twenty-two eligible newborns were selected by a systematic sampling method. Data were collected by four data collectors by using a pretested questionnaire. The distribution of vernix caseosa on the different regions of the neonate was assessed, by exposing their whole body for a minute. Data entry was done by EPI data version 4.6 and analyzed by using SPSS version 25. A logistic regression of P-value of < 0.25 during bivariate and P < 0.05 during multivariate analysis at a 95% confidence level was considered statistically significant.
Results: Out of 422 study participants 231 (54.7%) with 95% CI (49.8, 59.8) babies had vernix caseosa. Being primiparous with (AOR = 1.9, PV = 0.013, 95% CI: 1.141, 2.92), being multiparous with (AOR = 1.98, PV = 0.04, CI: 1.29, 3.225), being females with (AOR = 2.1, PV = 0.001, CI: 1.39, 3.18), being preterm with (AOR = 2.98, PV = 0.036, 95% CI: 1.08, 10.72), non-diseased newborns with (AOR = 1.6, PV = 0.046, 95% CI: 1.07, 2.7) were identified as associated factors for the distribution of vernix caseosa on the newborn skin.
Conclusion: This study showed that the distribution of vernix caseosa on the skin of the newborns was associated with parity, sex, gestational age, and absence of disease.
Keywords: epidermal barrier, stratum corneum, vernix caseosa
Introduction
Background
Vernix caseosa (VC) is a naturally occurring, complex, lipid-rich substance produced by fetal sebaceous glands and corneocytes, which covers the skin surface of the fetus during the third trimester of pregnancy.1–5 It develops in a cephalo-caudal pattern and is the consequence of an ordered sequence of epithelial maturation.6
Vernix plays on several roles on the biochemical adaptation of the newborn (NB) skin and some other visceral systems to the extrauterine environment.3 Vernix performs several overlapping biological activities, including moisturization, anti-infective, antioxidant, wound healing, and waterproofing. Detached vernix, when swallowed by the fetus, had potential effect on gut maturation.7,8 It also serves as a mechanical barrier and vaginal lubricant, facilitating parturition and protecting the womb and fetus from vertical transmission of any microbes. The color of vernix can reflect intrauterine problems.9,10 VC is composed of water-containing corneocytes (80%), lipids (10%) and (10%) proteins.11,12
The amount and distribution of VC differ by gestational age, delivery mode, gender, birth weight, and APGAR score of newborn.13 In a study in India on VC in neonatal adaptation, the average coverage of vernix on NB skin was 38% and found that the scalp, back, and folds had the most frequent coverage. The chest, lower limb, and the entire body of the infant having vernix are the least frequent coverage. The vernix distribution had a greater coverage on the back than on the chest and only 8.6% of NB had over the entire body.14,15 In another study on the prevalence of VC in Spain, 49.2% of the NB had vernix, with a higher prevalence observed in NB who was white, female, high birth weight, high Apgar score and born at term.13 In some settings, vernix was removed from NB right after they are born. According to the evidence, there may be a value in reconsidering this practice.16
So far, in Ethiopia, there is no research done related to the distribution of VC and associated factors on NB skin. Doing such research could reveal factors associated with the distribution of VC on the NB skin enabling the healthcare provider will give special attention to NB those born without vernix.
Methods and Materials
Study Area and Period
The study was conducted in ACSHMC, which is located 99km southeast of Addis Ababa (capital city of Ethiopia), Oromia region, and east Shoa zone. ACSHMC serves more than 540,000 people each year. According to the health management information system (HMIS) of this hospital, the department of obstetrics and gynecology is the one that covers the most maternity and neonatal services. On average, 720 mothers give birth in this department each month regardless of its outcome, and 9054 women are served in this ward each year. The study was conducted prospectively from November 1 to December 1, 2021 by using a hospital-based cross-sectional study design which was carried out on NB delivered at ACSHMC from November 1 to December 1, 2021.
Source Population
All newborns delivered at ACSHMC.
Study Population
All newborns delivered at ACSHMC in the obstetrics department during the study period.
Sample Size Determinations
The sample size was determined by using a single population proportion formula Using a 50% (p = 0.5) variability and 95% confidence interval with ±5% precision, and 10% non-respondents 422 was found to be the required sample size.
