Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Diabetes and Psoriasis: Different Sides of the Same Prism
Authors Abramczyk R , Queller JN, Rachfal AW , Schwartz SS
Received 22 July 2020
Accepted for publication 12 August 2020
Published 7 October 2020 Volume 2020:13 Pages 3571—3577
DOI https://doi.org/10.2147/DMSO.S273147
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Rachel Abramczyk,1 Jenna N Queller,2 Amy W Rachfal,3 Stanley S Schwartz4,5
1Main Line Health System, Wynnewood, PA, USA; 2Private Practice, Boca Raton, FL, USA; 3Stage Gate Partners, LLC, Ardmore, PA, USA; 4Stanley Schwartz, LLC, Main Line Health System, Ardmore, PA, USA; 5University of Pennsylvania, Philadelphia, PA, USA
Correspondence: Stanley S Schwartz
Stanley Schwartz, MD, LLC, 233 E Lancaster Ave, Suite 305, Ardmore, PA 19003, USA
Tel/Fax +1 610 642 6850
Email [email protected]
Abstract: Diabetes and psoriasis are prevalent conditions with a spectrum of serious adverse outcomes. Both diseases are common comorbidities for each other, and diabetes is considered as a risk factor for psoriasis and vice versa. However, it is our contention that these diseases are not merely comorbidities of each other but rather share common underlying pathophysiologies (ie, genes and epigenetic changes, inflammation, abnormal environment, and insulin resistance) that drive disease. As such, they can be viewed as facets of the same prism. Genes can cause or permit susceptibility to damage from abnormal external and internal environmental factors, inflammation, and insulin resistance which can also drive epigenetic changes. These co-existing mechanisms act in a vicious cycle over time to potentiate cell and tissue damage to ultimately drive disease. Viewing diabetes and psoriasis through the same prism suggests potential for therapies that could be used to treat both conditions. Although additional controlled trials and research are warranted, we believe that our understanding of the overlapping pathophysiologies continues to grow, so too will our therapeutic options.
Keywords: genes, epigenetics, inflammation, abnormal environment, insulin resistance
Introduction
Affecting more than 30 million people in the US, diabetes mellitus (DM) is a disease characterized by a chronic hyperglycemic state with an associated spectrum of complications.1 The complications and associated comorbidities of DM are well-established and range from retinopathy, nephropathy, neuropathy to cardiovascular disease, metabolic disease, cancers, dementia, nonalcoholic fatty liver disease, and beyond.1–4 Our understanding of the known pathophysiologic mechanisms of DM continues to evolve, moving away from the notion of discrete disease subtypes (eg, Type 1, Type 2, Latent Autoimmune Diabetes of Adults) to a common underlying pathway of inflammatory, metabolic, environmental, and genetic insults that result in beta cell injury and a spectrum of phenotypes.2,5
Psoriasis is a chronic, systemic inflammatory condition affecting approximately 8 million people in the US.6,7 The pathogenesis of psoriasis is thought to be a complex, immune-mediated process that results in damage that is more than skin deep.8 It is characterized by underlying inflammatory processes including regulatory immune cells, cytokines, and adipokines.7 Chronic inflammation with a dominant TNF-α-IL-23-Th17 axis leads to uncontrolled keratinocyte proliferation and dysfunctional differentiation, and neovascularization.8 Psoriasis has been associated with other chronic diseases including cardiometabolic disease (eg, DM, major adverse cardiovascular events, obesity, hypertension, dyslipidemia, metabolic syndrome), psoriatic arthritis, inflammatory bowel disease, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, chronic kidney disease, cancer, and mood disorders.6
Given its systemic nature and associated shared comorbidities with DM, it is not surprising that psoriasis has been associated with DM.6,8 Our own bias is not that psoriasis is a risk factor for DM but that the two diseases share overlapping pathophysiologies that link them. Here, we offer a coherent theory to explain the association between DM and psoriasis through our unified construct of DM, its pathophysiology, complications, and treatment. In this construct, we draw parallels between the genetic influences, inflammatory pathways, environmental influences, insulin resistance resulting in end-organ damage in DM and psoriasis, viewing the multifaceted aspects of the two diseases through the same prism (Figure 1). The specific phenotype of any individual will depend on which factors are involved and to what degree in each patient.
