Back to Journals » Cancer Management and Research » Volume 11
Distribution of circulating tumor cell phenotype in early cervical cancer
Authors Pan L, Yan G, Chen W, Sun L, Wang J, Yang J
Received 15 December 2018
Accepted for publication 18 March 2019
Published 17 June 2019 Volume 2019:11 Pages 5531—5536
DOI https://doi.org/10.2147/CMAR.S198391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Li Pan, Gaoshu Yan, Wenli Chen, Lei Sun, Jichuan Wang, Jialin Yang
Radiotherapy Department, Sichuan Cancer Hospital & Institute, School of Medicine, University of Electronic Science and Technology of China, Chengdu, 610041, People’s Republic of China
Background and objective: Circulating tumor cells (CTCs) can be classified into three phenotypes based on epithelial-to-mesenchymal transition (EMT) markers, including epithelial CTCs, mesenchymal CTCs, and mixed phenotypic CTCs. This study is aimed to analyze the correlation between CTC phenotypes and the clinicopathological features of patients with early cervical cancer.
Methods: Peripheral blood samples were obtained from 90 patients with early cervical cancer. CTCs were isolated and classified. The correlations of CTC counts and CTC phenotypes with clinicopathological features of patients were analyzed.
Results: The positivity rate for CTCs in patients with stage I-IIA cervical cancer was 90%. An increased CTC number was observed in patients with FIGO stage II, pelvic lymph node metastasis, and lymphovascular involvement. There were 38.89% epithelial CTCs, 23.33% mesenchymal CTCs, and 14.44% mixed phenotypic CTCs, Mesenchymal CTCs were more common in patients with FIGO stage II, pelvic lymph node metastasis, lymphovascular involvement, and deep stromal invasion.
Conclusion: CTCs with mesenchymal phenotypes are closely related to pelvic lymph node metastasis and lymphatic vascular invasion in stage I-IIA cervical cancer. Detection of circulating tumor cell phenotypes is helpful for the early diagnosis of cervical cancer micro-metastasis and for the assessment of disease status.
Keywords: circulating tumor cell, phenotype, cervical cancer
Introduction
Cervical cancer is a common malignant tumor in women. Its incidence is second only to breast cancer, and it has a tendency to occur in young women, thereby seriously threatening their reproductive health and quality of life.1,2 The early detection of cervical cancer is remarkably significant for achieving radical cure. The treatment of patients with advanced cervical cancer is poor, and the recurrence and metastasis rates are high.3 Therefore, the diagnosis, treatment, curative effect, and prognosis of cervical cancer have attracted worldwide attention. The recurrence and metastasis of cervical cancer can seriously perplex clinicians.
Recently, several researchers have reported that circulating tumor cells (CTCs) play an important role in metastasis.4–6 They penetrate the basement membrane into the peripheral circulatory system and eventually grow in distant organs to form metastases.7 CTCs are an important hub between primary and metastatic tumors in the process of tumor metastasis and function in the induction of metastatic tumor and preservation of biological characteristics similar to those of primary tumors.8 CTCs can further clarify the characteristics of the primary lesion to develop a more effective individualized treatment.9 There have been significant achievements in the treatment of various malignant tumors, especially breast cancer. It is now clear that the number of CTCs in breast cancer patients is related to the prognosis of patients. Cristofanilli et al10 reported that more than 5 CTCs for every 7.5 milliliter blood is a prognostic factor for metastatic breast cancer. In addition, CTCs are associated with the progression and poor prognosis of many other solid tumors including lung cancer,11,12 colorectal cancer,13,14 and pancreatic cancer.15,16 The detection of CTCs is non-invasive and more acceptable to patients. Moreover, CTC detection may facilitate an earlier and more effective diagnosis of tumor recurrence and metastasis than computed tomography and positron emission tomography.17–20 Therefore, the study of cervical cancer CTCs may play a significant role in clinical diagnosis and treatment.
There are different phenotypes of CTC in peripheral blood. The main phenotypes include epithelial CTCs, mesenchymal CTCs, and mixed epithelial–mesenchymal CTCs.21 Epithelial-to-mesenchymal transition (EMT) is an important characteristic of cancer with high metastatic ability.22,23 It is believed that EMT activation facilitates the generation of CTCs and improves their survival in the bloodstream.21,24,25 During EMT, the epithelial tumor cells can became more aggressive and migratory; these cells can easily enter distant organs by invading blood vessels or lymphatic vessels.
