Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Differential Diagnosis Of Multiple-System Atrophy With Parkinson’s Disease By External Anal- And Urethral-Sphincter Electromyography
Authors Qiu F, Wang K, Li T, Song D, Wang Z, Zhang H, Liu J, Ren M, Qi X
Received 2 June 2019
Accepted for publication 2 October 2019
Published 5 November 2019 Volume 2019:15 Pages 3061—3067
DOI https://doi.org/10.2147/NDT.S218073
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Feng Qiu,1,* Kunyu Wang,1,* Tingting Li,2 Dandan Song,3 Zhiwei Wang,1 Hailing Zhang,4 Jianguo Liu,1 Ming Ren,5 Xiaokun Qi1
1Department of Neurology, Sixth Medical Center of Chinese PLA General Hospital, Beijing, 100048, People’s Republic of China; 2Department of Gastroenterology, Second Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 3Department of Medicine, Beijing Northern Hospital of China North Industries Group Corporation, Beijing 100089, People’s Republic of China; 4Department of Neurology, Changhai Hospital, Shanghai 200433, People’s Republic of China; 5Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, Beihang University, Beijing 100191, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaokun Qi
Department of Neurology, Sixth Medical Center of PLA General Hospital, 6 Fucheng Road, Haidian District, Beijing 100048, People’s Republic of China
Tel +86 139 1079 7081
Email [email protected]
Ming Ren
Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, Beihang University, 37 Xueyuan Road, Haidian District, Beijing 100191, People’s Republic of China
Tel +86 139 1079 7081
Email [email protected]
Background: The differential diagnosis of Parkinson’s disease (PD) with multiple-system atrophy (MSA) is difficult because of their similarity in symptoms and signs. The objective of this study was to investigate the value of external anal-sphincter electromyography (EAS-EMG) and urethral-sphincter electromyography (US-EMG) in differentiating MSA from PD.
Methods: A total of 201 patients, — 101 MSA and 100 PD — were recruited in this study. Average duration and amplitude of motor unit potentials (MUPs), percentage of polyphasic MUPs, amplitude during strong contractions, and recruitment patterns during maximal voluntary contractions were recorded and analyzed to assess diagnostic efficiency of EAS-EMG and US-EMG for MSA.
Results: Significant differences in average MUP duration and recruitment patterns during maximal voluntary contractions were found between patients with MSA and patients with PD using both EAS-EMG (P<0.001, P<0.001) and US-EMG (P<0.001, P<0.001). The percentage of polyphasic MUPs and amplitude during strong contractions showed significant differences in MSA and PD using only EAS-EMG (P<0.001, P=0.005). Cutoff points for average MUP duration in EAS-EMG and US-EMG for differential diagnosis of MSA with PD were 10.9 and 11.1 milliseconds, respectively. With average MUP duration of EAS-EMG and US-EMG being applied jointly, sensitivity and specificity in distinguishing MSA from PD were 83.2% and 71.8%, respectively.
Conclusion: EAS-EMG and US-EMG were sensitive and specific methods for the diagnosis and differential diagnosis of MSA, and the combination of both would improve the diagnostic rate of MSA compared to only one method being used.
Keywords: differential diagnosis, MSA, PD, EAS-EMG, US-EMG
Introduction
Multiple-system atrophy (MSA) is a rare sporadic and progressive neurodegenerative disorder of the central nervous system characterized by various combinations of parkinsonism, cerebellar ataxia, autonomic dysfunction, and corticospinal symptoms,1,2 and the underlying mechanisms of the disorder are still poorly understood. The disorder is divided into two categories: MSA with predominant parkinsonism (MSA-P) and MSA with cerebellar features (MSA-C).3 The designation of MSA-P or MSA-C is determined by the dominant feature at the time of evaluation, which may change with time.