Sampling Procedures
ACSHMC was selected purposely, and a systematic sampling approach was used to pick 422 eligible neonates from the obstetrics department. Based on a previous HMIS report, the average number of NB delivered during the data collection period was approximated to get the study participants. In one month, an average of 720 NB was delivered, with 300 delivered through caesarian section (C/S) and 420 through spontaneous vaginal delivery (SVD). Proportional allocation was used for both caesarian section (C/S) and vaginal deliveries. The data were collected for the one-month duration. To get the sampling interval, the total number of NB delivered in one month is divided by the sample size given as a Kth interval of 2. The first NB was chosen at random or by lottery, and every second neonate was allocated to the study.
Inclusion and Exclusion Criteria
Inclusion Criteria
All newborns delivered at ACSHMC during the study period.
Exclusion Criteria
Stillbirth and newborns enrolled (wrapped) with cloth were excluded from the study.
Data Collection Tool and Procedure
Data were collected through observation and interviews with the mother by using a pretested questionnaire, which was adopted after reviewing different literatures. The tool generally contains three parts involving the distribution of VC, maternal factors, and neonatal factors. The distribution of VC was assessed by observing the NB skin before enrolling with cloth by exposing the whole body for a minute immediately after delivery. The body mapping technique (Figure 1) was used to assess the specific regions of NB skin covered by vernix, which is adopted from previously done research.17 According to the body mapping technique, the body of newborn is delineated into seven regions those are head, arm (upper limb), chest, back, inguinal, sacrum, and leg (lower limb). The distribution of VC was assessed by observing the NB skin for the presence or absence of vernix immediately after delivery. Then, if the NB had vernix, the specified region/location is identified by using a body map to assess the regional distribution of VC. This procedure was done by two trained midwifery nurses. At the same time, the sex, gestational age, birth weight, lanugo hair, and APGAR score were recorded during the examination of NB. Maternal variables (age, parity, and mode of delivery, history of medical illness, medication use, and dietary supplement during pregnancy) were collected by interviewing the mothers who had given childbirth. This interview was done by another two trained nurses. The NB is considered as diseased if the NB does not maintain an extrauterine environment and needs additional supportive or curative care and is transferred into the neonatal intensive care unit (ICU).
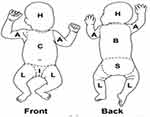 |
Figure 1 Body map showing different regions of the newborn’s body as used for assessing the distribution of vernix caseosa in each region.14 The distribution of VC in each region was identified by body map. Abbreviations: H, head; A, arm; C, chest; L, leg; I, inguinal; B, back; S, sacrum. |
Study Variable
Dependent Variable
Distribution of vernix caseosa.
Independent Variable
Maternal Factors
Age, Mode of delivery, Parity, Medical illness, Medication, Nutrition or dietary supplements during pregnancy (I, e iron, folic acid) and Toxic substance usage (smoking, alcohol, and chewing chat).
Neonatal Factors
Sex, Gestational age, Birth weight, APGAR score, Presence of illness, Developmental abnormality, and Lanugo hair.
Data Analysis and Interpretation
After each data collection, all information was checked for completeness. Then, the data were entered into EPI data version 4.6 and exported into SPSS (Statistical software) version 25 for analysis. Frequencies and percentages were used for the descriptive analysis of data. Bivariate logistic regression was done and variables with P-value less than 0.25 were exported to multivariate logistic regression. The odds ratio and 95% confidence level were used to evaluate the strength of association between the dependent and independent variables. Finally, variables with P-value less than 0.05 from multivariate logistic regression are taken as a statistically significant association with the distribution of VC. Data were presented by using both descriptive and inferential statistics. Graphs, texts, and tables are then used to present the results.
Data Quality Control
The English version tools were used for the data collection. A 5% pretest was done before data collection at Rift Valley Hospital, and adjustment was made for unclear questionnaire. The data collectors were trained for two days about the objective, purpose, methods, technique, how to approach study participants and the issue of confidentiality. Easy, short, and clear questionnaire paper is used. Close supervision was done by the principal investigator. The completeness of data was reviewed daily after data collection to maintain the consistency of data quality, and any difficulties encountered during data collection were addressed appropriately. Finally, the collected data were verified for completeness, consistency, and clarity by supervisors and the principal investigator.
Ethical Consideration
Ethical clearance was obtained from Addis Ababa University College of the Health Science Department of Human Anatomy and the institutional review board (IRB) research committee with ethical approval reference number (አናት 160/2021). After obtaining a supportive official letter, it was sent to ACSHMC to receive permission for data collection. Official permission is received from the ACSHMC. Written consent was obtained from the mother for guaranteeing their agreement of participation or refusal after the pregnant woman was informed about the objective, purpose, and outcome of the study.
