Back to Journals » International Journal of Nanomedicine » Volume 14
Dexamethasone-loaded injectable silk-polyethylene glycol hydrogel alleviates cisplatin-induced ototoxicity
Authors Chen Y, Gu J, Liu J, Tong L, Shi F, Wang X , Wang X , Yu D, Wu H
Received 20 November 2018
Accepted for publication 21 April 2019
Published 6 June 2019 Volume 2019:14 Pages 4211—4227
DOI https://doi.org/10.2147/IJN.S195336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Linlin Sun
Yuming Chen,1–3 Jiayi Gu,1–3 Jian Liu,4 Ling Tong,1 Fuxin Shi,5 Xiaoqin Wang,4 Xueling Wang,1–3 Dehong Yu,1–3 Hao Wu1–3
1Department of Otolaryngology-Head and Neck Surgery, Shanghai Ninth People’s Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200011, People’s Republic of China; 2Ear Institute, School of Medicine, Shanghai Jiao Tong University, Shanghai 200011, People’s Republic of China; 3Shanghai Key Laboratory of Translational Medicine on Ear and Nose Diseases (14DZ2260300), Shanghai 200011, People’s Republic of China; 4National Engineering Laboratory for Modern Silk, Soochow University, Suzhou 215123, People’s Republic of China; 5Department of Otology and Laryngology, Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Decibel Therapeutics, Boston, MA, 02215, USA
Background: Cisplatin is an extensively used anti-neoplastic agent for the treatment of various solid tumors. However, a high incidence of severe ototoxicity is accompanied by its use in the clinic. Currently, no drugs or therapeutic strategies have been approved for the treatment of cisplatin-induced ototoxicity by the FDA.
Purpose: The purpose of this study was to investigate the otoprotective effects of dexamethasone (DEX)-loaded silk-polyethylene hydrogel (DEX-SILK) following round window membrane administration in the cisplatin-induced ototoxicity mouse model.
Methods: The morphology, gelation kinetics, viscosity and secondary structure of the DEX-SILK hydrogel were analyzed. DEX concentration in the perilymph was tested at different time points following hydrogel injection on the RWM niche. Cultured cells (HEI-OC1), organ of Corti explants (C57/BL6, P0-2), and cisplatin-induced hearing loss mice model (C57/BL6) were used as in vitro and in vivo models for investigating the otoprotective effects of DEX-SILK hydrogel against cisplatin.
Results: Encapsulation of DEX with a loading of 8% (w/v) did not significantly change the silk gelation time, and DEX was evenly distributed in the Silk-PEG hydrogel as visualized by scanning electron microscopy (SEM). The concentration of Silk majorly influenced DEX distribution, morphological characteristics, viscosity, and gelation time. The optimized DEX-SILK hydrogel (8% w/v loading, 15% silk concentration, 10 μl) was administered directly onto the RWM of the guinea pigs. The DEX concentration in the perilymph was maintained above 1 μg/ml for at least 21 days for the DEX-SILK, while it was maintained for less than 6 h in the control sample of free DEX. DEX-SILK (5-60 ng/ml) exhibited significant protective effects against cisplatin-induced cellular ototoxicity and notably reduced the production of reactive oxygen species (ROS). Eventually, pretreatment with DEX-SILK effectively preserved outer hair cells in the cultured organ of Corti explants and demonstrated significant hearing protection at 4, 8, and 16 kHz in the cisplatin-induced hearing loss mice as compared to the effects noted following pretreatment with DEX.
Conclusion: These results demonstrated the clinical value of DEX-SILK for the therapy of cisplatin-induced ototoxicity.
Keywords: silk-polyethyleneglycol hydrogel, cisplatin-induced hearing loss, dexamethasone, round window membrane
Introduction
Ototoxicity is a side effect of cisplatin treatment characterized by bilateral, gradual, and severe hearing loss, with a prevalence of 40–80% in adult cancer patients and >50% in pediatric cancer patients.1 This ototoxicity can lead to a multifaceted decrease in the quality of life and notably impact the social and academic development of pediatric patients.2–4 Numerous protective compounds have been identified in preclinical studies and several are undergoing clinical trials.5,6 However, no Food and Drug Administration (FDA)-approved drugs or treatment strategies are currently available for treating cisplatin-induced ototoxicity.
Dexamethasone (DEX), a synthetic steroid analog, is widely used for the treatment of various inner ear diseases including sudden idiopathic sensory neural hearing loss, Meniere’s disease, and Bell’s palsy. Recently, DEX was reported as a candidate for otoprotection based on its ability to upregulate the antioxidant enzyme activity and activate the cell survival pathways.7–10 Although the exact molecular mechanism remains unclear, DEX binds to the glucocorticoid receptor and activates the signal transduction pathway that mediates inflammation and induces apoptosis by modulating nuclear factor kappa B (NF-kB) and inhibiting reactive oxygen species (ROS) production in the inner ear. Systemic administration of DEX exhibited otoprotective effects against cisplatin-induced hearing loss.8,9 However, glucocorticoids down-regulate apoptosis genes, which might induce cancer cell resistance during co-administration of DEX with cisplatin.11 The development of an inner ear drug administration technique has provided an alternative drug administration route, which is intratympanic administration.9,10 Cisplatin is usually administrated in several cycles with a few days recovery period.12 DEX should be administrated several times at a high concentration each time in order to achieve the desired efficacy against cisplatin-induced hearing loss. Our previous study indicated that DEX could be rapidly cleared from the middle ear down the Eustachian tube, with drug levels in the perilymph lasting approximately 6 h in guinea pigs.13 Repeated intratympanic administration of DEX can increase the incidence of tympanic membrane perforations, and can cause undesired infection during the experimental procedure.10 Moreover, reduced drug administration frequencies and higher drug concentration levels were maintained in the perilymph in order to achieve optimal clinical efficacy.
