Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Differences in health care outcomes between postdischarge COPD patients treated with inhaled corticosteroid/long-acting β2-agonist via dry-powder inhalers and pressurized metered-dose inhalers
Authors Wittbrodt ET , Millette LA, Evans KA , Bonafede M , Tkacz J, Ferguson GT
Received 14 June 2018
Accepted for publication 12 October 2018
Published 24 December 2018 Volume 2019:14 Pages 101—114
DOI https://doi.org/10.2147/COPD.S177213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Eric T Wittbrodt,1 Lauren A Millette,1 Kristin A Evans,2 Machaon Bonafede,2 Joseph Tkacz,2 Gary T Ferguson3
1Medical Affairs, AstraZeneca, Wilmington, DE, USA; 2Life Sciences, Value-Based Care, IBM Watson Health, Cambridge, MA, USA; 3Pulmonary Research Institute of Southeast Michigan, Farmington Hills, MI, USA
Purpose: The aim of this study was to examine real-world differences in health care resource use (HRU) and costs among COPD patients in the USA treated with a dry powder inhaler (DPI) or pressurized metered-dose inhaler (pMDI) following a COPD-related hospitalization.
Methods: This retrospective analysis used the Truven MarketScan® databases. Eligibility criteria included 1) age ≥40 years, 2) COPD diagnosis, 3) inpatient admission with a diagnosis of COPD exacerbation, 4) inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA) prescription within 10 days of hospital discharge (index date), and 5) continuous enrollment for 12 months preindex and 90 days postindex. Outcomes included pre- and postindex HRU and costs. DPI and pMDI groups were compared on postindex outcomes via multivariate models controlling for demographic and baseline characteristics.
Results: The sample included 1,960 DPI and 1,086 pMDI ICS/LABA patients. During the preindex period, pMDI patients were significantly more likely to be prescribed a short-acting β-agonist, experienced more COPD exacerbation-related hospital days, and had a greater number of pulmonologist visits compared to DPI patients (P<0.05), all suggestive of greater disease severity. However, multivariate models revealed that pMDI patients incurred 10% lower all-cause postindex costs (predicted mean costs [2016 US dollars]: $2,673 vs $2,956) and 19% lower COPD-related costs (predicted mean costs: $138 vs $169; P<0.05). Additionally, pMDI patients were 28% less likely to experience a COPD exacerbation-related hospital readmission within 60 days postdischarge compared to the DPI patients (OR: 0.72, 95% CI: 0.52–0.99, P<0.05).
Conclusion: Despite greater COPD-related HRU and costs preceding index hospitalization, US patients using a pMDI after hospital discharge incurred significantly lower all-cause and COPD-related health care costs compared with those using a DPI, in addition to a decreased likelihood of a COPD exacerbation-related hospital readmission. Results suggest that inhaler device type may influence COPD outcomes and that COPD patients may derive greater clinical benefit from treatment delivered via pMDI vs DPI.
Keywords: COPD, inhaler, inhaled corticosteroid, long-acting β2-agonist, utilization, costs
Introduction
COPD is a common, progressive pulmonary disease characterized by persistent airflow limitation.1 The leading risk factor for COPD is extended inhalation of noxious particles or gases, usually from cigarette smoking, although pollution and occupational exposures can also be sources.2 In 2014, COPD and other chronic lower respiratory diseases were the third leading cause of mortality in the USA,3 with mortality rates increasing more than 30% between 1980 and 2014.4
Patients with COPD often have periods of acute worsening of symptoms or COPD exacerbations.5 Moderate exacerbations require the use of antibiotics and/or systemic corticosteroids, whereas severe exacerbations result in hospitalization, which accounts for as much as 70% of COPD-related medical costs.6–8 Reduction in exacerbation severity and frequency is a primary clinical objective. The total US national medical cost of COPD was estimated at $32.1 billion for 2010 and is projected to rise to $49 billion by 2020.9
Current COPD guidelines recommend treating symptomatic, stable COPD patients with inhaled long-acting bronchodilators, such as a long-acting β2-agonist (LABA).10 For the prevention of acute exacerbations in patients at risk for exacerbations, especially those with COPD-related hospitalizations, guidelines recommend the use of an inhaled corticosteroid (ICS) in combination with inhaled long-acting bronchodilators.11 Selection of the specific pharmaceutical agent and delivery mechanism is left to the prescribing clinician based on patient preference, cost, and adverse effect profile.