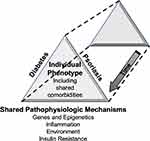 |
Figure 1 Diabetes and psoriasis: Different sides of the same prism. |
Epidemiology
There has been increasing evidence over the past several years describing the association between DM and psoriasis.6,9-11 Indeed it has been proposed that psoriasis is a risk factor for the development of DM and, conversely, that DM is an intrinsic risk factor for the exacerbation of psoriasis.9–11 In 2013, Armstrong et al performed a systematic review and meta-analysis of 27 observational studies, describing a 59% overall increased prevalence of DM in psoriasis patients, with up to 97% increased prevalence in those with severe psoriasis.9 In those studies that assessed incident cases of DM, patients with psoriasis were 27% more likely to develop DM compared to those without.9 Wan et al further evaluated the association between psoriasis severity and DM, describing significantly increased incidence of DM in psoriasis patients with more disease per body surface area.12 More recently, a systematic review and meta-analysis of 38 studies by Mamizadeh et al found a similar significant association of DM in nearly 1 million psoriasis patients, reinforcing the notion that psoriasis is not limited to the skin.11 It is our own bias that these 2 diseases are not merely co-morbidities of each other but rather that they share the same pathophysiologies, and are facets of the same prism, which we aim to bring to light.
Pathophysiology of DM – 4 Basic Pathways
We believe the spectrum of DM is due to a common denominator, the defective beta cell.2 This core defect results from four basic pathophysiologic processes comprising genes and epigenetic changes, inflammation, environment, and insulin resistance.2 As a result, hyperglycemia arises through multiple pathways, the so-called Egregious Eleven.2 The beta-cell dysfunction and related mechanisms of hyperglycemia and resultant endogenous fuel excess cause oxidative stress and epigenetic changes in tissues throughout the body, including worsening beta-cell dysfunction.2 It is our view that some conditions associated with DM, such as psoriasis, are frequently concurrent due to overlapping pathophysiologies, especially shared genes and epigenetic changes (Figure 1).
Genes and Epigenetic Changes
The most important common pathophysiologic mechanism relating diabetes and psoriasis is the shared genetic mechanisms. Over the past 10 years, it has been increasingly evident that genes related to both Type 1 DM, Type 2 DM, and psoriasis share overlapping genes and susceptibility loci. In 2017, Wang et al examined 89 DM susceptibility loci in nearly 4500 psoriasis patients and 6000 controls in China, identifying the PTPN22, ST6GAL1, and JAZF1 genes as significant in both psoriasis and DM.13 The PTPN22 gene is known to encode an intracellular phosphatase that is thought to affect T-cell receptor signaling pathways in autoimmune diseases including Type 1 DM, Graves disease, rheumatoid arthritis, and systemic lupus erythematosus.14 The JAZF1 gene was found to contain an intron variant that was associated with psoriasis through an unknown mechanism, and has also been found to be a transcriptional repressor, negatively influencing glucose metabolism with associated beta-cell impairment.13 Finally, a noncoding variant was found within the first intron of the ST6GAL1 gene which is thought to encode a glycosyltransferase that participates in cell-surface carbohydrate determinants and antigen differentiation thought to be necessary in T cell activation, but the role in both DM and psoriasis is unclear.13 Furthermore, Quaranta et al noted different single nucleotide polymorphisms of the CDKAL1 gene were present in psoriasis and Type 2 DM cases.15 The function of the protein encoded by this gene is not definitively known but is thought to be associated with insulin production under glucotoxic conditions.15 Transcripts of this gene were found to be virtually absent from keratinocytes but clearly expressed in immune cells although downregulated in actively proliferating immune cells.15
With regard to epigenetics, dysregulation of miRNA expression or mutation in miRNA genes is associated with a wide variety of human diseases. Post-transcriptional changes by microRNAs (miRNAs) have also been implicated in DM and psoriasis.3,5 For example, Granata et al describes a link between DM and psoriasis through miR-21 which is up-regulated in T helper cells within psoriatic skin lesions, and activated as promoter of genes implicated in Type 1 DM.16 In addition, in pancreatic β cells, when the miR-21 gene promoter is activated, subsequent downregulation of programmed cell death protein 4 (PDCD4) occurs, thus protecting pancreatic cells from apoptosis.17 As miR-21 is also upregulated in psoriasis, the miR-21-PDCD4 axis may play a crucial role in both DM and psoriasis, representing a potential therapeutic target for treating both diseases.17
