Back to Journals » Drug Design, Development and Therapy » Volume 13
Dexmedetomidine Promotes Hippocampal Neurogenesis and Improves Spatial Learning and Memory in Neonatal Rats
Authors Zhang Y, Gao Q, Wu Z, Xue H, Liu B, Zhao P
Received 22 August 2019
Accepted for publication 2 December 2019
Published 3 January 2020 Volume 2019:13 Pages 4439—4449
DOI https://doi.org/10.2147/DDDT.S228220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Yahan Zhang,1 Qiushi Gao,1 Ziyi Wu,1 Hang Xue,1 Bo Liu,2 Ping Zhao1
1Department of Anesthesiology, Shengjing Hospital, China Medical University, Shenyang, People’s Republic of China; 2Department of Animal Laboratory of Experimental Research Center, Shengjing Hospital, China Medical University, Shenyang, People’s Republic of China
Correspondence: Ping Zhao
Department of Anesthesiology, Shengjing Hospital, China Medical University, No. 36 Sanhao Street, Heping District, Shenyang, People’s Republic of China
Tel +86-189-4025-8971
Email [email protected]
Background: Dexmedetomidine (Dex) is a highly selective α2-adrenoceptor agonist used as an off-label medication for pediatric sedation and analgesia. Recently, Dex was reported to exhibit neuroprotective efficacy in several brain injury models. Here we investigate whether neonatal Dex administration promotes hippocampal neurogenesis and enhances hippocampus-dependent spatial learning and memory under physiological conditions.
Methods: Postnatal day 7 (P7) pups were administered saline (vehicle control) or Dex (10, 20, or 40 μg/kg) by intraperitoneal injection. Neurogenesis and astrogenesis were examined in brain slices by BrdU immunostaining on P8 and changes in the expression levels of GDNF, NCAM, CREB, PSD95, and GAP43 were assessed by Western blotting on P35, respectively. Open field and Morris water maze (MWM) tests were conducted from P28 to P36 in order to assess effects on general motor activity and spatial learning, respectively.
Results: Dexmedetomidine at 20 μg/kg significantly enhanced neurogenesis and astrogenesis in hippocampus and upregulated GDNF, NCAM, CREB, PSD95, and GAP43 compared to vehicle and other Dex doses. Moreover, 20 μg/kg Dex-injected rats showed no changes in motor or anxiety-like behavior but performed better in the MWM test compared to all other groups.
Conclusion: Neonatal injection of Dex (20 μg/kg) enhances spatial learning and memory in rat pups, potentially by promoting hippocampal neurogenesis and synaptic plasticity via activation of GDNF/NCAM/CREB signaling.
Keywords: dexmedetomidine, neurogenesis, GDNF, spatial learning and memory, neonate
Introduction
Dexmedetomidine (Dex) is a potent α2-adrenoceptor agonist used in Europe as an adjunct to general anesthesia for prevention of pain and agitation during various pediatric medical procedures.1 It is used independently or in combination with other regimens like propofol or ketamine and has been shown to be relatively safe, exhibiting no marked effects on hemodynamic or respiratory activity.2 However, broad pharmacological and safety spectra are incomplete, so use in children is restricted mainly to sedation during radiological procedures and mitigation of pain and shivering in the pediatric intensive care unit (PICU).3
In recent years, the neuroprotective effect of Dex has been proved.2,4,5 For instance, Dex reduced production of the inflammatory cytokine IL-1β and modulated ex pression levels of the antioxidant glutathione (GSH) and neurotrophic factors in premature infants with hyperoxia-induced brain injury.4–6 In addition, Wang et al reported that Dex can mitigate postoperative cognitive dysfunction (POCD) in rats by promoting neurogenesis.7 Furthermore, Perez-Zoghbi et al have clarified that co-administration of Dex dose-dependently was able to alleviate the injury of extensive brain regions against sevoflurane neurotoxicity.2 However, the evidence above only indicated the effect of Dex against injuries under physiological conditions but neglecting its own neuroprotective efficacy. The conclusion of Wang et al illustrated that suitable dose of sevoflurane alone could improve neurogenesis which inspired us to test whether Dex used alone could promote the production of hippocampal new neuron. As we know, new neurons are produced throughout life in the hippocampal dentate gyrus (DG) subgranular zone (SGZ) and cortical subventricular zone (SVZ), and maintenance of neurogenesis is considered critical for neural plasticity, memory, and protection against metabolic insults.8,9 However, whether Dex exerts neuroprotection by promoting neurogenesis, release of neurotrophic factors, antioxidant activity, anti-inflammatory activity, or some combination is still unclear.