For home use, inhaled COPD medications are most commonly delivered using either a dry powder inhaler (DPI) or a pressurized metered-dose inhaler (pMDI). These devices can be challenging to use for some patients, with administration errors commonly occurring and potentially resulting in inadequate dose delivery.12,13 Each delivery system has requirements and limitations, making appropriate device selection and education a critical component of COPD care.10,14
Poor technique for various inhaler devices can be addressed with educational interventions; however, effective device usage is also dependent on patient physical characteristics.15 For example, a systematic review found that 45% of pMDI users had suboptimal hand-breath coordination for optimal drug delivery.16 Coordination limitations can be addressed by the use of holding chambers or spacers;17 however, errors in handling, execution, and breath technique are still common.16 Effective drug delivery via a DPI requires that the patient generates levels of inspiratory flow sufficient to overcome the resistance of the device.18 In other words, the energy required to aerosolize a DPI medication comes from the user, and adequate DPI medication delivery relies on proper technique, sufficient effort from a patient, and a lack of medical conditions that might otherwise prevent adequate inspiratory flows. Importantly, peak inspiratory flow needed for DPI administration is often limited by lung hyperinflation, especially after an acute exacerbation. Recent studies of peak inspiratory flow after recovery from an acute exacerbation found that 19%–52% of COPD patients had insufficient peak inspiratory flow for effective DPI use, and those patients were more likely to be older and have more severe disease.19–21
Successful delivery of medication is required to achieve the desired benefit of reduced exacerbation and hospitalization frequency.22 Poor inhalation technique has been estimated to increase direct medical costs of COPD by 2.2%–7.7%.23 A randomized controlled trial of inhaled ICS/LABA combination therapy of fluticasone propionate/salmeterol xinafoate (FP/SAL) found no difference in clinical benefit between patients using a DPI or a pMDI.24 However, patients enrolled in clinical trials are subject to strict inclusion criteria, receive more consistent training in inhaler use and are excluded if they are unable to effectively use a study device, have had a recent exacerbation, or suffer from very severe lung disease. In a real-world, retrospective matched cohort observational study of 236 patient pairs treated with a 500 μg/day dose of FP/SAL, those using a pMDI had fewer moderate-to-severe exacerbations compared with those using a DPI at equivalent dosage.25
This retrospective claims-based study examined real-world differences in all-cause and acute exacerbation of COPD (AECOPD)-related readmission rates within 30 and 60 days of discharge after hospitalization for an AECOPD among patients in the USA treated with an ICS/LABA combination delivered via DPI or pMDI. Treatment groups were also compared based on all-cause and COPD-related health care resource use (HRU), such as inpatient admissions, emergency room (ER) visits, outpatient office visits, and the associated costs. Results will provide much-needed evidence on the association between different types of inhaled medication delivery devices and health care outcomes in a real-world setting.
Methods
Data sources
This observational retrospective cohort analysis utilized de-identified US administrative claims data from the Truven Health Analytics MarketScan® Commercial Claims and Encounters database (Commercial) and MarketScan® Medicare Supplemental and Coordination of Benefits database (Medicare Supplemental) for the period from January 1, 2009, to July 29, 2016. Each database captures the inpatient medical, outpatient medical, and outpatient prescription drug data for its respective covered population and together forms a nationally representative sample of insured individuals living in the USA.
Ethics approval and informed consent
All study data were accessed with protocols compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996 regulations (HIPAA). As the database is fully de-identified and compliant with the HIPAA, this study was exempted from Institutional Review Board approval.
Patient selection criteria
To be eligible for the current study, patients were required to have at least two nondiagnostic claims with a diagnosis of COPD (ICD-9-CM: 490.xx–492.xx and 496.xx; ICD-10: J40, J41.0, J41.1, J41.8, J42, J43.0, J43.1, J43.2, J43.8, J43.9, J44.0, J44.1, and J44.9) between January 1, 2010, and April 30, 2016, and at least one prescription claim for an ICS/LABA combination therapy during the same period. Eligible therapies include budesonide and formoterol, fluticasone furoate and vilanterol, FP/SAL, and mometasone and formoterol. Patients had to be aged ≥40 years on the date of the first prescription claim for an ICS/LABA combination therapy, which must have been within 10 days after an inpatient discharge with a primary diagnosis of an AECOPD (ICD-9-CM: 491.21, 491.22, and 492.8; ICD-10: J44.1). The date of the prescription was set as the index date, and the date of the hospital discharge was set as the index discharge date.