The purpose of this study was to analyze the correlation between CTC phenotypes and the clinicopathological features of patients with stage I-II A (International Federation of gynecology and obstetrics, FIGO) cervical cancer.
Material and methods
Patients
A total of 90 primary cervical cancer patients with stage I-IIA who underwent radical surgical resection in the Sichuan Cancer Hospital & Institute & School of Medicine, University of Electronic Science and Technology of China, between 2015 and 2017 were selected. All patients were confirmed by preoperative cervical biopsy pathology and postoperative pathological diagnosis. The clinicopathological features of patients are listed in Table 1. Twenty healthy controls were enrolled. The study is approved by the University of Electronic Science and Technology of China according to the Declaration of Helsinki (6th revision, 2008), and all subjects were asked to sign an informed consent form.
 | Table 1 CTC number and clinicopathological features in early cervix cancer patients |
CTC detection
A total of 5 mL of peripheral blood was collected from each patient in the morning before surgery. CTCs were isolated via optimized CanPatrol™ CTC enrichment technique implemented using CD45 antibody.26,27 First, erythrocytes were removed by carrying out red blood cell lysis and CD45+ leukocytes were depleted using magnetic bead separation method. CTCs were enriched by passing them through calibrated membrane filters having 8-μm-diameter pores; CTCs were identified and characterized by RNA-in situ hybridization (ISH). Based on branched DNA (bDNA) signal amplification technology, CTCs were classified using EMT markers into three subpopulations including epithelial CTCs (CTCs with the epithelial marker EpCAM or CK8), mesenchymal CTCs (CTCs with the mesenchymal marker vimentin or TWIST), and mixed phenotypic CTCs (CTCs with both epithelial and mesenchymal markers) as previously described.28 Cellsearch® detection was also performed.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM, USA). The comparison between CTCs and clinicopathological features were analyzed using Student’s t-test or one-way ANOVA test. The correlation between CTCs and clinicopathological features was examined using Pearson’s chi-square test. A P-value of <0.05 was considered to be statistically significant.
Results
CTCs in cervical cancer patients
The positive rate of CTCs in patients with stage I-IIA cervical cancer was 90% (80 out of 90 samples). The CTC count per 5 mL of peripheral blood in patients with positive CTCs ranged from 1 to 39 (median 10).
Correlation between CTCs and clinicopathological features of cervical cancer
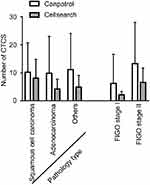
We assessed the correlation between clinicopathological features and CTC count (Table 1). There were no significant differences in CTC count between patients of different ages or those with different pathological types (P>0.05). An increased CTC count was observed in patients with FIGO stage II, pelvic lymph node metastasis and lymphovascular involvement (P<0.01). There was no relationship between CTC count and patients with deep stromal invasion or parametrial extension (P>0.05). CTCs could not be detected in healthy controls. The rate of CTC detection using Cellsearch® was lower than that of optimized CanPatrol™ CTC enrichment technique (Figure 1).
Correlation between CTC phenotypes and clinicopathological features of cervical cancer
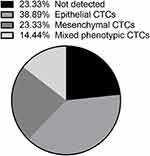
RNA-in situ hybridization led to the identification of EMT markers in an epithelial CTC (red dots), a mesenchymal CTC (green dots), and a mixed phenotypic CTC (red and green dots) (Figure 2). Thus, we classified CTC phenotypes of patients into three subpopulations based on EMT markers, including epithelial CTCs, mesenchymal CTCs, and mixed phenotypic CTCs. As shown in Figure 3, there were 38.89% epithelial CTCs, 23.33% mesenchymal CTCs, 14.44% mixed phenotypic CTCs, and 23.33% CTCs without detectable phenotypes. The correlation between CTC phenotypes and clinicopathological features was also analyzed (Table 2). There was no significant relationship between CTC phenotypes and age, pathology type, or parametrial extension (P>0.05). However, mesenchymal CTCs were more common in patients with FIGO stage II, pelvic lymph node metastasis, lymphovascular involvement, and deep stromal invasion.