GDNF is a soluble neurotrophic factor secreted by astrocytes that contributes to repair following neurological injury and sustains cortical dopaminergic (DAergic) neuron survival under normal physiological conditions.10 For instance, Dex dose-dependently triggered GDNF release from cultured astrocytes and activated downstream cAMP-response element-binding protein (CREB) to protect neurons from oxygen and glucose deprivation (OGD) injury.5 Hippocampal CREB is also a critical modulator of synaptic plasticity and learning and memory capacity,11 and GDNF can trigger CREB activation through NCAM binding to influence neurite outgrowth, synaptic plasticity, and memory.12
To date, however, there is no direct evidence that Dex administration can trigger neurogenesis in the neonatal DG, activate GDNF/NCAM/CREB signaling, or promote hippocampus-dependent learning and memory. We examined these questions in neonatal rats by immunohistochemical assessment of neuronal and astroglial proliferation in the DG, Western blot analysis of GDNF, NCAM, and CREB expression in hippocampus, and behavioral tests of spatial learning and memory.
Materials and Methods
Ethical Approval and Animal Preparation
Postnatal day 7 (P7) Sprague-Dawley rat pups were used in this study due to peak neurogenesis of hippocampus. Housing conditions complied with the regulations of the National Animal Experiment Center. Briefly, pups were housed under a 12 h:12 h light: dark cycle with ad libitum access to food and water. All experimental procedures were approved by The Laboratory Animal Care Committee of China Medical University (Shenyang, China; no. 2016PS337K) and conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Grouping
Total 200 pups of both sexes were randomly divided into four groups (n=50 per group): a saline vehicle (Control, C) group and three Dex dose groups, 10 µg/kg (DEX10), 20 µg/kg (DEX20), and 40 µg/kg (DEX40). Dexmedetomidine (Hengrui Medicine Co, Ltd, Jiangsu, China) was injected intraperitoneally at the indicated dose in 0.1−0.2 mL saline, while group C received equal volume of 0.9% saline.
Bromodeoxyuridine Injections
Bromodeoxyuridine (BrdU, 5-bromo-2-deoxyuridine; B5002, Sigma, USA) was intraperitoneally injected at 200 mg/kg 30 min before DEX injection to investigate effects on hippocampal neurogenesis. Rats were sacrificed and brains were harvested for staining 24 h after BrdU administration (P8).
Tissue Processing and Immunostaining
Pups (n=5 for BrdU stain at P8 and P35, n=5 for BrdU/GFAP stain at P8, respectively) treated as indicated were deeply anesthetized with sodium pentobarbital (80 mg/kg) and transcardially perfused with 0.9% sodium followed by 4% paraformaldehyde. Brains were then removed, post-fixed overnight in 4% paraformaldehyde at 4°C, embedded in paraffin, and sectioned in the coronal plane at 3.0−3.5 um thickness. Slices were then deparaffinized, and immunostained. Briefly, slices from BrdU-injected rats were treated with 2 N HCl for 30 min at 37°C for DNA denaturation and with 0.1 M borate buffer (pH 8.5) for neutralization. These slices were then blocked by incubation in 5% fetal bovine serum for 30 min, washed in PBS, and incubated overnight with anti-BrdU (1:500; Abcam, Cambridge, UK) or anti-BrdU mixed with anti-GFAP (1:100, Millipore, MAB377, Germany) at 4°C. Immunolabeled slices were then incubated with one or two kinds of secondary antibodies for single or double staining for 2 h at room temperature and cell nuclei were counterstained with DAPI. In order to evaluate neurogenesis level via BrdU stain at P8 and P35, we counted the positively stained BrdU-labeled cells then divided by the total cell number in a fixed area (1000 ×1000) pixels by NIS-Elements AR Analysis 4.50.00 software from every 60 µm slice coronal sections, totally 5 sections per brain. In order to quantify the newly generated astrocyte number, double labelled BrdU/GFAP positive cells were counted in a fixed area at a magnification of X400 using a confocal scanning laser microscope (Zeiss LSM 880) from every 60 µm slice coronal sections, totally 5 sections per brain. Representative photographs of the hippocampal DG were captured using a Nikon C1 microscope.