Patients were required to fill a prescription for an ICS/LABA combination therapy dispensed by either a DPI or a pMDI, but not both, on the index date. Patients were required to have at least 15 months of continuous enrollment with medical and pharmacy benefits (12 months prior and 90 days following and including the index date). Patients were excluded if they had an asthma diagnosis during the 12-month preindex period or a diagnosis of cystic fibrosis, pulmonary fibrosis, bronchiectasis, or respiratory tract cancer anytime during the study period. Finally, patients with a prescription claim for any tiotropium medication within 90 days before or on the index date were excluded. Patients were not excluded based on short-acting β-agonist (SABA) usage in either the pre- or postindex periods, as these medications are commonly prescribed as rescue medications.5
Outcome measures
All-cause and AECOPD-related readmissions within 30 and 60 days after the index discharge date were assessed for all patients. Results were reported as the proportion of patients with one or more readmissions by the selected time point and as the time to the first readmission after the index discharge date.
All-cause and COPD-related HRU and costs were measured during both the 12-month pre-index and the 90-day postindex periods. HRU was reported for inpatient admissions, ER visits, physician office visits, outpatient laboratory and radiology services, outpatient prescriptions, and other outpatient services. Costs were calculated based on the paid amounts for adjudicated claims including portions paid by both insurers and the patient. All costs are reported as per person per month (PPPM) and were adjusted to 2016 US dollars using the medical care component of the consumer price index.26
Demographic and clinical characteristics
Patients’ demographic characteristics were assessed on the index date and included age, age group, sex, geographic region, insurance plan type, payer type, and rural residence indicator. Urban or rural residence classification was based on whether the primary subscriber’s address was located within a metropolitan statistical area.
Clinical characteristics were assessed during the 12-month preindex period and included the Deyo–Charlson comorbidity score, selected comorbid conditions (acute bronchitis and bronchiolitis, anxiety, asthma, cardiovascular disease, acute myocardial infarction, congestive heart failure, ischemic stroke, depression, diabetes, gastroesophageal reflux disease, hypertension, osteoporosis, osteoarthritis, and pneumonia), COPD severity indicators (hospitalization days due to AECOPD, pulmonologist visits, SABA prescription fills, and oral corticosteroid prescription fills), respiratory treatments (oxygen therapy, nebulizer use, and COPD medications), and medications for other common chronic conditions (antihypertensive, diabetes, and lipid-lowering medications).
Statistical analyses
Patients were segmented by their index device for all analyses. Descriptive statistics was calculated for demographics, clinical characteristics, and all outcome measures. For continuous variables, the mean and SD were calculated, with statistically significant differences between device groups assessed via Student’s t-test. For categorical variables, the counts and percentages were calculated, with statistically significant differences between device groups assessed via Chi-squared test. The alpha level for all statistical tests was 0.05.
Logistic regression models were built to assess the association between inhaler type and binary outcomes including all-cause and AECOPD-related readmissions within 30 and 60 days in the postindex period. Cox proportional hazards models were used to assess the association between inhaler type and time to readmission (all-cause and AECOPD related) within 30 and 60 days of the index discharge date. Generalized linear models with gamma distributions were used to assess the association between inhaler type and costs (all-cause and AECOPD related) during the postindex period. The vector of covariates included in all models were as follows: age, sex, geographic region, insurance plan type, rural residence indicator, preindex myocardial infarction, preindex ischemic stroke, preindex SABA prescription, number of preindex AECOPD-related inpatient days, preindex experience with the index inhaler type, and number of preindex pulmonologist visits.