This was conducted prior to data collection at the time when a pregnant mother was admitted to the delivery room for bearing a child. Confidentiality of information and privacy of the study participants was maintained during the examination of the newborn and throughout all information. Each participant was informed about the voluntariness, risk, and benefits of participation. This study is in line with the declaration of Helsinki.
Operational Definition
Distribution of Vernix Caseosa
The presence of fatty substance fixed on the NB skin (ie, on the head, arm, chest, back, inguinal, sacrum, and leg regions).
Observation of Newborns
Inspection of the presence of VC on the NB skin, while the newborn is exposed all over the body immediately after delivery before enrolling with cloth.
Toxic Substance Usage
Having any intake of alcohol, smoking, and khat chewing during pregnancy that affects the NB.
Spontaneous Vaginal Delivery
Procedure conducted to allow birth throughout the vaginal canal.
Caesarian Section
Abdominal incision performed on a pregnant mother to bring out the fetus.
Primiparous
Mother gave birth only for the first time which is a candidate for this study.
Multiparous
Mother who gave birth to one, two, or three infants before the current birth.
Grand Multiparous
A mother who has delivered five or more infants.
Diseased Newborn
NB who has an illness or any deviation from normal health after delivery and transferred to the neonatal intensive care unit.
Dietary Supplement
A woman who had an intake of folic acid, iron, etc., during pregnancy at antenatal care (ANC) follow-up.
Dissemination of Result
The finding of this study finally will be presented and submitted to Addis Ababa University College of Health Science School Medicine Department of Anatomy. Then, it will be disseminated to the Addis Ababa research unit and ACSHMC. Finally, the finding of the study will be disseminated through publication for further utilization.
Results
Regional Distribution of Vernix Caseosa on the Skin of Newborns
In this study, among 422 study participants, 231 (54.7%) with 95% CI (49.8, 59.8) babies had VC, whereas 191 (45.3%) babies did not. VC was found distributed on the inguinal, arm, back, sacrum, head, leg, and chest regions within decreasing order of frequency. The inguinal 185 (21.1%) and arm 184 (21%) regions were the most frequently observed regions covered by VC. The next frequently observed regions are back 151 (17.3%), sacrum 130 (14.9%), and head 103 (11.8%). The leg 57 (6.5%) and chest 42 (4.8%) were the least frequently observed regions. Twenty-three (2.6%) NB had VC over the entire body (Figure 2).
 |
Figure 2 Regional distribution of vernix caseosa among newborns delivered at ACSHMC, Oromia regional state, Ethiopia, November to December 1, 2021 (n = 875). |
Maternal Related Characteristics
Characteristics of the Mode of Delivery, Age, and Parity
Regarding mode of delivery, out of 246 babies born by spontaneous vaginal delivery (SVD), 125 (54.1%) babies were born with VC while 121 (63.3%) babies were without VC. Likewise, out of 176 NB delivered by caesarian section (CS) 106 (45.9%) babies were born with VC, and 70 (36.6%) babies were without VC. In this study, the majority of the study participants (308 NB) were born to women in the age group of less than 29 years old. Among this group, 159 (68.8%) of NB were born with VC, and 149 (78%) of NB were born without VC. According to parity, out of 167 primiparous women, 84 (36.4%) mothers gave NB with VC while 83 (43.5%) mothers gave NB without VC. Among the babies born from grand parous mothers, 85 (36.8%) babies had VC, and 45 (23.6%) babies had no VC (Table 1).
 |
Table 1 Characteristics of Mode of Delivery, Age, and Parity Among Newborns Delivered at ACSHMC, Oromia Regional State Ethiopia, November to December 1, 2021 (n = 244) |
Characteristics of Medical Illness, Medications, Toxic Substances, and Dietary Supplements
Among neonates whose mothers had no medical conditions, 188 (81.4%) of them had VC whereas 159 (83.2%) did not. Babies delivered to moms with no history of medication use included 169 (84.4%) with VC and 164 (84.9%) without VC. Regarding toxic substances user, only seven (3%) of NB had and 6(3.1%) of NB had no VC. Mothers who do not utilize toxic substance gave birth to 185 kids (96.9%) without VC and 234 babies (97%) with the condition. Regarding NB delivered from mothers who had taken dietary supplements 213 (92.2%) babies had VC and 172 (90.1%) babies had no VC (Table 2).