Inflammation
The known underlying pathophysiologies of psoriasis are complex in nature with a strong focus on the immune response, both innate and adaptive.6,10 Inflammation is common, associated with both DM and psoriasis, and serves as a mechanistic link between the skin lesions and comorbidities in psoriasis and metabolic derangements in DM. Inflammation is necessary for the immune system to protect against various environmental and pathogenic insults, however when it becomes chronic it can do more harm than good, increasing the risk of developing other comorbidities, including metabolic syndrome, cardiovascular disease, inflammatory bowel disease, cancer, and notably DM.18 The inflammatory cytokines implicated in psoriasis can increase the amount of insulin-like growth factor (IGF) in the skin and body.19 Binding of IGF to IGF receptors results in proliferation of keratinocytes and fibroblasts.19 Further, Davidovici et al demonstrated TNF-α secreted by macrophages in dermal and adipose tissue has been associated with altered adipokine gene transcription, increasing expression of pro-inflammatory leptin, and anti-inflammatory adiponectin.18
Environment
The pathogenesis of both DM and psoriasis is thought to be impacted by the environment, both externally and internally. Indeed an abnormal environment influences patients based on their unique genetic makeup and also triggers epigenetic modifications and impacts the immune system.20 Lifestyle factors like diet and exercise are well-established modifiable risk factors in the development of DM and are largely related to the inflammatory state associated with obesity.21 Environmental risk factors that are thought to play a critical role in psoriasis include UV exposure, medications, smoking, diet and obesity, alcohol intake, infections, and stress.20 Many of these are associated with downstream dysregulation of the immune system/immune responses, likely through epigenetic modifications.20
The gastrointestinal (GI) environment has been shown to play a major role in glucose homeostasis, mediating up to 70% of postprandial insulin release via incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP).22 These hormones have also been shown to promote proliferation and prevent apoptosis of beta cells, however their levels and function are noted to be reduced in Type 2 DM.22 Interestingly, Gyldenlove et al noted significantly reduced incretin effect (39%) in 12 non-diabetic psoriasis patients after oral glucose tolerance tests compared to matched controls (57%), suggesting GI-related mechanisms of glucose control (eg, incretin response) are impacted in psoriasis patients as well.23
Insulin Resistance
Insulin resistance is an important pathophysiologic mechanism involved in DM and psoriasis. Importantly, insulin resistance and inflammation are closely intertwined. Indeed, inflammatory mediators (eg, cytokines and adipokines) involved in the development of insulin resistance are dysregulated in patients with psoriasis.18,24 It stands to reason that insulin resistance has been found in patients with psoriasis and is significantly correlated with area and severity, even in patients without concomitant metabolic syndrome.18,24 Moreover, during times of hyperinsulinemia due to insulin resistance as often seen in Type 2 DM, insulin has the potential to bind to IGF receptors, resulting in keratinocyte and fibroblast proliferation.19 Further, levels of adiponectin, an enhancer of insulin sensitivity and inducer of anti-inflammatory cytokines, are found to be reduced in both DM and psoriasis.18
Associated Comorbidities
In addition to the link between DM and psoriasis through the four described overlapping pathophysiologies, both conditions have other similar associated comorbidities. When considering DM and psoriasis through the prism of these overlapping drivers, it is understandable that other comorbidities that are driven by similar influences are also shared. Below we focus on common comorbidities and their link with psoriasis, with the understanding that DM shares these links as well.1,2,4
Psoriasis and Metabolic Syndrome and Obesity
Metabolic syndrome (MS), the co-existence of central obesity, hypertension, dyslipidemia, and hyperglycemia, are significant risk factors for DM and its associated complications.1,2 Although psoriasis patients are 2–3% of the population, over 30% of them have been found to meet criteria for MS, with up to 76% higher risk in certain populations, especially in women and those over age 40.19,25 Furthermore, when analyzing the prevalence of individual MS components, obesity, dyslipidemia, and hyperglycemia are each found more commonly in those with psoriasis compared to those without.26