For glial fibrillary acid protein (GFAP) immunostaining at P8 (n=5 per group), paraffin-embedded brain slices prepared as described were deparaffinized, heated in citrate buffer for 7 min 30 s at 120°C for antigen recovery, and immunostained with anti-GFAP (1:200, Ab53554, Abcam, UK) using a commercial immunohistochemistry kit (ZB2050, zsbio, China). Immunolabeling was visualized by chromogenic staining using diaminobenzidine (DAB) followed by nuclear counterstaining with hematoxylin (ZSGB-BIO, ZLI-9610) and imaging using a Nikon C1 microscope. For the purpose of evaluating the number of astrocytes at P8, we counted positive cells divided by the total cell number in a fixed area (1000 ×1000) pixels by NIS-Elements AR Analysis 4.50.00 software from every 60 µm slice coronal sections, totally 5 sections per brain.
Western Blotting Analysis
At 24 h after DEX injection, bilateral hippocampi of P8 pups for GDNF/NCAM/CREB analysis (n=5 per group) and P35 pups for PSD95/GAP43 analysis (n=5 per group) were rapidly harvested, flash-frozen, and stored at −80°C until use. Frozen hippocampi were cut and then lysed on ice for 30 min. The lysate was centrifuged and total protein concentration measured using a BCA Protein Assay Kit (P0010; Beyotime, China). Equal amounts of protein per gel lane were separated by electrophoresis using 12.5% SDS-polyacrylamide gels and electrotransferred to polyvinylidene fluoride membranes (IPVH0010; Millipore, Germany). The membranes were blocked in 5% non-fat milk diluted in Tris-buffered saline with Triton X (TBST) for 1 h, incubated with the indicated primary antibodies overnight at 4°C, and then incubated in secondary antibodies for 2 h at room temperature. Protein bands were visualized and photographed using SuperSignal R West Pico Chemiluminescent Substrate (34080; Thermo, USA) and a GE Amersham Imager 600. The primary antibodies used were anti-GDNF (1:500 Ab18956; Abcam, Cambridge, UK), anti-NCAM (1:1000, 3606S; Cell Signaling Technology, Danvers, MA, USA), anti-PSD95 (1:1000, 3450S; Cell Signaling Technology), anti-GAP43 (1:1000, 8945S; Cell Signaling Technology), and anti-CREB (1:1000, 9197S; Cell Signaling Technology).
Ethological Tests
Rats in every group were housed separately after weaning until postnatal day 28. Two behavioral tests were conducted as previously described on days P28–P34, the open-field test and Morris water maze test.13
Open Field Test
The open-field test was used to evaluate anxiety-like and locomotor behaviors.14 Rats (n=10 per group) were placed in a 100 cm × 100 cm × 40 cm (W × D × H) arena with opaque sidewalls under dim lighting and tracked by infrared detectors to observe horizontal movement and rearing (Noldus Ethovision XT, Netherland). Rats tend to avoid the center region of the arena in favor of the wall.15 Rats in each group were allowed to explore the arena for 10 min and movement distance, time spent in the center and wall, movement velocity, and fecal pellets were recorded for anxiety mood evaluation.