Fetal Related Characteristics
Characteristics of Sex, Gestational Age, Birth Weight, and Disease
In terms of NB babies’ sex, 194 (46%) of study participants were female, and above half 228 (54%) of NB were male. Out of 194 (46%) female NB, 124 (53.7%) babies were born with VC, whereas 70 (36.6%) babies were born without VC. Likewise referring to gestational age, among 306 term babies, one hundred seventy-nine (77.5%) babies had and 127 (66.5%) babies had no VC. One hundred sixty-eight (72.7%) of NB with VC, whereas 127 (66.5%) of NB without VC were among normal birth weight babies. Regarding NB disease, 286 of the participants were non-diseased. Out of them, 172 (74.5%) babies had VC, whereas 77 (40.3%) babies had no VC (Table 3).
 |
Table 3 Characteristics of Sex, Gestational Age, Birth Weight, and Disease Among Newborns Delivered at ACSHMC, Oromia Regional State, Adama, Ethiopia, November to December 1, 2021 (n = 244) |
Characteristics of APGAR SCORE at 1st and 5th Minutes, Developmental Anomaly, and Lanugo Hair
Out of 231 (54.7%) of NB with VC, 15 (6.5%), 110 (47.6%), and 106 (45.9%), NB scored APGAR of 6, 7, and 8 at the 1st minute, respectively. On the other hand, out of 191 (45.3%) newborns without VC 48 (25.1%), 75 (39.3%), and 68 (35.6%) NB scored APGAR 6, 7, and 8, respectively, at the 1st minute. Two (0.9%), 12 (5.2%), 28 (12.1%), and 189 (81.8%) neonates with VC simultaneously received APGAR scores of 6, 7, 8, and 9 at fifth minute. Among NB without VC 191 (45.3%), 10 (5.2%), 31 (6.2%), 19 (9.9%), and 131 (68.6%) of them scored APGAR of 6, 7, 8, and 9. VC was found in 8(3.5%) of NB with the developmental anomaly and 47 (20.3%) of NB with lanugo hair. Moreover, it was found in 223 (96.5%) of newborns had not a developmental anomaly and 184 (79.8%) of NB without lanugo hair (Table 4).
Associated Factors for the Distributions of Vernix Caseosa
The distribution of VC was found in 231 (54.7%) of NB. Maternal and neonatal variables were included in the study. A bivariate logistic regression analysis was done for each independent variable to select candidate variables for multivariate logistic regression. Variables such as age, mode of delivery, parity, sex, gestational age, birth weight, and disease had an association with the distribution of VC with a p-value less than 0.25. Variables that have an association with the bivariate analysis of (P-value < 0.25) were identified as a candidate for multivariate analysis. However, the result of the multivariate analysis revealed that parity, sex, gestational age, and absence of disease were associated with the distribution of the VC with a p-value less than 0.05.
According to the analysis of the study, parity was identified as the associated factor for the distribution of VC. The odds of having VC in newborns delivered from a primiparous mother were 1.9 times (AOR = 1.9, PV = 0.013, 95% CI = 1.141, 2.92) higher than NB delivered from a grand parous mother. Similarly, the odds of having VC in NB delivered from a multiparous mother were 1.9 times (AOR = 1.98, PV = 0.04, 95% CI: 1.29, 3.225) higher than in NB delivered from a grand parous mother.
This study also indicated that being female babies is significantly associated with the distribution of VC. The odds of having VC in females are 2 times (AOR = 2.1, PV = 0.001, CI: 1.39, 3.18) higher than in males. The odds of developing VC in preterm were almost 3 times (AOR = 2.98, PV = 0.036, 95% CI: 1.08, 10.72) higher than in post-term. Lastly, the study showed that the distribution of VC is significantly associated with the presence or absence of disease in the NB. It indicated that the odds of having VC in non-diseased NB are 1.6 times (AOR = 1.6, PV = 0.046, 95% CI: 1.07, 2.7) higher than in diseased babies (Table 5).