Results
Baseline patient characteristics
A total of 3,046 patients were eligible for study inclusion, with 64.3% (n=1,960) prescribed a DPI, and 35.7% (n=1,086) prescribed a pMDI (Figure 1). Demographic characteristics as assessed on the index date are listed in Table 1. DPI and pMDI groups did not differ with respect to age or sex, with approximately 46% of the sample being male and 53% aged 65 years or older. The majority of patients lived in urban areas (79%), and they were insured by a Medicare supplemental plan (55%). There were slight differences in the type of coverage between groups (±2%, P<0.01), with pMDI users being more likely to have comprehensive/indemnity, health maintenance organization, or consumer-driven/high-deductible health plans.
Clinical characteristics in the 12-month preindex period are listed in Table 1. Both cohorts had a mean Deyo-Charlson comorbidity index of 2.4 and also a similar prevalence of the measured comorbid conditions, except for a higher prevalence of acute myocardial infarction in the DPI cohort (4.0% DPI vs 2.3% pMDI, P<0.05) and a higher prevalence of ischemic stroke in the pMDI cohort (1.7% DPI vs 2.8% pMDI, P<0.05). Rates of prescription medication fills for common chronic conditions (hypertension, diabetes, and high cholesterol) were similar for both groups. A significantly larger proportion of pMDI users filled a SABA prescription during the preindex period (36.4% DPI vs 40.1% pMDI, P<0.05), but otherwise there was no significant difference in respiratory treatments. Patients prescribed a pMDI had significantly more hospitalization days due to AECOPD (3.7±2.5 DPI vs 4.0±2.9 pMDI, P<0.02) and more outpatient pulmonologist visits (1.6±3.8 DPI vs 1.9±4.1 pMDI, P<0.05) in the preindex period. The pMDI group was significantly more likely than DPI users to have preindex experience with their index device type (4.8% DPI vs 37.8% pMDI, P<0.0001).
Baseline all-cause and COPD-related HRU and costs are listed in Table 2. During the 12-month preindex period, DPI users were more likely to visit the ER for both all-cause (48.0% DPI vs 43.6% pMDI, P<0.05) and COPD-related (19.4% DPI vs 15.7% pMDI, P<0.02) encounters. However, pMDI users had higher COPD-related HRU including longer in-patient hospitalizations (3.5±2.2 days DPI vs 3.7±2.3 days pMDI, P<0.05), more physician office visits (33.7% DPI vs 37.6% pMDI, P<0.05), and more prescriptions filled (5.3% DPI vs 6.0% pMDI, P<0.05). There were no statistically significant differences in all-cause or COPD-related costs during the 12-month preindex period.
Postindex period outcomes
Postindex period all-cause and COPD-related HRU and costs are shown in Table 3. Bivariate analyses revealed that DPI users were more likely to initiate tiotropium use within 30 days of treatment index compared to the pMDI group (6.0% DPI vs 4.3% pMDI, P<0.05). Groups did not differ with respect to remaining HRU outcomes. Regarding health care expenditures, compared to the DPI group, pMDI patients incurred lower PPPM all-cause outpatient ($1,495±$3.641 DPI vs $1,222±$2,114 pMDI, P<0.01), COPD-related outpatient ($75±$232 DPI vs $58±$129 pMDI, P<0.02), COPD-related total medical ($112±$461 DPI vs $85±$247 pMDI, P<0.05), COPD-related total health care ($169±$467 DPI vs $136±$253 pMDI, P<0.02), and outpatient pharmacy costs ($57±$44 DPI vs $51±$40 pMDI, P<0.0001). Other health care expenditures did not differ between groups.
There was no significant difference in the frequency of all-cause or AECOPD-related readmissions within 30 days of discharge or all-cause readmission within 60 days of discharge between DPI and pMDI groups (Table 4). The bivariate analysis suggested that the pMDI group experienced a longer time to a COPD exacerbation-related hospital readmission within 30 days postdischarge compared to DPI patients (P<0.05). Results of logistic models of hospital readmissions, controlling for patient demographics and clinical characteristics, revealed that pMDI patients were 28% less likely to experience a COPD exacerbation-related hospital readmission within 60-day postdischarge compared to DPI patients (OR: 0.72, 95% CI: 0.52–0.99, P<0.05; Table 4). Kaplan–Meier plots for the time to 60-day all-cause and AECOPD-related readmissions are shown in Figure 2. Results of a Cox proportional hazards model confirmed that the time to AECOPD-related readmission within 60 days was significantly different between groups after multivariate adjustment (HR [pMDI vs DPI]: 0.73; 95% CI: 0.54–0.99). Increased age was associated with a greater likelihood of all-cause and COPD-related hospital readmissions in all models, whereas preperiod acute myocardial infarction and ischemic stroke were each associated with greater likelihood of 30- and 60-day all-cause readmissions.