Morris Water Test
Spatial learning and memory were tested in the Morris water maze (MWM) from P29 to P34. The MWM consisted of a steel pool 160 cm in diameter and 60 cm deep filled with opaque water at 20 ± 1°C and surrounded by salient visual cues. Rats (n=10 per group) were trained to find a hidden (submerged) escape platform in a fixed quadrant for 5 consecutive days (training phase). On each trial, the rat was placed at the side of the pool facing the edge and allowed to search for the submerged platform for 90 s. The time spent before the rats reached the submerged platform was recorded as Escape latency.16 In case of failure, the rat was guided to the platform and allowed to remain on it for another 20 s.
During the spatial memory probe test on the 6th day, the platform was removed. Rats were released from the quadrant opposite to the former platform location and permitted to swim for 90 s. The swim paths of each animal were recorded by infrared detectors and data analyzed using image analysis software (Noldus Ethovision XT, Netherland)
Statistical Analysis
Data are presented as mean ± standard deviation (SD). All data were analyzed using SPSS 20.0 or GraphPad Prism 6.0. Datasets were first assessed for equal variance using Bartlett’s test and for normality using the Shapiro–Wilk test. Group means were compared by one-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc tests for pair-wise comparisons. Escape latencies during MWM training trials were compared by two-way repeated measures ANOVA. In all tests, a P < 0.05 (two-tailed) was considered significant.
Result
Dexmedetomidine Promotes Neurogenesis in the Dentate Gyrus of Neonatal Rats
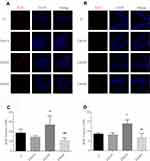
To investigate whether Dex alters neurogenesis, hippocampal slices were prepared from rats following intraperitoneal injection of Dex and the mitosis indicator BrdU and immunostained for BrdU detection at P8 and P35, respectively. Immunostaining revealed enhanced BrdU-positive cell number in the DG of rats injected with 20 μg/kg Dex (DEX20 group) compared to the control (C) group (Figure 1A and C; p < 0.01 at P8, Figure 1B and D; p < 0.05 at P35, respectively). BrdU-positive cell number was slightly lower in the DEX40 group compared to the DEX20 group (Figure 1A and C; p < 0.001 at P8, Figure 1B and D; p < 0.01 at P35, respectively), while there was no significant difference between the C group and the DEX10 group. Therefore, a Dex dose of 20−40 μg/kg accelerated hippocampal neurogenesis with peak efficacy at 20 μg/kg, possibly because higher doses may also induce apoptosis of developing neurons.17
Dexmedetomidine Increases Astrogenesis in Hippocampus of Neonatal Rats
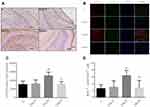
Astrocytes are critical for maintaining neuronal survival under physiological conditions and for repair following injury. Whether Dex can trigger astrogenesis in the hippocampus was examined by immunochemistry and BrdU/GFAP double immunostaining. According to immunochemistry results, the overall astrocyte number was significantly higher in the DEX20 group compared to the C group (Figure 2A and C; p < 0.01); however, GFAP expression was significantly lower in the DEX40 group compared to the DEX20 group (Figure 2A and C; p < 0.01). In order to clearly present newborn astrocyte, double staining BrdU/GFAP was conducted at P8. Immunostaining revealed increased BrdU/GFAP positive cell number in the DG of rats in DEX20 group compared to the C group (Figure 2B and D; p < 0.01). However, BrdU/GFAP positive cell number was lower in the DEX40 group in comparison with the DEX20 group (Figure 2B and D; p < 0.01). There was no significant difference in newborn astrocyte number between the C group and the DEX10 group.