 | Table 2 CTC phenotypes and clinicopathological features in early cervix cancer patients |
Discussion
At present, the diagnosis of recurrence and metastasis of cervical cancer is dependent on pathological tissues. As an emerging molecular marker, CTCs facilitate individualized treatment of tumor and act as guiding tools for early clinical diagnosis as well as the evaluation of treatment efficacy and prognosis.29 Moreover, the presence of peripheral blood CTCs, as a noninvasive indicator, will enable clinicians to design an individualized postoperative adjuvant therapy for patients with cervical cancer for reducing recurrence and metastasis in and improving the over survival and quality of life of cancer patients.30 A poor prognosis has been suggested in patients with early breast cancer who have ≥5 CTCs per 30 mL of blood.10 When the CTC count in the peripheral blood of patients with nonmetastatic colorectal cancer is ≥1/7.5 mL, it greater risk of recurrence and metastasis and worse prognosis have been indicated.31 This study revealed that patients with early cervix cancer who had higher CTC count in their peripheral blood also were at a higher risk of reaching FIGO stage II or experiencing pelvic lymph node metastasis, lymphovascular invasion, and deep stromal invasion.
Lecharpentier32 found that none of the CTCs extracted from six patients with non-small cell lung cancer metastasis expressed only epithelial markers but presented with both mesenchymal and epithelial markers. Moreover, in three other cases, only mesenchymal CTCs were expressed; although this study confirmed the existence of EMT, it did not primarily focus on tumor tissues that express only epithelial markers. Mego et al33 examined the expression of EMT markers such as TWIST1, SNAIL1, SLUG, ZEB1, and FOXC2 in breast cancer CTCs and showed that the chemotherapy did not change the EMT markers present in CTCs. With the development of CTC detection, RNA-in situ hybridization technology is gradually applied in the clinic. This technique makes use of both epithelial and mesenchymal markers to specifically identify the EMT phenotypes of CTCs. In 2013, Yu et al34 used RNA-in situ hybridization to study the effect of epithelial–mesenchymal ratio in patients with breast cancer and found that higher the proportion of epithelial phenotype, higher is the effectiveness of this technique. On the contrary, poor prognosis was suggested in associated with a high proportion of mesenchymal phenotype. We classified the CTC phenotypes of patients into three subpopulations based on EMT markers, including epithelial CTCs, mesenchymal CTCs, and mixed phenotypic CTCs. There were 38.89% epithelial CTCs, 23.33% mesenchymal CTCs, and 14.44% mixed phenotypic CTCs. There were 23.33% CTCs without detectable phenotypes, which might be owing to the concealing of markers. Other markers should be considered in the future. The mesenchymal CTCs were more common in patients with FIGO stage II, pelvic lymph node metastasis, lymphovascular involvement, and deep stromal invasion. We speculated that tumor cells may enter peripheral blood by invading lymphatic vessels, blood vessels, and lymphatic vessels as positive CTCs. The infiltration of the uterus may penetrate the deep muscle layer and result in the infiltration of surrounding interstitial tissues, but the tumor cells do not invade the lymphatic and blood vessels and do not enter the peripheral blood circulation. Postoperative pathology suggests that pelvic lymph node metastasis and lymphatic vessel infarction are associated with a high risk of recurrence and metastasis. It is recommended to perform adjuvant radiotherapy and chemotherapy in clinical practice, and CTCs are associated with pelvic lymph node metastasis and lymphatic vascular invasion. Cervical cancer patients with CTCs are at a higher risk of distant metastasis. For these patients, it is recommended to further improve the implementation of relevnt examinations to exclude metastasis and consider strengthening the intensity of chemotherapy. Thus, monitoring of CTCs is helpful for the diagnosis and evaluation of the prognosis of cervical cancer. Owing to the nature of cervical cancer that is usually accompanied by HPV infection, a definitive study should be performed to understand the HPV genome, RNA, or protein sequences in the CTCs of selected HPV16/18-positive patients in the future.