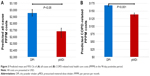
Finally, results of the generalized linear gamma models of total health care costs controlling for patient demographics and clinical characteristics revealed that, compared to DPI patients, pMDI patients incurred lower all-cause ($2,673 vs $2,956) and COPD-related PPPM costs ($138 vs $169; P<0.05; Figure 3) during the postperiod. The presence of baseline acute myocardial infarction was associated with greater all-cause and COPD-related costs, whereas baseline SABA use and a greater number of visits to a pulmonologist were associated with increased COPD-related costs (P<0.05).
Discussion
This study demonstrated that patients prescribed a pMDI to deliver an ICS/LABA combination therapy after hospitalization for COPD had 10% lower all-cause health care costs and 18% lower COPD-related health care costs compared to patients prescribed a DPI. This was despite greater baseline disease severity of the pMDI cohort, as indicated by a greater number of AECOPD-related in-patient hospitalization days, outpatient pulmonologist visits, and SABA prescriptions filled prior to the index hospitalization. Mutivariate analysis was used to control for these baseline differences in indicators of disease severity. Notably, COPD-related prescription costs were lower for the pMDI cohort, potentially due to the lower percentage of pMDI patients being prescribed tiotropium-based medications. Patients using tiotropium alone delivered by soft-mist inhaler or DPI or in combination with olodaterol delivered by soft-mist inhaler (Respimat, Boehringer Ingelheim microParts GmbH, Dortmund, Germany) concurrent with their index hospitalization were excluded from the current analysis to avoid confounding the comparison between DPI and pMDI delivery of ICS/LABA.
The multivariate analysis revealed a lower frequency of AECOPD readmission within 60 days for the pMDI cohort, and this was confirmed by Kaplan–Meier curves and multivariate analysis comparing time to first readmission within 60-day postdischarge. Although the pMDI cohort had a lower frequency of readmission and longer times to readmission, the differences between groups were not statistically significant for any of the evaluated all-cause readmission outcomes or the AECOPD readmission outcomes evaluated at 30 days. We hypothesize that this is due to an inability to adequately control for the differences in COPD severity between groups without access to spirometry results. Several covariates (preindex SABA fills, AECOPD inpatient days, and pulmonologist visits) were chosen as surrogate markers of disease severity based on the descriptive analysis; however, none of these are a direct measure of lung function. Also, two variables controlling for differences in pre-existing cardiovascular disease between groups were included in the models. The observed lag in statistical significance of AECOPD readmission outcomes is consistent with the hypothesis that the selected covariates were unable to completely account for differences in disease severity and overall health.
In this study, patients in the pMDI cohort were significantly more likely to have had recent experience with their index device type. This may be due to the larger number of formulations available in this format, the lower cost of pMDI devices, or their common use in rescue inhalers.27 Concurrent use of multiple device types has been shown to negatively impact patient outcomes as has nonconsensual switching of device types.17,28,29 To control for the possibility that device continuity contributed to the improved outcomes observed in the pMDI cohort, preindex experience with the index inhaler type was included as a covariate in the multivariate modeling. It is notable that the difference in time to AECOPD readmission within 60 days did not become statistically significant until preindex device utilization was added as a covariate. This highlights the impact of real-world experience with an inhaler type on health outcomes.