Dexmedetomidine Enhances Neural Cell Expression of GNDF, NCAM, and CREB
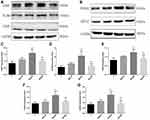
The GDNF/NCAM/CREB signaling pathway is implicated in hippocampal neurogenesis, neuronal survival, and synaptic plasticity, processes essential for hippocampus-dependent learning and memory.18 Given the upregulation of neurogenesis and gliogenesis observed following Dex administration, we examined if Dex also upregulates the expression of these signaling factors. Indeed, the DEX20 group exhibited significantly upregulated GDNF and NCAM expression compared to the C group (Figure 3A, C and D; p < 0.001, p < 0.0001, respectively). Consistent with effects on neurogenesis and gliogenesis, expression levels of both GDNF and NCAM were lower in the DEX40 group compared to the DEX20 group (Figure 3A, C and D; p < 0.001, p < 0.001, respectively). Similarly, 20 μg/kg Dex also upregulated CREB expression compared to the C group (Figure 3A and E; p < 0.001) and compared to the DEX40 group (Figure 3A and E; p < 0.01). These findings suggest that 20 μg/kg Dex may enhance GDNF/NCAM/CREB signaling.
Dexmedetomidine Facilitates Expression of Synapse-Associated Proteins in the Hippocampi
We then examined the effects of Dex injection on the expression of the synaptic markers PSD95 and GAP43 in the hippocampus at P35 by Western blot. Indeed, in parallel with GDNF/NCAM/CREB upregulation, 20 μg/kg Dex injection also elevated the expression levels of PSD95 and GAP43 compared to the C group (Figure 3B, F and G; p < 0.001, p < 0.0001, respectively) and the DEX40 group (Figure 3B, F and G; p < 0.001, p<0.001, respectively), while expression levels in the latter group did not differ from the C group or DEX10 group.
Dexmedetomidine Treatment in Neonates Facilitates Later Spatial and Memory Performance
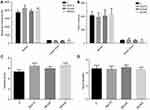
The long-term effects of Dex on learning and memory ability were examined in P−35rats by the MWM test. To exclude anxiety and locomotor interference produced by Dex administration, rats were first examined in the open field test at P28. There were no differences in movement distance, time spent in the center and wall, movement velocity, and fecal pellets among groups (Figure 4A–D). There were also no significant differences in swimming velocities among groups, consistent with open field results suggesting that Dex has no effect on motor function (Figure 5A). However, rats in the DEX20 group demonstrated significantly reduced escape latencies during hidden-platform training trials compared to the control group (Figure 5C, DEX20 group versus C group, p < 0.01 on the 2nd, 3rd, and 4th training days, p < 0.05 on the 5th day), indicating superior spatial learning. In addition, the DEX20 group demonstrated a greater number of crossings over the former platform location during the probe trial compared to the C group (Figure 5B, p<0.01), suggesting superior spatial memory for platform location. However, there were no significant differences in training or probe trial performance among controls and the other DEX groups.
Discussion
Intraperitoneal injection of dexmedetomidine (20 µg/kg) promoted hippocampal neurogenesis and astrogenesis, upregulated expression of the neurotrophic factor GDNF, the GDNF receptor NCAM, the downstream transcription factor CREB, and the synaptic proteins PSD95 and GAP43, and this sustained neurogenesis resulted in enhancing both spatial learning and memory in later life. These findings suggest that Dex can enhance hippocampal synaptic plasticity via activation of GDNF/NCAM/CREB signaling, resulting in improved spatial memory capacity.
Dexmedetomidine is a potent and selective alpha-2-adrenergic agonist with superior analgesic efficacy compared to clonidine.19 Neither a general anesthetic nor pure analgesic, it is used as an open-label drug across Europe for pediatric sedation, an application supported by a wealth of clinical research on its pharmacological activity in children.3,20,21 However, there is still a need for additional information on pharmacological and safety profiles, especially as many anesthetics can induce post-operative cognitive dysfunction. Contrary to this effect, Dex actually promoted cognitive function when administered alone to neonatal rats.