Possible, probable, and definite MSA are three diagnostic levels for the disorder. Possible or probable MSA is diagnosed based mainly on clinical presentation and image manifestations.4 Additionally, probable MSA is characterized by poor levodopa-responsive parkinsonism. A definite diagnosis of MSA relies on pathological findings of high-density of α-synuclein-positive glial cytoplasmic inclusions associated with degenerative changes in striatonigral and olivopontocerebellar pathways. Progressive atrophy in the cerebral cortex, brain stem, and cerebellum can be detected on brain magnetic resonance imaging.5 The existence of relatively specific imaging findings, such as hot cross–bun sign of the pons, putaminal slit syndrome, and hypointensity signal of posterolateral putamen on T2-weighted images, increases the likelihood of a diagnosis of MSA in long-standing cases.6,7 In the early phase of the disease, patients with MSA often present with symptoms and signs of autonomic nervous system dysfunction, but without findings on imaging.
It has been suggested that both MSA and Parkinson’s disease (PD) have similar autonomic nerve damage; however, the underlying pathophysiological mechanisms are different.8,9 One of the pathological hallmarks of MSA is the degeneration of Onuf’s nucleus,10–12 and the pathogenesis of MSA is related to the loss of motor neurons in Onuf’s nucleus.13,14 External anal-sphincter electromyography (EAS-EMG) and urethral sphincter (US) EMG can detect the denervation and reinnervation of the sphincter muscle in motor unit potentials (MUPs), reflecting basically the loss of neurons. Therefore, EAS-EMG may have diagnostic value for MSA.15 Mean duration of MUPs in EAS-EMG is most sensitive in the differential diagnosis of MSA with other parkinsonism-related diseases, such that it may be taken as a supportive diagnostic method for MSA.16 US-EMG may help monitor the function of the US in maintaining urinary continence,17,18 and US-EMG has been proposed as routine testing for patients with suspected MSA in some hospitals.5,19 Although several studies have reported potential diagnostic value of EAS-EMG and US-EMG for MSA, the findings of those studies were limited by small samples.5,10,19 Further study of the differentiation of MSA from PD by using EAS-EMG and US-EMG is needed. To investigate whether EAS-EMG and US-EMG are helpful for the diagnosis of MSA and the differentiation of MSA from PD, we recruited 201 patients: 101 with MSA and 100 with PD. Relevant parameters of EAS-EMG and US-EMG were recorded and analyzed.
Methods
Participants
Study patients with suspected MSA or PD were recruited from in- and outpatients and interviewed by two experienced neurologists in the Department of Neurology at Sixth Medical Center of Chinese PLA General Hospital from March 2015 to March 2018. This study was approved by the ethics committees of the Sixth Medical Center of Chinese PLA General Hospital and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Criteria
Patient sex was not confined, and clinical diagnoses of MSA and PD were in strict accordance with the diagnostic criteria of MSA by Gilman et al and the UK PD Society Brain Bank Clinical Diagnostic Criteria by Daniel and Lees,4,20 respectively. Exclusion criteria were consistent with those for MSA diagnostic criteria by Gilman et al and the UK PD Society Brain Bank Clinical Diagnostic Criteria by Daniel et al; patients with mental disorders that may compromise the evaluation of MSA; uncooperative patients due to disturbance of consciousness and severe cognitive impairment; cardiac, hepatic, renal, or multiple-organ insufficiency; use of heart pacemakers; deafness where unable to communicate or cooperate with the examination; inability to cooperate with EMG testing due to local infection around the anus or urethra; and sphincter-dysfunction syndrome caused by combined sacral disorders.
Study Design
Enrolled patients had electrophysiological examinations done by technicians blind to diagnoses and were followed up by neurologists (also blind to diagnoses) every 6 months. During follow-up of at least 1 year, diagnoses were confirmed by three experienced neurologists based on diagnostic criteria and EMG results. Patients with probable MSA and PD would be considered final for diagnoses and follow-up. Patients with possible MSA would be followed up until reaching final diagnosis.