The relationship between psoriasis and obesity is largely based on a chronic inflammatory state.27 White adipose tissue is metabolically active, consisting of adipocytes and immune cells like macrophages.28 It is these cells that secrete adipokines, which are pro-inflammatory cytokines such as adiponectin, leptin, chemerin, resistin, visfatin, vaspin, free fatty acids, TNF-ɑ, and several interleukins, most of which have also been implicated in the chronic diseases described above.28 For example, levels of leptin and resistin levels are higher in patients with psoriasis and correlated with disease severity and higher concentrations of TNF-α and IL-6 are considered biomarkers for psoriasis.29 Dysregulation of both pro- and anti-inflammatory adipokines that occurs with obesity leads to chronic inflammation.29 This inflammation is thought to lead to a wide array of metabolic disorders and chronic complications spanning not only psoriasis and DM, but also hypertension, lipid disorders, insulin resistance, infertility, and cancer.29 In the case of psoriasis, inflammation in the fatty tissue related to metabolic dysfunction associated with obesity can directly affect inflammatory processes in psoriatic skin lesions.29 Finally, certain epidemiological studies note that obesity might be an independent risk factor for psoriasis while others suggest that obesity is a consequence of psoriasis.27 Therefore, the association between the two appears to be a bidirectional one.27
Psoriasis and Cardiovascular Disease
With increased MS prevalence comes increased cardiovascular morbidity and mortality. Atherosclerosis associated with cardiovascular disease has been described as a process driven by inflammation.30 Moreover, the underlying pathophysiology of psoriasis is deeply rooted in inflammatory cytokines that inhibit normal functioning of insulin receptors with eventual downstream endothelial dysfunction and hyperproliferation, bridging a link between atherosclerosis and the chronic skin condition.31 Additionally, psoriasis has been found to be a risk factor for myocardial infarction, with increased psoriasis severity being associated with greater risk of an event.32 In fact, severe psoriasis has been found to be an independent risk factor for death from cardiovascular disease.33
Psoriasis and Cancer
A recent systematic review and meta-analysis of 58 observational studies by Trafford et al has found significant associations between psoriasis and both cancer incidence and mortality.34 The cohorts that were analyzed demonstrated an 18% increased risk of developing cancer in psoriasis patients compared to those without, with 22% increased risk in those with severe psoriasis. Incidence of lymphoma, keratinocyte, esophageal, liver, and pancreatic cancers was noted to be higher in the psoriatic population.34 Overall, psoriasis patients were found to have a 22% increased risk of dying from these cancers compared to the psoriasis-free populations.34 This increased risk is thought to be in part due to the chronic inflammation associated with psoriasis.34 Not surprisingly, however, the study authors deemed part of this increased risk due to lifestyle factors like obesity and smoking, as the studies that adjusted for these factors noted considerable attenuation of cancer risk.34 Nonetheless, it is becoming clear to us that the cumulative effects of inflammation and lifestyle factors are implicated in cancer, psoriasis, and DM.4,34
Potential Treatment Implications
It has become evident that the current DM classification of discrete categories (eg, Type 1 versus Type 2) is outdated as we have learned so much more about the complexities of the disease spectrum.2,5 A co-author of this current paper has proposed a new model of classification around the abnormal beta cell and its pathophysiologic causes including genetic predisposition and epigenetic changes, inflammation, environmental influences, and insulin resistance – the aforementioned base of the DM-Psoriasis prism (Figure 1).2,5 The unique contributions of these factors that exist in any individual will define their diabetic phenotype which includes the presentation of their hyperglycemic state, the mechanisms behind their hyperglycemia, and the implications for more specialized therapeutic approaches. These common pathophysiologic mechanisms also contribute to cell and tissue damage within psoriasis.