In summary, CTCs with mesenchymal phenotypes are closely related to pelvic lymph node metastasis and lymphatic vascular invasion in stage I-IIA cervical cancer. Detection of circulating tumor cell phenotypes is helpful for the early diagnosis of cervical cancer micro-metastasis and assessment of disease status. In the future, the clinical value of CTC detection in cervical cancer needs to be estimated via a large-scale prospective randomized study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Watson M, Benard V, Thomas C, Brayboy A, Paisano R, Becker T. Cervical cancer incidence and mortality among American Indian and Alaska Native women, 1999-2009. Am J Public Health. 2014;104(Suppl 3):S415–S422. doi:10.2105/AJPH.2014.302167
2. Obel J, Souares Y, Hoy D, et al. A systematic review of cervical cancer incidence and mortality in the Pacific Region. Asian Pac J Cancer Prev. 2014;15(21):9433–9437.
3. Benard VB, Thomas CC, King J, et al. Vital signs: cervical cancer incidence, mortality, and screening - United States, 2007-2012. MMWR Morb Mortal Wkly Rep. 2014;63(44):1004–1009.
4. Li TT, Liu H, Yu J, Shi GY, Zhao LY, Li GX. Prognostic and predictive blood biomarkers in gastric cancer and the potential application of circulating tumor cells. World J Gastroenterol. 2018;24(21):2236–2246. doi:10.3748/wjg.v24.i21.2236
5. Chistiakov DA, Chekhonin VP. Circulating tumor cells and their advances to promote cancer metastasis and relapse, with focus on glioblastoma multiforme. Exp Mol Pathol. 2018;105(2):166–174. doi:10.1016/j.yexmp.2018.07.007
6. Burz C, Pop VV, Buiga R, et al. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget. 2018;9(36):24561–24571. doi:10.18632/oncotarget.25337
7. Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–456. doi:10.1038/nrc1370
8. Alixpanabières C, Pantel K. Liquid biopsy in cancer patients: advances in capturing viable CTCs for functional studies using the EPISPOT assay. Expert Rev Mol Diagn. 2015;15(11):1411–1417. doi:10.1586/14737159.2015.1091729
9. Krawczyk N, Fehm T, Banys-Paluchowski M, Janni W, Schramm A. Liquid biopsy in metastasized breast cancer as basis for treatment decisions. Oncol Res Treat. 2016;39(3):112–116. doi:10.1159/000444605
10. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. doi:10.1056/NEJMoa040766
11. Bu XM, Xu FF, Ma J, Jiang B. The expression of circulating tumor cells in peripheral blood of patients with non-small cell lung cancer and its detection. J Biol Regul Homeost Agents. 2018;32(4):843–849.
12. Bracht JWP, Mayo-de-Las-Casas C, Berenguer J, Karachaliou N, Rosell R. The present and future of liquid biopsies in non-small cell lung cancer: combining four biosources for diagnosis, prognosis, prediction, and disease monitoring. Curr Oncol Rep. 2018;20(9):70. doi:10.1007/s11912-018-0720-z
13. Wang W, Wan L, Wu S, et al. Mesenchymal marker and LGR5 expression levels in circulating tumor cells correlate with colorectal cancer prognosis. Cell Oncol (Dordr). 2018;41(5):495–504. doi:10.1007/s13402-018-0386-4
14. Hashimoto M, Tanaka F, Yoneda K, et al. The clinical value of circulating tumour cells (CTCs) in patients undergoing pulmonary metastasectomy for metastatic colorectal cancer. J Thorac Dis. 2018;10(3):1569–1577. doi:10.21037/jtd.2018.03.05
15. Liu X, Li C, Li J, et al. Detection of CTCs in portal vein was associated with intrahepatic metastases and prognosis in patients with advanced pancreatic cancer. J Cancer. 2018;9(11):2038–2045. doi:10.7150/jca.23989
16. Court CM, Ankeny JS, Sho S, et al. Circulating tumor cells predict occult metastatic disease and prognosis in pancreatic cancer. Ann Surg Oncol. 2018;25(4):1000–1008. doi:10.1245/s10434-017-6290-8