EAS-EMG And US-EMG Examination
A Viking Quest 4 EMG/evoked-potential instrument (Nicolet, Madison, Wisconsin, USA) with standard settings (filters 20–10 k Hz) was used. The scanning speed for mild contractions was 5 ms/D, with sensitivity 100 V/D, while that for strong contractions was 200 ms/D, with sensitivity 0.5 V/D. EMG was performed as described previously.5 For EAS-EMG, patients were in the left lateral recumbent position. Hips were kept apart, and at the back of the left outer anus (about 4:30) — 10 mm in from the mucocutaneous junction — a concentric needle electrode was inserted into shallow strata of the subcortex of the external anal sphincter.10 Electrical activities of the anal sphincter while relaxed (mimicking defecation) and with mild and strong contractions (mimicking discontinuous defecation) were recorded. For US-EMG, examinations were performed with patients in the horizontal position. For male patients, a concentric needle electrode of length 50 mm and diameter 0.45 mm was inserted into the middle of the anus and bulbospongiosus. For female patients, a concentric needle electrode 75 mm in length and 0.65 mm in diameter was inserted 5 mm beside the external urethral orifice.21 Sensations of resistance and myoelectricity were the signs of insertion into the US. Electrical activity of the US in holding urine phase and urinating phase were recorded.
Statistical Analysis
Statistical analyses were completed with SPSS 22.0. Age, course of disease, sex composition, average MUP duration and amplitude, percentage of polyphasic MUPs, and amplitude and recruitment patterns during maximal contraction were analyzed. Kolmogorov–Smirnov one-sample test and Levene's test were applied for normality-of-distribution and equality-of-variance tests, respectively. Data with normal distribution are expressed as means ± SD, and were analyzed using independent-sample t-tests when in equal variance. For data abnormally distributed, medians and IQRs (first–third quartiles) are used to express central tendency and dispersion, separately. Mann–Whitney U tests were used to compare two groups with abnormal distributions or data showing heterogeneity of variance. Pearson's χ2 was used for comparing proportions. Indicators of sensitivity, specificity, area under the receiver-operating characteristic (ROC) curve (AUC), 95% CI, and Youden index were calculated to assess the diagnostic efficiency of EAS-EMG and US-EMG for MSA according to drawn ROC curves. Finally, the largest Youden-index value for the electrophysiological index was found to be critical, thereby being the cutoff point. P<0.05 was regarded as significant.
Results
Sample Characteristics
Two groups of patients, — 101 (52 male) with MSA, median age 56 (52–64.5) years, median course 2.4 (1.5–4) years, and 100 (65 male) with PD, mean age 64.7±10 years, median course 3 (1.075–5) years — were involved in the study. All cases had various levels of dysurination and dysdefecation. Sex composition and course of disease were not statistically different between the two groups, while a significant difference in age was found (Table 1).
 |
Table 1 Sex, Age, And Course Of Disease Between MSA And PD Groups |
EAS-EMG Results Of Patients With MSA And PD
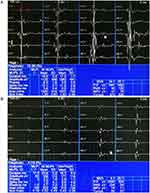
A total of 99 cases with MSA and 100 with PD underwent EAS-EMG examinations. Two cases were excluded, because of perianal abscess and hemorrhoids. There were significant differences in average MUP duration, percentage of polyphasic MUPs, amplitude during strong contractions, and ratio of simple phase and simple-mix phase between MSA and PD by EAS-EMG. EAS-EMG in the MSA group mainly showed prolonged average MUP duration, increased percentage of polyphasic MUPs, lower amplitude during strong contractions (Figure 1A), and abnormal recruitment patterns during maximal voluntary contractions compared with the PD group. There was no statistically significant difference for average MUP amplitude between the two groups (Table 2).