Viewing these 2 conditions through the same prism suggests that some therapies may be potentially used to treat both psoriasis and DM. Indeed, non-pharmacologic approaches such as lifestyle modification (eg, diet, weight reduction, smoking cessation, increased physical activity) have beneficial effects in both patients with DM and psoriasis.21,35,36,37 This concept also applies to pharmacologic treatment. For example, several case studies have described significantly improved psoriasis severity in patients receiving GLP-1 agonists for DM.38 Additionally, thiazolidinediones (TZDs), antidiabetic drugs that improve insulin sensitivity and decrease inflammation through peroxisome proliferator-activated receptors (PPAR)-ɣ, inhibit proliferation of keratinocytes (which express PPAR receptors).39 A systematic review and meta-analysis found that the TZD pioglitazone significantly decreased psoriasis severity compared to placebo suggesting efficacy for the treatment of psoriasis.39 Finally, metformin, a drug commonly used by patients with DM and metabolic syndrome based on its ability to decrease the rate of hepatic gluconeogenesis and potentially decrease insulin resistance, may also provide potential benefit with regard to psoriasis.40–42 In a large case-control cohort study, metformin use was associated with reduced risk of psoriasis, however this reduction in risk was only observed in males.42 It is hypothesized that this potential benefit may be conferred by the insulin-sensitizing effect as well as anti-inflammatory effects of metformin through activation of adenosine monophosphate-activated protein kinase (AMPK).40–42 Based on this, some have suggested metformin as a possible add-on therapy in overweight male patients with metabolic syndrome, however further research in psoriasis patients with and without metabolic syndrome is clearly warranted.41
Conversely, current psoriasis therapies may potentially be used to treat DM. For example, TNF ɑ-blockers that are approved for the treatment of psoriatic arthritis and psoriasis vulgaris are also being evaluated in DM due to the potential to improve insulin sensitivity.43 A prospective study found that the anti-TNF-ɑ therapy adalimumab showed significantly improved insulin sensitivity in psoriasis patients after 6 months of therapy.44 In addition, first approved for the treatment of moderate-to-severe psoriasis in the US (but not available ex-US), alefacept is a biological drug tested in the T1DAL (inducing remission in new-onset Type 1 DM with alefacept) trial. This study has shown that alefacept is able to target pathogenic effector T cells, preventing β cell destruction in patients with new-onset Type 1 DM.45 The beneficial effects reported for many drugs on both psoriasis and DM may underline the correlation between these diseases. More research is needed to evaluate the efficacy of shared treatments in both diseases.
We believe that as our understanding of the overlapping pathophysiologies between DM and psoriasis continues to grow, so too will our treatment armamentarium. We encourage further investigations of DM and psoriasis through the lens of our prism.
Funding
No funding was used for the generation of this manuscript.
Disclosure
We certify that any affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript (eg, employment, consultancies, stock ownership, honoraria, expert testimony) is disclosed below. Any financial project support of this work is identified in an acknowledgment in the manuscript. Note, no funding source was used for this manuscript.
Dr. Abramczyk has no potential conflicts of interest.
Dr. Queller has no potential conflicts of interest.