Discussion
The result of this study showed that out of 422 study participants 231 (54.7%) with 95% CI (49.8, 59.8) had VC, whereas 191 (45.3%) had no VC. This is somewhat higher than the study done in Spain and India, in which the prevalence was reported, respectively, as 49.2%13 and 38%.15
The discrepancy between the current study with the Spain’s study could be due to a difference in the time of examination. In this study, NB was examined immediately after delivery for a minute, while in the study from Spain NB was examined during their first three days of life. Once the NB is enrolled with cloth, the vernix could be removed from the skin surface of the NB. Vernix is also expected to dry and shed spontaneously within 24 hours of birth.18,19
In the latter study which was conducted on VC in neonatal adaptation in India, the discrepancy could be due to the methods they used to classify the vernix retained and vernix removed group. In their study, a small amount of vernix that was wiped with a cotton towel was classified as non-vernix NB.13 In the current study, even the presence of a small amount of vernix was included as the presence of VC. As the result, the time of examination and methods of classifying the presence versus absence of vernix might have contributed to the difference in the prevalence of VC.
Furthermore, in this study, there was a regional difference in the distribution of VC. The inguinal (21.7%) and arm (21.6%) regions were the most frequently observed regions that are covered by VC. Similarly, the back, sacrum, and head regions had frequently observed as 17.7%, 15.3%, and 12.1%, respectively. The least frequent coverage was found in the leg (6.7%) and chest (4.9%) regions. This finding is in agreement with the studies carried out in Spain by Vischer et al, which found that VC coverage was significantly higher on the back than on the chest.15 This might be explained by the pattern of sebaceous gland distribution in the body. Sebaceous glands are most numerous on the face and scalp, least over the thorax and abdomen, and few on the extremities.20 Additionally, this might be due to the progress of vernix in development different from region to region which begins to coat the skin from head to toe and back to front during the third trimester.21
The distribution of VC on the newborn skin is associated with the parity, sex, gestational age, and absence of disease of the NB. Parity was identified as an associated factor for the distribution of VC. Babies born from a primiparous and multiparous mother had higher odds of developing VC than grand parous mothers with (AOR = 1.9, AOR = 1.98) respectively. This finding is consistent with the study done in Brazil.22 This could be due to the risk of low birth weight being higher in grand parous women than multiparous women. Primiparous women have a lower probability of having low birth weight babies compared to grand parous women.23 This might be due to repeated pregnancies that will damage the uterine blood vessel walls. This condition will affect the nutrition of the fetus in subsequent pregnancies causing fetal growth disorders leading to low birth weight.24 According to scholars, very low birth-weight infants lack VC and insufficient formation of the stratum corneum.25
Moreover, the current study identified that female NB had higher odds (AOR = 2.1) of developing VC than male NB. This finding is in agreement with the study done in Prague, Turkey and India.26,28,29 This is due to the activities of cutaneous barrier formation, distribution of VC, and sebaceous glands being controlled by sex hormones and gender-related. Radka Mikova reported that differential temporal dynamics in the skin development in boys and girls are controlled by steroid hormones.26 Other previous studies in fetal rats had documented that the formation of the cutaneous barrier is enhanced by estrogen and delayed by testosterone.30 As a result, the observed sex-related differences are most likely associated with the activities of sebaceous glands in the skin of the NB.
In this study, gestational age was identified as an associated factor for the distribution of VC. Babies born prematurely had higher odds (AOR = 2) of developing VC than post-term. This finding is in agreement with the study conducted in India.23 In contrast, a study carried out in the Hamilton and USA found that preterm NB lacks VC.30,31 However, preterm NB is generally covered with a generous amount of VC, approaching the term it remains in the skin creases and hair.32 This could be due to the interaction of pulmonary surfactant and VC resulting in a progressive increment in phospholipid concentration. During the third trimester, pulmonary surfactant (phospholipid) released into the amniotic fluid resulted in increased vernix detachment as the gestational age reaches term and post-term.33
In this study, non-diseased NB had higher odds (AOR = 1.6) of having VC than diseased babies. This finding might be consistent with the study done in Sweden that confirmed VC had an innate antimicrobial effect.34 This could be again, the skin of full-term NB is well prepared to immediately protect the infant from water loss, light, irritants, and infectious agents, as well as to provide innate immunity, tactile discrimination, thermal regulation, and acid mantle formation.35,36 Stratum corneum is intact impermiable layer which is found on the outer layer of the epidermis. So it compares with vernix to indicate how much the vernix is important for the newborn to adapt extrauterine environment.2
On the other hand, this study showed that distribution of VC had no association with the NB birth weight and APGAR score. This finding disagrees with the Spain's finding concluded that prevalence of VC is higher in high birth weight NB and high APGAR score.13 This study revealed that mode of delivery had no association with the distribution of VC. This finding is inconsistent with the study conducted in India showed that the prevalence VC higher in the NB delivered through caesarian section (C/S) than vaginal delivery.15
Strength and Limitations
This study used primary data gathered via observation and interview. The scarcity of available works of literature related to the distribution of VC in Africa and Ethiopia makes the investigation of VC challenging. Lack of alternative available techniques for investigating the regional distribution of VC other than the body mapping makes the finding a little bit unclear.