 |
Table 2 EAS-EMG Results Between MSA And PD Groups |
US-EMG Results Of Patients With MSA And PD
In sum, 81 cases with MSA and 96 with PD underwent US-EMG examinations. A total of 24 cases were not examined with US-EMG, due to patient refusal or noncooperation. The average MUP duration and ratio of simple phase and simple-mix phase of MSA showed significant differences compared with those of PD. US-EMG of MSA group mainly showed prolonged average MUP duration (Figure 1B) and abnormal recruitment patterns during maximal voluntary contractions compared with the PD group. There were no statistical differences in average MUP amplitude, percentage of polyphasic MUPs, or amplitude during strong contractions between the two groups (Table 3).
 |
Table 3 US-EMG Results Between MSA And PD Groups |
Comparison Of Indices Obtained In EAS-EMG And US-EMG Of Patients With MSA
A total of 99 cases with MSA underwent EAS-EMG examinations, while 81 cases with MSA underwent US-EMG examinations. EAS-EMG showed more obvious changes in average MUP amplitude, percentage of polyphasic MUPs, and amplitude during strong contractions than US-EMG for patients with MSA. There was no statistical difference in other variables (Table 4).
 |
Table 4 Comparison Of Indices Obtained In EAS-EMG And US-EMG Of MSA Group |
Cutoff Points Obtained In EAS-EMG And US-EMG
Cutoff points of average MUP duration and percentage of polyphasic MUPs for the differential diagnosis of MSA with PD in EAS-EMG were 10.9 ms and 40.9%, respectively (Table 5 and Figure 2A). The cutoff point of average MUP duration for distinguishing MSA from PD in US-EMG was 11.1 ms (Table 5 and Figure 2B). With the average MUP duration of EAS-EMG and US-EMG being applied jointly using parallel testing, the sensitivity and specificity for distinguishing MSA from PD were 83.2% and 71.8%, respectively.
 |
Table 5 Cutoff Points, Area Under ROC curve, Sensitivity, Specificity, And 95% CIs Of Some Parameters In EAS-EMG And US-EMG For Differentiating MSA From PD |
Discussion
We found that EAS-EMG and US-EMG possessed practical value for the diagnosis and differential diagnosis of patients with MSA. Due to the much lower prevalence of MSA than PD, ranging from one in 50,000 to one in 20,000,22,23 it took us >3 years to recruit enough patients for identifying the cutoff point for a diagnosis of MSA. No other studies on this topic with a large sample of MSA patients have been published. Sensitivity and specificity of the average MUP duration for the differential diagnosis of MSA with PD were calculated at the optimal cutoff point. EAS-EMG was more sensitive than US-EMG in terms of diagnosis, and showed more significant changes in most of the indices than US-EMG, except for average MUP amplitude. The combined application of both EAS-EMG and US-EMG would improve the diagnostic rate of MSA.
Autonomic failure, such as in urination and defecation disorders, is the prominent clinical feature and primary reference point in diagnostic criteria for MSA,4 and early and severe autonomic nervous dysfunction are poor prognostic factors.3 However, a definite diagnosis can only be made with postmortem histopathological study.4 Onuf’s nucleus in the anterior horn cells of the sacral cord can innervate the external sphincter muscle of the anus and urethra, and can be influenced by neuronal cell loss in MSA.24,25 Neurogenic injury can be observed in EAS-EMG and US-EMG containing prolonged average MUP duration, increased percentage of polyphasic MUPs, and occurrence of spontaneous activity, and some may discover satellite potential.26
Compared with PD, the parameters of EAS-EMG in patients with MSA, including average MUP duration, percentage of polyphasic MUPs, amplitude during strong contractions and abnormal recruitment patterns during maximal voluntary contractions, showed significant differences in this study. Meanwhile, there were significant differences for average MUP duration and abnormal recruitment patterns in US-EMG between MSA and PD. Specifically, average MUP duration showed more significant difference between MSA and PD in EAS-EMG and US-EMG (both P<0.001). Therefore, the authors of this study would consider that EAS-EMG and US-EMG could be of value for the differential diagnosis of MSA with PD, especially in the early course of the disease.