17. Willecke-Hochmuth R, Pachmann K, Drevs J. Treatment of advanced solid tumours with NSAIDs: correlation of quantitative monitoring of circulating tumour cells and positron emission tomography-computed tomography imaging. Oncol Lett. 2016;12(3):1711–1716. doi:10.3892/ol.2016.4878
18. Ma B, King AD, Leung L, et al. Identifying an early indicator of drug efficacy in patients with metastatic colorectal cancer-a prospective evaluation of circulating tumor cells, 18F-fluorodeoxyglucose positron-emission tomography and the RECIST criteria. Ann Oncol. 2017;28(7):1576–1581. doi:10.1093/annonc/mdx149
19. Bayarri-Lara CI, de Miguel Perez D, Cueto Ladron de Guevara A, et al. Association of circulating tumour cells with early relapse and 18F-fluorodeoxyglucose positron emission tomography uptake in resected non-small-cell lung cancers. Eur J Cardiothorac Surg. 2017;52(1):55–62. doi:10.1093/ejcts/ezx049
20. Abrahamsson J, Aaltonen K, Engilbertsson H, et al. Circulating tumor cells in patients with advanced urothelial carcinoma of the bladder: association with tumor stage, lymph node metastases, FDG-PET findings, and survival. Urol Oncol. 2017;35(10):606e609–606e616. doi:10.1016/j.urolonc.2017.05.021
21. Han D, Chen K, Che J, Hang J, Li H. Detection of epithelial-mesenchymal transition status of circulating tumor cells in patients with esophageal squamous carcinoma. Biomed Res Int. 2018;2018:7610154. doi:10.1155/2018/7610154
22. Zeng YE, Yao XH, Yan ZP, Liu JX, Liu XH. Potential signaling pathway involved in sphingosine-1-phosphate-induced epithelial-mesenchymal transition in cancer. Oncol Lett. 2016;12(1):379–382. doi:10.3892/ol.2016.4661
23. Zeng Y, Yao X, Chen L, et al. Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/syndecan-1/TGF-beta autocrine loop. Oncotarget. 2016;7(39):63324–63337. doi:10.18632/oncotarget.11450
24. Yin LC, Luo ZC, Gao YX, Li Y, Peng Q, Gao Y. Twist expression in circulating hepatocellular carcinoma cells predicts metastasis and prognoses. Biomed Res Int. 2018;2018:3789613. doi:10.1155/2018/3789613
25. Qi LN, Xiang BD, Wu FX, et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res. 2018;78(16):4731–4744. doi:10.1158/0008-5472.CAN-17-2459
26. Liu YK, Hu BS, Li ZL, He X, Li Y, Lu LG. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol Int. 2016;10(4):640–646. doi:10.1007/s12072-016-9732-7
27. Jin XR, Zhu LY, Qian K, et al. Circulating tumor cells in early stage lung adenocarcinoma: a case series report and literature review. Oncotarget. 2017;8(14):23130–23141. doi:10.18632/oncotarget.15506
28. Ou H, Huang Y, Xiang L, et al. Circulating tumor cell phenotype indicates poor survival and recurrence after surgery for hepatocellular carcinoma. Dig Dis Sci. 2018. doi:10.1007/s10620-018-5124-2
29. King JD, Casavant BP, Lang JM. Rapid translation of circulating tumor cell biomarkers into clinical practice: technology development, clinical needs and regulatory requirements. Lab Chip. 2014;14(1):24–31. doi:10.1039/c3lc50741f
30. Alixpanabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor dna as liquid biopsy. Cancer Discov. 2016;6(5):479–491. doi:10.1158/2159-8290.CD-15-1483
31. Bork U, Rahbari NN, Scholch S, et al. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer. 2015;112(8):1306–1313. doi:10.1038/bjc.2015.88
32. Lecharpentier A, Vielh P, Perezmoreno P, Planchard D, Soria JC, Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011;105(9):1338–1341. doi:10.1038/bjc.2011.405
33. Mego M, Mani SA, Lee BN, et al. Expression of epithelial-mesenchymal transition-inducing transcription factors in primary breast cancer: the effect of neoadjuvant therapy. Int J Cancer. 2012;130(4):808–816. doi:10.1002/ijc.26037
34. Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi:10.1126/science.1228522
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.