Dr. Rachfal is a clinical drug development consultant. None of her past or current clients has provided compensation related to this manuscript. Dr. Rachfal has been compensated by Dr. Schwartz for medical editorial assistance with the manuscript and reports personal fees from Stanley Schwartz, LLC, during the conduct of the study. Note, Dr. Rachfal meets authorship criteria.
Dr. Schwartz is employed and receive financial compensation only from his clinical practice, Stanley Schwartz, MD, LLC (affiliated with Main Line Health [with no monetary connection]) with no compensation related to the manuscript. Dr. Schwartz is on advisory boards for Salix Pharmaceuticals and Arkay Therapeutics and on the Speaker’s Bureau for Salix Pharmaceuticals, Janssen Pharmaceuticals, Boehringer Ingelheim, Eli Lilly, Merck and Novo; and reports personal fees from Merck, Salix, Janssen, Lilly, B-I, outside the submitted work.
The authors report no other potential conflicts of interest for this work.
References
1. National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. Available from: https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html.
2. Schwartz SS, Epstein S, Corkey BE, et al. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol Metab. 2017;28(9):645–655.
3. de la Monte SM. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer’s disease. Drugs. 2017;77(1):47–65.
4. Schwartz SS, Grant SFA, Herman ME. Intersections and clinical translations of diabetes mellitus with cancer promotion, progression and prognosis. Postgrad Med. 2019;131(8):597–606.
5. Schwartz SS, Epstein S, Corkey BE, Grant SFA, Gavin JR, Aguilar R. The time is right for a new classification system for diabetes: rationale and implications of the β-cell-centric classification schema. Diabetes Care. 2016;39(2):179–186.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390.
7. National Psoriasis Foundation. Statistics; 2019. Available from: https://www.psoriasis.org/content/statistics.
8. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475.
9. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91.
10. Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci. 2019;20(18):
11. Mamizadeh M, Tardeh Z, Azami M. The association between psoriasis and diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13(2):1405–1412.
12. Wan MT, Shin DB, Hubbard RA, Noe MH, Mehta NN, Gelfand JM. Psoriasis and the risk of diabetes: a prospective population-based cohort study. J Am Acad Dermatol. 2018;78(2):315–322.e1.
13. Wang H, Wang Z, Rani PL, et al. Identification of PTPN22, ST6GAL1 and JAZF1 as psoriasis risk genes demonstrates shared pathogenesis between psoriasis and diabetes. Exp Dermatol. 2017;26(11):1112–1117.
14. Mishra R, Chesi A, Cousminer DL, et al. Relative contribution of type 1 and type 2 diabetes loci to the genetic etiology of adult-onset, non-insulin-requiring autoimmune diabetes. BMC Med. 2017;15(1):88.
15. Quaranta M, Burden AD, Griffiths CEM, et al. Differential contribution of CDKAL1 variants to psoriasis, Crohn’s disease and type II diabetes. Genes Immun. 2009;10(7):654–658. doi:10.1038/gene.2009.51
16. Granata M, Skarmoutsou E, Trovato C, Rossi GA, Mazzarino MC, D’Amico F. Obesity, type 1 diabetes, and psoriasis: an autoimmune triple flip. Pathobiology. 2017;84(2):71–79.
17. Ruan Q, Wang T, Kameswaran V, et al. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc Natl Acad Sci U S A. 2011;108(29):12030–12035.
18. Davidovici BB, Sattar N, Prinz JC, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130(7):1785–1796.
19. Hu Y, Zhu Y, Lian N, Chen M, Bartke A, Yuan R. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10.
20. Zeng J, Luo S, Huang Y, Lu Q. Critical role of environmental factors in the pathogenesis of psoriasis. J Dermatol. 2017;44(8):863–872.
21. Khazrai YM, Defeudis G, Pozzilli P. Effect of diet on type 2 diabetes mellitus: a review. Diabetes Metab Res Rev. 2014;30(S1):24–33.