It is worth noting that the sensitivity and specificity for differentiating MSA from PD in EAS-EMG were 75.8% and 83.0% when average MUP duration was prolonged >10.9 ms, while these in US-EMG were 63.0% and 86.5% when average MUP duration was prolonged >11.1 ms, and an AUC for both of >0.7 indicates they have high value in clinical application. The sensitivity for average MUP duration in distinguishing MSA from PD would improve to 83.2% if EAS-EMG and US-EMG are used in combination.
There were significant differences in average MUP amplitude, percentage of polyphasic MUPs, and amplitude during strong contractions between EAS-EMG and US-EMG in patients with MSA. It is difficult to obtain data for percentage of polyphasic MUPs in US-EMG during a change in location, because the anatomic location of the US is specific. Our previous research with a small sample showed that average MUP amplitude on US-EMG was more sensitive than on EAS-EMG for the diagnosis of MSA.19 In this study, it was evaluated with a larger sample. Our results showed a higher average MUP amplitude on EAS-EMG than on US-EMG.
The current study had some limitations. The association of the natural course of MSA with the dynamics of EAS-EMG or US-EMG was not explored, which may provide more information for the diagnosis of MSA. Also, we compared only patients with MSA and patients with PD, and studies with even larger samples may still be needed in future.
Conclusion
Our results confirmed that EAS-EMG and US-EMG were highly sensitive and specific methods for the diagnosis of MSA and differential diagnosis of MSA with PD, and that the combination of both studies is helpful in improving the sensitivity of MSA diagnosis compared to only one method being used. The two electrophysiological methods could serve as a supplement and also substitution for each other in cases where either is not available.
Acknowledgments
This study received grants from the Capital Foundation of Medical Development and Research (2011-5031-02) and Beijing Municipal Science and Technology Commission (Z151100004015017).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vodusek DB. How to diagnose MSA early: the role of sphincter EMG. J Neural Transm. 2005;112(12):1657–1668. doi:10.1007/s00702-005-0377-2
2. Sun Z, Jia D, Shi Y, et al. Prediction of orthostatic hypotension in multiple system atrophy and Parkinson disease. Sci Rep. 2016;6:21649. doi:10.1038/srep21649
3. Sako W, Abe T, Murakami N, et al. Imaging-based differential diagnosis between multiple system atrophy and Parkinson’s disease. J Neurol Sci. 2016;368:104–108. doi:10.1016/j.jns.2016.06.061
4. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi:10.1212/01.wnl.0000324625.00404.15
5. Qiu F, Liu JG, Li LP, Song DD, Yao W, Qi XK. Comparative study on diagnostic significance of urethral sphincter versus external anal sphincter electromyography in patients with multiple system atrophy. Zhonghua Yi Xue Za Zhi. 2013;93(25):1958–1961.
6. Sitburana O, Ondo WG. Brain magnetic resonance imaging (MRI) in parkinsonian disorders. Park Relat Disord. 2009;15(3):165–174. doi:10.1016/j.parkreldis.2008.04.033
7. Horimoto Y, Aiba I, Yasuda T, et al. Longitudinal MRI study of multiple system atrophy - when do the findings appear, and what is the course? J Neurol. 2002;249(7):847–854. doi:10.1007/s00415-002-0734-0
8. Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Park Relat Disord. 2011;17(10):724–729. doi:10.1016/j.parkreldis.2011.04.016
9. Roy S, Jaryal AK, Srivastava AK, Deepak KK. Cardiovagal baroreflex sensitivity in Parkinson’s disease and multiple-system atrophy. J Clin Neurol. 2016;12(2):218–223. doi:10.3988/jcn.2016.12.2.218
10. Tison F, Arne P, Sourgen C, Chrysostome V, Yeklef F. The value of external anal sphincter electromyography for the diagnosis of multiple system atrophy. Mov Disord. 2000;15(6):1148–1157.