Hoffman reported that Dex improved neurologic outcome following transient partial brain ischemia in rats,22 a finding that inspired numerous subsequent studies on the neuroprotective efficacy of Dex under other pathogenic conditions. Indeed, DEX may decrease the severity of neuroinflammation by reducing the release of the pro-inflammatory cytokines IL-1β and IL-6 in systemic inflammatory response syndrome (SIRS) and alleviate perinatal hypoxic brain injury by upregulating neurotrophic factor expression.23,24 Moreover, Dex dose-dependently attenuated isoflurane-induced neural apoptosis in multiple brain regions by upregulating autophagy.25,26 However, most of these studies have focused on the promotion of neurogenesis and neuroprotection under pathological conditions. For instance, Endesfelder reported that Dex pre-treatment promoted hippocampal neural proliferation and plasticity in P7 rat pups subjected to hyperoxia-induced injury.27 Furthermore, Wang demonstrated pro-neurogenic activity and improved cognitive recovery following POCD in adult rats via activation of the PKA/CREB/BDNF signaling pathway and consequent promotion of neurogenesis.7 Thus, Dex appears to have pro-neurogenic, neuroprotective, and pro-synaptoplastic effects under pathological conditions, but effects during normal brain development are less clear.
Postnatal neurogenesis is a multi-step process involving neural stem cell proliferation, differentiation, migration to the programmed niche, and integration into existing neural circuits.28 The two neurogenic niches, the dentate gyrus SGZ and cortical SVZ, produce neuronal progenitors throughout life. The DG, a part of the hippocampal formation critical for spatial memory, reaches peak neurogenesis around postnatal week one in rodents. However, the rate of neurogenesis may be modulated by pathological events. Following ischemic or chemically induced neuronal death, neurogenesis rate may increase due to enhanced neurotrophic factor secretion by astrocytes.29 Therefore, we examined the effects of Dex alone at P7 under normal physiological conditions and demonstrate that Dex at 20 µg/kg i.p. can indeed promote neurogenesis and astrogenesis in the DG. In contrast, higher and lower doses were less effective and also did not substantially enhance spatial learning, suggesting that promotion of neurogenesis contributes to the superior spatial learning demonstrated by rats administered 20 µg/kg i.p. Dex.
Astrocyte proliferation is an important reparative response to brain injury. These reactive astrocytes can scavenge cell debris and secrete neurotrophic factors to promote neuronal repair and metabolism.30 Yan reported that Dex dose-dependently stimulated astrocytes to release GDNF via downstream activation of PKC and CREB.12 Consistent with these findings, 20 µg/kg i.p. Dex increased astrocyte numbers as revealed GFAP immunostaining and upregulated GDNF expression. Alternatively, high-dose Dex administration had less marked effects on astrogenesis and GDNF expression as well as on spatial memory, again suggesting that GDNF signaling is necessary for improved spatial memory by 20 µg/kg i.p. Dex. While higher doses did not appear beneficial, they also did not appear to have deleterious effects on hippocampal function.
Synaptic plasticity is believed to be the primary neurocellular mechanism for information storage in the hippocampus.31 CREB is a key transcription factor involved in long-lasting forms of hippocampal plasticity dependent on de novo protein synthesis. The enhanced expression of CREB and synaptic proteins suggests that Dex enhances spatial memory by promoting neural plasticity. It is widely accepted that the hippocampus is responsible for the early storage of episodic memories, and a large body of evidence suggests that the continuous supply of newborn hippocampal cells is necessary for the maintenance of spatial learning and memory over time.32 However, it is still unclear how these effects are initiated by Dex and if neurogenesis is directly related to synaptic plasticity underlying spatial memory. Peak upregulation of the synaptic proteins PSD95 and GAP43 by 20 µg/kg Dex concomitant with peak enhancement of neurogenesis and MWM performance suggests such as relationship, but further studies are needed for confirmation. Finally, we found no differences in open field activity among groups, consistent with the report of Groves that newborn cell quantity fluctuation did not affect rodent performance in this task,33 and suggesting that Dex has no effect on locomotor function or anxiety over the entire dose range tested.