22. Holst JJ, Vilsbøll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297(1–2):127–136.
23. Gyldenløve M, Vilsbøll T, Zachariae C, Holst JJ, Knop FK, Skov L. Impaired incretin effect is an early sign of glucose dysmetabolism in nondiabetic patients with psoriasis. J Intern Med. 2015;278(6):660–670.
24. Polic MV, Miskulin M, Smolic M, et al. Psoriasis severity—a risk factor of insulin resistance independent of metabolic syndrome. Int J Environ Res Public Health. 2018;15(7):1486.
25. Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77(4):657–666.e8.
26. Gui XY, Yu XL, Jin H-Z, Zuo YG, Wu C. Prevalence of metabolic syndrome in Chinese psoriasis patients: a hospital-based cross-sectional study. J Diabetes Investig. 2018;9(1):39–43.
27. Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232(6):633–639.
28. Kong Y, Zhang S, Wu R, et al. New insights into different adipokines in linking the pathophysiology of obesity and psoriasis. Lipids Health Dis. 2019;18(1):171.
29. Zorena K, Jachimowicz-Duda O, Ślęzak D, Robakowska M, Mrugacz M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int J Mol Sci. 2020;21(10):3570.
30. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695.
31. Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. 2018;9:579.
32. Mosca S, Gargiulo P, Balato N, et al. Ischemic cardiovascular involvement in psoriasis: a systematic review. Int J Cardiol. 2015;178:191–199.
33. Nasrin GM, Mohammed T, Nigel JC. Double trouble: psoriasis and cardiometabolic disorders. Cardiovasc J Afr. 2018;29(3):189–194.
34. Trafford AM, Parisi R, Kontopantelis E, Griffiths CEM, Ashcroft DM. Association of psoriasis with the risk of developing or dying of cancer: a systematic review and meta-analysis [published online ahead of print, 2019 Oct 16]. JAMA Dermatol. 2019;155(12):1390–1403.
35. García-Molina L, Lewis-Mikhael AM, Riquelme-Gallego B, Cano-Ibáñez N, Oliveras-López MJ, Bueno-Cavanillas A. Improving type 2 diabetes mellitus glycaemic control through lifestyle modification implementing diet intervention: a systematic review and meta-analysis. Eur J Nutr. 2020;59(4):1313–1328.
36. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 Suppl 1):S15–S21.
37. Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database Syst Rev. 2019;7(7):CD011972.
38. Al-Badri MR, Azar ST. Effect of glucagon-like peptide-1 receptor agonists in patients with psoriasis. Ther Adv Endocrinol Metab. 2014;5(2):34–38.
39. Malhotra A, Shafiq N, Rajagopalan S, Dogra S, Malhotra S. Thiazolidinediones for plaque psoriasis: a systematic review and meta-analysis. Evid Based Med. 2012;17(6):171–176.
40. Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol. 2013;27(11):1329–1335.
41. Glossmann H, Reider N. A marriage of two “Methusalem” drugs for the treatment of psoriasis?: arguments for a pilot trial with metformin as add-on for methotrexate. Dermatoendocrinol. 2013;5(2):252–263.
42. Brauchli YB, Jick SS, Curtin F, Meier CR. Association between use of thiazolidinediones or other oral antidiabetics and psoriasis: a population based case-control study. J Am Acad Dermatol. 2008;58(3):421–429.
43. Barra L, Pope JE, Payne M. Real-world anti-tumor necrosis factor treatment in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: cost-effectiveness based on number needed to treat to improve health assessment questionnaire. J Rheumatol. 2009;36(7):1421–1428.
44. Pina T, Armesto S, Lopez-Mejias R, et al. Anti-TNF-α therapy improves insulin sensitivity in non-diabetic patients with psoriasis: a 6-month prospective study. J Eur Acad Dermatol Venereol. 2015;29(7):1325–1330.
45. Rigby MR, Harris KM, Pinckney A, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 1205;125(8):3285–3296.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