11. Paviour DC, Williams D, Fowler CJ, Quinn NP, Lees AJ. Is sphincter electromyography a helpful investigation in the diagnosis of multiple system atrophy? A retrospective study with pathological diagnosis. Mov Disord. 2005;20(11):1425–1430. doi:10.1002/mds.20584
12. Yamamoto T, Sakakibara R, Uchiyama T, et al. When is Onuf’s nucleus involved in multiple system atrophy? A sphincter electromyography study. J Neurol Neurosurg Psychiatry. 2005;76(12):1645–1648. doi:10.1136/jnnp.2004.061036
13. Winge K, Jennum P, Lokkegaard A, Werdelin L. Anal sphincter EMG in the diagnosis of parkinsonian syndromes. Acta Neurol Scand. 2010;121(3):198–203. doi:10.1111/j.1600-0404.2009.01169.x
14. Caruso S, Panella MM, Cianci S, et al. TOT does not affect the urethral sphincter innervation: a pilot study. Int Urogynecol J. 2011;22(6):739–742. doi:10.1007/s00192-011-1364-9
15. Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Sphincter EMG as a diagnostic tool in autonomic disorders. Clin Auton Res. 2009;19(1):20–31. doi:10.1007/s10286-008-0489-5
16. Yamamoto T, Sakakibara R, Uchiyama T, et al. Receiver operating characteristic analysis of sphincter electromyography for parkinsonian syndrome. Neurourol Urodyn. 2012;31(7):1128–1134. doi:10.1002/nau.22208
17. Steward JE, Clemons JD, Zaszczurynski PJ, Butler RS, Damaser MS, Jiang HH. Quantitative evaluation of electrodes for external urethral sphincter electromyography during bladder-to-urethral guarding reflex. World J Urol. 2010;28(3):365–371. doi:10.1007/s00345-009-0463-4
18. Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170(3):1027–1031. doi:10.1097/01.ju.0000079492.09716.43
19. Qi XK, Li LP, Yao W, Liu JG, Qiu F. The diagnostic value of urethral sphincter electromyography in patients with multiple system atrophy. Zhonghua Nei Ke Za Zhi. 2012;51(12):975–977.
20. Daniel SE, Lees AJ. Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl. 1993;39:165–172.
21. Hansen J, Borau A, Rodríguez A, Vidal J, Sinkjaer T, Rijkhoff NJ. Urethral sphincter EMG as event detector for Neurogenic detrusor overactivity. IEEE Trans Biomed Eng. 2007;54(7):1212–1219. doi:10.1109/TBME.2007.890739
22. Mathevosian S, Singh SR, Pu CY. Multiple system atrophy mistaken for autoimmune cerebellar degeneration. Am J Med. 2016;129(9):e183–e184. doi:10.1016/j.amjmed.2016.03.034
23. Carrozzino D, Morberg BM, Siri C, Pezzoli G, Bech P. Evaluating psychiatric symptoms in Parkinson’s Disease by a clinimetric analysis of the Hopkins Symptom Checklist (SCL-90-R). Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:131–137. doi:10.1016/j.pnpbp.2017.10.024
24. Iwata M, Hirano A. Sparing of the Onufrowicz nucleus in sacral anterior horn lesions. Ann Neurol. 1978;4(3):245–249. doi:10.1002/ana.410040309
25. Lee EA, Kim BJ, Lee WY. Diagnosing multiple system atrophy with greater accuracy: combined analysis of the clonidine-growth hormone test and external anal sphincter electromyography. Mov Disord. 2002;17(6):1242–1247. doi:10.1002/mds.10225
26. Jian F, Pan H, Zhang Z, et al. Sphincter electromyography in diabetes mellitus and multiple system atrophy. Neurourol Urodyn. 2015;34(7):669–674. doi:10.1002/nau.22639
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.