Overall, the experimental data demonstrates that Dexmedetomidine promotes hippocampal neurogenesis and improves spatial learning and memory, however, it cannot be excluded that Dex exerted its neurogenic function directly or indirectly by neuroprotective, anti-oxidative, anti-apoptotic and anti-inflammatory effects in drug-induced brain injury and acute kidney injury model.34,35 It is likely that Dexmedetomidine may bring about such effect by modulating signaling pathways like BMP/SMAD, Wnt/β-catenin signaling cooperates through LEF1, SHH, and thereby modulating neurogenic niches or microenvironment in the brain to enhance physiologically function like memory35–37. Overall, the data suggest that Dexmedetomidine promotes the neurogenesis and warrants further investigation in hippocampal and SVZ neurogenic niche and advanced genetic models.38
This study has several limitations. First, in this report, we only put our emphasis on clarifying neurogenesis promotive effect and molecular mechanisms of Dex on DG, regardless of SVZ, the other neural stem niche supplying newborn neural cells throughout the whole life. However, more experiments should be conducted to evaluate the hidden association of Dexmedetomidine with stem cells in SVZ via target gene detection approach and transgenic animal tool. In addition, we also did not provide evidence that any of these changes in protein expression or neurogenesis are necessary for improved cognition in Dex-treated rats.
Conclusion
Our findings illustrate that Dex alone can promote neurogenesis in the DG, upregulate expression of GDNF, NCAM, and CREB through astrogenesis, components of a critical signaling pathway involved in synaptic plasticity, and improve spatial learning and memory in later life. Collectively, these results suggest that a suitable dosage of Dex is a promising potential treatment for neural repair via facilitating newborn neural cells into function state and functional recovery following brain insult. Further studies are needed to examine the underlying target gene-based mechanisms by advanced approaches and the clinical potential and safety of Dex, especially in neonatal medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bhana N, Goa KL, McClellan KJ Dexmedetomidine. Drugs.2000;59:263–268. doi:10.2165/00003495-200059020-00012
2. Perez-Zoghbi JF, Zhu W, Grafe MR. Brambrink AM Dexmedetomidine-mediated neuroprotection against sevoflurane-induced neurotoxicity extends to several brain regions in neonatal rats. Br J Anaesth. 2017;119:506–516. doi:10.1093/bja/aex222
3. Mason KP, Lerman J. Review article: dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113:1129–1142. doi:10.1213/ANE.0b013e31822b8629
4. Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi:10.1038/nn.2136
5. Yan M, Dai H, Ding T, et al. Effects of dexmedetomidine on the release of glial cell line-derived neurotrophic factor from rat astrocyte cells. Neurochem Int. 2011;58:549–557. doi:10.1016/j.neuint.2011.01.013
6. Sifringer M, von Haefen C, Krain M, et al. Neuroprotective effect of dexmedetomidine on hyperoxia-induced toxicity in the neonatal rat brain. Oxid Med Cell Longev. 2015;2015:530371. doi:10.1155/2015/530371
7. Wang WX, Wu Q, Liang SS, et al. Dexmedetomidine promotes the recovery of neurogenesis in aged mouse with postoperative cognitive dysfunction. Neurosci Lett. 2018;677:110–116. doi:10.1016/j.neulet.2018.03.043
8. Steiner B, Klempin F, Wang L, Kott M, Kettenmann H. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi:10.1002/glia.20407
9. Neurogenesis FJ. Gliogenesis in nervous system plasticity and repair. Annu Rev Cell Dev Biol. 2016;32:127–141. doi:10.1146/annurev-cellbio-111315-124953
10. Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J Neurosci. 2001;21:8108–8118. doi:10.1523/JNEUROSCI.21-20-08108.2001
11. Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res. 2002;133:135–141. doi:10.1016/S0166-4328(01)00470-3
12. Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi:10.1016/S0092-8674(03)00435-5
13. Xu Y, Tian Y, Tian Y, Li X, Zhao P. Autophagy activation involved in hypoxic-ischemic brain injury induces cognitive and memory impairment in neonatal rats. J Neurochem. 2016;139:795–805. doi:10.1111/jnc.13851