One confounding factor to the above results is the difference in medication formulations between groups. Although we restricted our analysis to those patients newly prescribed an ICS/LABA combination therapy, manufacturer and regulatory restrictions created the scenario in which no formulations are available in the USA for the treatment of COPD in both device types. FP/SAL is available in both delivery devices; however, the pMDI format is only approved for use in asthma.30 Although medications from the same class are anticipated to perform similarly,24,31 the impact of the absence of formulations or combinations in either group is unknown. In addition, our analysis did not include patients using ICS and LABA monotherapies in combination. This decision was made to avoid additional confounding factors as LABA monotherapy is not available in a pMDI format, and ICS monotherapy is only available through off-label usage of asthma products. Furthermore, neither monotherapy is recommended for patients with previous hospitalizations for AECOPD.14
There are a number of studies that have examined the cost-effectiveness of various COPD medications, with the frequency of AECOPD-related hospitalizations being the primary driver of costs.32–34 Analysis of the 3-year multicenter Towards a Revolution in COPD Health study of 6,112 participants found that the ICS/LABA combination of FP/SAL was more cost-effective than placebo or either treatment alone.32 In that study, all medications were delivered via DPI.35 A 2005 systematic review of COPD and asthma clinical trials by the American College of Chest Physicians and the American College of Asthma, Allergy, and Immunology, which relied heavily on data from LABA studies, found no significant difference in clinical outcomes between device types.36 However, patients can only be included in clinical trials if they are able to use the study device correctly so these results cannot necessarily be extrapolated to real-world performance. Limited data exist from real-world practice regarding if and how the choice of inhalation device impacts outcomes in COPD; however, a 2011 retrospective matched cohort study of asthma patients (N=1,567 pairs) reported that pMDI users had significantly higher odds of achieving asthma control and treatment success (ie, no exacerbations and no change in therapy) compared to DPI users.37 In addition, although it is known that use of inhalers containing ICS increases a patient’s risk of developing oral thrush,38 two recent real-world studies have reported that pMDI use is associated with a lower risk of thrush compared to DPI use.39,40
The findings of this study are consistent with those of the only other real-world observational study on the impact of inhaler type on ICS/LABA control of AECOPD.25 In that matched cohort study by Jones et al, patients treated with a 500 μg/day dose of FP/SAL delivered via pMDI had fewer moderate-to-severe exacerbations than patients using a DPI. This effect was not present at the higher dose of 1,000 μg/day of FP/SAL. The authors hypothesized that the higher dosage compensated for any problems in minimum effective dose delivery due to suboptimal peak inspiratory flow for DPI usage. The study did not evaluate costs; however, hospitalization for AECOPD has been shown to be a leading driver of high costs in COPD treatment. Two possible reasons why pMDIs may be more effective than DPIs are that peak inspiratory flow rates have been shown to be lower during an AECOPD, which may reduce the efficacy of DPIs immediately following an exacerbation,41 and some DPIs are sensitive to environmental moisture, which reduces the delivery of fine aerosol particles.42,43
Limitations
Studies based on administrative claims data, such as that found in the MarketScan® Research Databases, have several inherent limitations. First, these datasets are subject to miscoding and undercoding, which may introduce bias or measurement error. Previous studies have demonstrated that the claims-based approach of combining advanced age (≥40 or ≥55 years old) and a primary discharge diagnosis of AECOPD (ICD-9-CM 491.21) to identify patients hospitalized for AECOPD has a positive predictive value of 97% compared to manual chart review by a physician.44,45 However, this selectivity for true-positive patients comes at the expense of excluding a large number of patients with the symptoms of an AECOPD but a different discharge code (sensitivity =12.5%). Stein et al44 tested three other algorithms but found them to be inferior in performance and recommended the algorithm above for comparative effectiveness research.
Second, this study was limited to individuals in the US with commercial or employer-sponsored Medicare supplemental insurance; therefore, the results may not be generalizable to patients outside the USA, or US patients with other insurance coverage or no coverage who may experience different patterns of health care utilization and costs. Third, the costs represented in these databases reflect the paid amounts of adjudicated claims to individual hospitals and providers and do not include indirect costs, which are a substantial portion of the economic burden of COPD. Fourth, claims data only indicate that a prescription was filled and not that the medication was utilized as directed. Additionally, medication obtained without a concomitant insurance claim, such as samples from health care providers, or delivered in a clinical trial, would not be captured in the databases. Fifth, not all medication formulations were available with both inhaler types, so the comparisons are between different formulations and different devices. Additionally, the dosage of the ICS/LABA medication was not assessed in this study, and so unmeasured differences in medication dosage may have contributed to observed differences between groups. Sixth, only patients using DPIs or pMDIs were included in the analysis, so these results may not extend to patients using other types of inhalers such as a soft-mist inhaler. Inclusion of soft-mist inhalers would have complicated the analysis by both adding another device type and requiring inclusion of monotherapy combinations due to the lack of an ICS/LABA combination in this format. Seventh, this study excluded patients aged <40 years, those with asthma-COPD overlap syndrome, those with other major respiratory diseases, and those who had filled a prescription for a tiotropium medication in the 90 days leading up to the index date. The findings, therefore, may not extend to these populations. Finally, multivariate analysis was used to control for differences in baseline demographic and clinical characteristics and several known factors that influence device selection, such as spirometry results, are not captured in administrative claims and therefore could not be controlled for.