Conclusions
The current study showed that the distribution of VC is associated with maternal and neonatal variables. Regarding the maternal variables, the parity of the mother showed a significant association. Primiparity and multiparity showed higher odds of developing VC than grand parous. Likewise, from the neonatal factors, sex, gestational age, and absence of disease in NB showed significant association. On the other hand, variables such as age, mode of delivery, medical illness, medication, toxic substance use, birth weight, APGAR score, developmental anomaly, and lanugo hair are not associated with the distribution of VC.
Acknowledgments
We are very much grateful to study participants and data collectors for their principal involvement in this study. The health care teams in the department of obstetrics and gynecology and ACSHMC administrations are also acknowledged for their persistent support and encouragement during data collection.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Dunglison R. Medical lexicon: a dictionary of medical science; 1846.
2. Singh G, Archana G. Unraveling the mystery of vernix caseosa. Indian J Dermatol. 2008;53(2):54. doi:10.4103/0019-5154.41645
3. Tollin M, Bergsson G, Kai-Larsen Y, et al. Vernix caseosa as a multi-component defence system based on polypeptides, lipids, and their interactions. Cell Mol Life Sci CMLS. 2005;62(19–20):2390–2399. doi:10.1007/s00018-005-5260-7
4. Bamalan OA, Menezes RG. Vernix Caseosa. In: StatPearls [Internet]. StatPearls Publishing; 2021.
5. Liu S, Zhang H, Duan E. Epidermal development in mammals: key regulators, signals from beneath, and stem cells. Int J Mol Sci. 2013;14(6):10869–10895. doi:10.3390/ijms140610869
6. Team HJ. Vernix Caseosa Composition, Biology, Functions & Clinical Implications. Health Jade; 2019.
7. Haubrich KA. Role of Vernix caseosa in the neonate: potential application in the adult population. AACN Adv Crit Care. 2003;14(4):457–464.
8. Hoath SB, Pickens W. The biology of vernix. In: Neonatal Skin Struct Funct.
9. Jha AK, Baliga S, Kumar HH, Rangnekar A, Baliga BS. Is there a preventive role for vernix caseosa?: an in vitro study. J Clin Diagn Res JCDR. 2015;9(11):SC13. doi:10.7860/JCDR/2015/14740.6784
10. Mallory S, Bree AF, Chern P. Illustrated Manual of Pediatric Dermatology: Diagnosis and Management. Taylor & Francis; 2005.
11. Vavrušová A, Vrkoslav V, Plavka R, Bosáková Z, Cvačka J. Analysis of (O-acyl) alpha- and omega-hydroxy fatty acids in vernix caseosa by high-performance liquid chromatography-Orbitrap mass spectrometry. Anal Bioanal Chem. 2020;420:2291–2302.
12. Hoeger PH, Schreiner V, Klaassen IA, Enzmann CC, Friedrichs K, Bleck O. Epidermal barrier lipids in human vernix caseosa: corresponding ceramide pattern in vernix and fetal skin. Br J Dermatol. 2002;146(2):194–201. doi:10.1046/j.1365-2133.2002.04584.x
13. Monteagudo B, Labandeira J, León-Muiños E, et al. Influence of neonatal and maternal factors on the prevalence of vernix caseosa. Actas Dermo Sifiliográficas Engl Ed. 2011;102(9):726–729. doi:10.1016/j.ad.2011.01.006