14. Scobie KN, Hall BJ, Wilke SA, et al. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–9887. doi:10.1523/JNEUROSCI.2260-09.2009
15. Kuniishi H, Ichisaka S, Yamamoto M, et al. Early deprivation increases high-leaning behavior, a novel anxiety-like behavior, in the open field test in rats. Neurosci Res. 2017;123:27–35. doi:10.1016/j.neures.2017.04.012
16. Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi:10.1371/journal.pone.0005464
17. Pancaro C, Segal BS, Sikes RW, et al. Dexmedetomidine and ketamine show distinct patterns of cell degeneration and apoptosis in the developing rat neonatal brain. J Matern Fetal Neonatal Med. 2016;29:3827–3833. doi:10.3109/14767058.2016.1148132
18. Kutlu MG, Gould TJ. Nicotinic modulation of hippocampal cell signaling and associated effects on learning and memory. Physiol Behav. 2016;155:162–171. doi:10.1016/j.physbeh.2015.12.008
19. McMorrow SP, Abramo TJ. Dexmedetomidine sedation: uses in pediatric procedural sedation outside the operating room. Pediatr Emerg Care. 2012;28:292–296. doi:10.1097/PEC.0b013e3182495e1b
20. Sakurai Y, Obata T, Odaka A, Terui K, Tamura M, Miyao H. Buccal administration of dexmedetomidine as a preanesthetic in children. J Anesth. 2010;24:49–53. doi:10.1007/s00540-009-0863-z
21. Zub D, Berkenbosch JW, Tobias JD. Preliminary experience with oral dexmedetomidine for procedural and anesthetic premedication. Paediatr Anaesth. 2005;15:932–938. doi:10.1111/j.1460-9592.2005.01623.x
22. Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–332.
23. Alam A, Hana Z, Jin Z, Suen KC, Surgery MD. Neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. doi:10.1016/j.ebiom.2018.10.021
24. Degos V, Charpentier TL, Chhor V, et al. Neuroprotective effects of dexmedetomidine against glutamate agonist-induced neuronal cell death are related to increased astrocyte brain-derived neurotrophic factor expression. Anesthesiology. 2013;118:1123–1132. doi:10.1097/ALN.0b013e318286cf36
25. Luo C, Ouyang MW, Fang YY, et al. Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1alpha. Front Cell Neurosci. 2017;11:197. doi:10.3389/fncel.2017.00197
26. Sanders RD, Xu J, Shu Y, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi:10.1097/ALN.0b013e31819daedd
27. Endesfelder S, Makki H, von Haefen C, Spies CD, Buhrer C, Sifringer M. Neuroprotective effects of dexmedetomidine against hyperoxia-induced injury in the developing rat brain. PLoS One. 2017;12:e0171498. doi:10.1371/journal.pone.0171498
28. Kronenberg G, Reuter K, Steiner B, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi:10.1002/(ISSN)1096-9861
29. Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A. 2007;104:17169–17173. doi:10.1073/pnas.0708228104
30. Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia. 2005;50:307–320. doi:10.1002/glia.20204
31. Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi:10.1038/nrn2303
32. Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi:10.1038/nn1707
33. Groves JO, Leslie I, Huang GJ, et al. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genet. 2013;9(9):e1003718
34. Hsing CH, Lin CF, So E, et al. Alpha2-Adrenoceptor agonist dexmedetomidine protects septic acute kidney injury through increasing BMP-7 and inhibiting HDAC2 and HDAC5. Am J Physiol Renal Physiol. 2012;303:F1443–53. doi:10.1152/ajprenal.00143.2012
35. Shan Y, Yang F, Tang Z, et al. Dexmedetomidine ameliorates the neurotoxicity of sevoflurane on the immature brain through the BMP/SMAD signaling pathway. Front Neurosci. 2018;12:964. doi:10.3389/fnins.2018.00964
36. Gajera CR, Emich H, Lioubinski O, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123:1922–1930. doi:10.1242/jcs.065912
37. Armenteros T, Andreu Z, Hortiguela R, Lie DC. Mira H BMP and WNT signalling cooperate through LEF1 in the neuronal specification of adult hippocampal neural stem and progenitor cells. Sci Rep. 2018;8:9241. doi:10.1038/s41598-018-27581-0
38. Lena B, Annabel C, Chandresh G, Annette HL, Thomas W. LRP2 in SHH-dependent adult neurogenesis. Mech Dev. 2017;145(Supplement):S116.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.