Conclusion
In this real-world retrospective cohort study, US patients initiating ICS/LABA combination therapy delivered by a pMDI after discharge from the hospital for an AECOPD had lower all-cause and COPD-related health care costs in the 90-day follow-up period despite having more severe disease during the preindex period, compared to those receiving a DPI. Reduced follow-up costs suggest that inhaler device type may influence COPD outcomes and that COPD patients may derive greater clinical benefit from treatment delivered via pMDI vs DPI, although this requires confirmation by future prospective studies.
Abbreviations
AECOPD, acute exacerbation of COPD; DPI, dry powder inhaler; ER, emergency room; FP/SAL, fluticasone propionate/salmeterol; HRU, health care resource use; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; pMDI, pressurized metered-dose inhaler; PPPM, per person per month; SABA, short-acting β-agonist.
Acknowledgments
Editorial support was provided by Jessamine P Winer-Jones, PhD, of IBM Watson Health, which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca. This research was presented in part at the American Thoracic Society Annual International Conference, San Diego, CA, USA, May 18–23, 2018.
Disclosure
ETW and LAM are employed by AstraZeneca. KAE, MB, and JT are employed by IBM Watson Health as consultants and received funding from AstraZeneca to conduct this study. GTF is a paid consultant of AstraZeneca. All authors report no other conflicts of interest in this work.
References
Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. | ||
Soriano JB, Rodríguez-Roisin R. Chronic obstructive pulmonary disease overview. Proc Am Thorac Soc. 2011;8:363–367. | ||
Murphy SL, Kochanek KD, Xu JQ, Arias E. Mortality in the United States, 2014. NCHS data brief, no 229. Hyattsville, MD: National Center for Health Statistics. 2015. | ||
Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Trends and patterns of differences in chronic respiratory disease mortality among us counties, 1980–2014. JAMA. 2017;318(12):1136–1149. | ||
Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Foster TS, Miller JD, Marton JP, Caloyeras JP, Russell MW, Menzin J. Assessment of the economic burden of COPD in the US: A review and synthesis of the literature. COPD. 2006;3:211–218. | ||
Schwab P, Dhamane AD, Hopson SD, et al. Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis. 2017;12:735–744. | ||
Dalal AA, Christensen L, Liu F, Riedel AA. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341–349. | ||
Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. | ||
Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. | ||
Criner GJ, Bourbeau J, Diekemper RL, et al. Executive summary: prevention of acute exacerbation of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):883–893. | ||
Mahon J, Fitzgerald A, Glanville J, et al. Misuse and/or treatment delivery failure of inhalers among patients with asthma or COPD: A review and recommendations for the conduct of future research. Respir Med. 2017;129:98–116. | ||
Arora P, Kumar L, Vohra V, et al. Evaluating the technique of using inhalation device in COPD and bronchial asthma patients. Respir Med. 2014;108(7):992–998. | ||
Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. | ||
Klijn SL, Hiligsmann M, Evers S, Román-Rodríguez M, van der Molen T, van Boven JFM. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim Care Respir Med. 2017;27(1):24. | ||
Sanchis J, Gich I, Pedersen S. Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: Has patient technique improved over time? Chest. 2016;150(2):394–406. | ||
Kaplan A, Price D. Matching inhaler devices with patients: The role of the primary care physician. Can Respir J. 2018;2018:9473051. | ||
Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213–218. | ||
Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305–1311. | ||
Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174–179. | ||
Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for copd exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217–224. | ||
Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938. | ||
Lewis A, Torvinen S, Dekhuijzen PN, et al. The economic burden of asthma and chronic obstructive pulmonary disease and the impact of poor inhalation technique with commonly prescribed dry powder inhalers in three European countries. BMC Health Serv Res. 2016;16:251. | ||
Koser A, Westerman J, Sharma S, Emmett A, Crater GD. Safety and efficacy of fluticasone propionate/salmeterol hydrofluoroalkane 134a metered-dose-inhaler compared with fluticasone propionate/salmeterol diskus in patients with chronic obstructive pulmonary disease. Open Respir Med J. 2010;4:86–91. | ||
Jones R, Martin J, Thomas V, et al. The comparative effectiveness of initiating fluticasone/salmeterol combination therapy via pMDI versus DPI in reducing exacerbations and treatment escalation in COPD: a UK database study. Int J Chron Obstruct Pulmon Dis. 2017;12:2445–2454. | ||
Aramaki E, Miura Y, Tonoike M, et al. Extraction of adverse drug effects from clinical records. Stud Health Technol Inform. 2010;160(Pt 1):739–743. | ||
van Aalderen WM, Garcia-Marcos L, Gappa M, et al. How to match the optimal currently available inhaler device to an individual child with asthma or recurrent wheeze. NPJ Prim Care Respir Med. 2015;25:14088. | ||
Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184–191. | ||
Thomas M, Price D, Chrystyn H, Lloyd A, Williams AE, von Ziegenweidt J. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009;9:1. | ||
Advair hfatm (fluticasone propionate and salmeterol) [US package insert]. Research triangle park, NC: Glaxosmithkline; 2017. | ||
Spencer S, Evans DJ, Karner C, Cates CJ. Inhaled corticosteroids versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD007033. | ||
Briggs AH, Glick HA, Lozano-Ortega G, et al. Is treatment with ICS and LABA cost-effective for COPD? Multinational economic analysis of the TORCH study. Eur Respir J. 2010;35(3):532–539. | ||
Hertel N, Kotchie RW, Samyshkin Y, Radford M, Humphreys S, Jameson K. Cost-effectiveness of available treatment options for patients suffering from severe COPD in the UK: a fully incremental analysis. Int J Chron Obstruct Pulmon Dis. 2012;7:183–199. | ||
Rutten-van Mölken MPMH, Goossens LMA. Cost effectiveness of pharmacological maintenance treatment for chronic obstructive pulmonary disease. Pharmacoeconomics. 2012;30(4):271–302. | ||
Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. | ||
Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371. | ||
Price D, Roche N, Christian Virchow J, et al. Device type and real-world effectiveness of asthma combination therapy: an observational study. Respir Med. 2011;105(10):1457–1466. | ||
Rachelefsky GS, Liao Y, Faruqi R. Impact of inhaled corticosteroid-induced oropharyngeal adverse events: results from a meta-analysis. Ann Allergy Asthma Immunol. 2007;98(3):225–238. | ||
Dekhuijzen PNR, Batsiou M, Bjermer L, et al. Incidence of oral thrush in patients with COPD prescribed inhaled corticosteroids: Effect of drug, dose, and device. Respir Med. 2016;120:54–63. | ||
van Boven JF, de Jong-van den Berg LT, Vegter S. Inhaled corticosteroids and the occurrence of oral candidiasis: a prescription sequence symmetry analysis. Drug Saf. 2013;36(4):231–236. | ||
Broeders ME, Molema J, Hop WC, Vermue NA, Folgering HT. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respir Med. 2004;98(12):1173–1179. | ||
Borgström L, Asking L, Lipniunas P. An in vivo and in vitro comparison of two powder inhalers following storage at hot/humid conditions. J Aerosol Med. 2005;18(3):304–310. | ||
Janson C, Lööf T, Telg G, Stratelis G, Nilsson F. Difference in resistance to humidity between commonly used dry powder inhalers: an in vitro study. NPJ Prim Care Respir Med. 2016;26:16053. | ||
Stein BD, Bautista A, Schumock GT, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141(1):87–93. | ||
Ginde AA, Tsai CL, Blanc PG, Camargo CA. Positive predictive value of ICD-9-CM codes to detect acute exacerbation of COPD in the emergency department. Jt Comm J Qual Patient Saf. 2008;34(11):678–680. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.