14. Archana G Clinical study of surface distribution of vernix caseosa in the newborn; 2008.
15. Visscher MO, Narendran V, Pickens WL, et al. Vernix caseosa in neonatal adaptation. J Perinatol. 2005;25(7):440–446. doi:10.1038/sj.jp.7211305
16. Fikree FF, Ali TS, Durocher JM, Rahbar MH. Newborn care practices in low socioeconomic settlements of Karachi, Pakistan. Soc Sci Med. 2005;60(5):911–921. doi:10.1016/j.socscimed.2004.06.034
17. Keenan E, Karmakar CK, Palaniswami M. The effects of asymmetric volume conductor modeling on non-invasive fetal ECG extraction. Physiol Meas. 2018;39(10):105013. doi:10.1088/1361-6579/aae305
18. Colwell A. To bathe or not to bathe: the neonatal question. Neonatal Netw. 2015;34(4):216–219. doi:10.1891/0730-0832.34.4.216
19. World Health Organization. WHO Recommendations on Postnatal Care of the Mother and Newborn. World Health Organization; 2014.
20. Fukuchi M, Tsukagoshi R, Sakurai S, et al. Ectopic sebaceous glands in the esophagus: endoscopic findings over three years. Case Rep Gastroenterol. 2012;6(1):217–222. doi:10.1159/000338651
21. Visscher MO, Adam R, Brink S, Odio M. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33(3):271–280. doi:10.1016/j.clindermatol.2014.12.003
22. Krüger EMM, Sinkos F, Uhry JF, et al. Dermatoses in the early neonatal period: their association with neonatal, obstetric and demographic variables. Rev Paul Pediatr. 2019;37:297–304. doi:10.1590/1984-0462/;2019;37;3;00012
23. Bekele A, Seyoum G, Tesfaye K, Fantahun Y. The effects of maternal age and parity on the birth weight of newborns among mothers with singleton pregnancies and at term deliveries. Ethiop J Health Dev. 2019;33(3):5.
24. Afifah I, Ariningtyas N, Djalilah G, Anas M. Maternal age and parity associated with low birth weight infants. Gac Médica Caracas. 2021;3:129.
25. Neri I, Ravaioli GM, Faldella G, Capretti MG, Arcuri S, Patrizi A. Chlorhexidine-induced chemical burns in very low birth weight infants. J Pediatr. 2017;191:262–265. doi:10.1016/j.jpeds.2017.08.002
26. Míková R, Vrkoslav V, Hanus R, et al. Newborn boys and girls differ in the lipid composition of vernix caseosa. PLoS One. 2014;9(6):e99173. doi:10.1371/journal.pone.0099173
27. Ö E, Ü G, Mollamahmutoğlu L, Gönül M. Skin findings in newborns and their relationship with maternal factors: observational research. Ann Dermatol. 2013;25(1):1–4. doi:10.5021/ad.2013.25.1.1
28. Haveri FTTS, Inamadar AC. A Cross-sectional prospective study of cutaneous lesions in newborn. ISRN Dermatol. 2014;20(2014):1–8. doi:10.1155/2014/360590
29. Hanley K, Rassner U, Jiang Y, et al. Hormonal basis for the gender difference in epidermal barrier formation in the fetal rat. Acceleration by estrogen and delay by testosterone. J Clin Invest. 1996;97(11):2576–2584. doi:10.1172/JCI118706
30. Tansirikongkol A, Visscher MO, Wickett RR. Water-handling properties of vernix caseosa and a synthetic analogue. J Cosmet Sci. 2007;58(6):651.
31. Pickens WL, Warner RR, Boissy YL, Boissy RE, Hoath SB. Characterization of vernix caseosa: water content, morphology, and elemental analysis. J Invest Dermatol. 2000;115(5):875–881. doi:10.1046/j.1523-1747.2000.00134.x
32. Tansirikongkol A. Development of a synthetic vernix equivalent, and its water handling and barrier protective properties in comparison with vernix caseosa. University of Cincinnati; 2006.
33. Adair CD, Sanchez-Ramos L, McDyer DL, Gaudier FL, Del Valle GO, Delke I. Predicting fetal lung maturity by visual assessment of amniotic fluid turbidity: comparison with fluorescence polarization assay. South Med J. 1995;88(10):1031–1033. doi:10.1097/00007611-199510000-00006
34. Marchini G, Lindow S, Brismar H, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. 2002;147(6):1127–1134. doi:10.1046/j.1365-2133.2002.05014.x
35. Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31(1):5–19. doi:10.1038/emm.1999.2
36. Hoath SB, Narendran V, Visscher MO. The biology and role of vernix. Newborn Infant Nurs Rev. 2001;1(1):53–58. doi:10.1053/nbin.2001.21839
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