Silk fibroin (SF) hydrogel is a natural protein derived from the domestic silkworm Bombyx mori (B. mori). This protein has been extensively studied for local, controlled and sustained drug release due to its versatility, biocompatibility, and biodegradation.14 One of the most important physical properties of SF is the spontaneous transformation from solution to gel by environmental stimuli.15 Previously, we evaluated the efficacy and safety of an injectable Silk-polyethylene glycol (PEG) hydrogel as a vehicle for inner ear delivery by round window membrane (RWM) administration.13 This hydrogel can easily attach to RWM without requiring biological adhesion. The high affinity between the SILK-PEG hydrogel and RWM enables the drug-loaded hydrogel to attach to the surface of the RWM so that the drug can penetrate from the outermost layers of the stratum corneum into the inner ear.13
In the present study, we investigated whether DEX-loaded silk-PEG hydrogel (DEX-SILK) exhibited a potential as a protective and/or therapeutic agent for cisplatin-induced hearing loss in mice. The results indicated that DEX-SILK could improve the drug concentration and maintain an efficient level in the inner ear over time in order to prevent cisplatin-induced hearing loss. In order to prolong the retention of DEX-SILK on RWM, the preparation of DEX-SILK was optimized to improve the drug loading and sustained release. Histological and physiological analyses were performed using cisplatin-treated HEI-OC1 cells, mouse cochlea organ of Corti explants, and ototoxicity mouse model in order to assess the effects of DEX-SILK in vitro and in vivo.
Methods
Materials, cell culture, and animals
Silk fibers isolated from the cocoons of B. mori silkworm were purchased from Xihe Silk Textile Co., Ltd. (Shengzhou, China). Lipid-soluble dexamethasone powder was purchased from Damas-beta (Shanghai, China). Water-soluble dexamethasone powder was purchased from Sigma (St. Louis, MO, USA).PEG was purchased from Sigma-Aldrich (Shanghai, China). Ketamine hydrochloride was purchased from Fujian Gutian Pharmaceutical Co., Ltd (Fuzhou,China), Xylazine was purchased from Sangon biotech (Shanghai, China). Cisplatin was purchased from Aladdin (Shanghai, China). Cell Counting Assay Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies (Japan). Anti-Myosin7a antibody was purchased from Proteus Bioscience (San Diego, CA, USA).
The House Ear Institute-Organ of Corti 1 (HEI-OC1) cell line (generated at House Ear Institute, Los Angeles, CA, USA) is a widely used progenitor hair cell line derived from the auditory organ of the transgenic mouse ImmortomouseTM.16 This cell line was developed as an in vitro system to investigate the cellular mechanisms involved in cisplatin-induced ototoxicity. The HEI-OC1 cells were cultured in high-glucose DMEM (Gibco BRL, USA) containing 10% FBS (Gibco,Waltham, MA, USA) without antibiotics under permissive conditions (33 °C, 10% CO2), as previously described.16
Male guinea pigs (3–4 week old, weighing 200–250 g) and C57BL/6J mice (about 8-week old) were purchased from the Shanghai Laboratory Center (Shanghai, China). The animal experiments were reviewed and approved by the Ethics Committee of the Shanghai Jiaotong University, School of Medicine. All animal experiments complied with the 3R principles (Reduction, Replacement, and Refinement).
Optimized preparation of silk-PEG hydrogel
Dex-loaded SILK-PEG hydrogel (DEX-SILK) preparation
PEG solution was prepared with a defined weight percentage (w/w) by mixing liquid PEG, MW 8000 g/mol (PEG-8000) with deionized water. After mixing and filtration through a 0.22 μm filter, the PEG solutions were stored at room temperature. PEG was mixed with silk in a glass vial at a weight ratio of 8:3 (PEG:SF) in order to form PEG–silk hydrogels. The vial was gently mixed and the solution was loaded in a penicillin bottle for lyophilization. The solution was freeze-dried using a lyophilizer (CHRIST Alpha 2–4 LSCplus, Martin Christ Gefriertrocknungsanlagen GmbH, Germany). Briefly, the samples were first cooled from ambient temperature and held at 5°C for 1 hr, then cooled to −45°C at 0.05 °C/min, and held for 720 min. The primary drying occurred at −20°C for 2400 min and the secondary drying at 25°C for 600 min. A lyophilized powder of PEG:SF mixture was generated. Subsequently, DEX-loaded SILK-PEG hydrogel (DEX-SILK) was prepared by dissolving the lyophilized powder of PEG:SF mixture with deionized water to extract the gel.
Scanning electron microscopy (SEM)
The hydrogel morphology was determined by (SEM (Hitachi S-4800, Tokyo, Japan). Different concentrations of PEG-Silk loaded with 8% DEX were prepared in a 24-well plate. The hydrogel was rinsed three times with water to remove PEG,17 then frozen at −80 °C overnight, and eventually lyophilized for 48 hrs. The samples were sprayed with gold before imaging and observed at a voltage of 3.0 kV.
Optical density measurement (OD)
Gelation kinetics was assessed by OD measurements. After preparing the drug-loaded hydrogel, the samples were added into a 24-well plate, with 200 μL of liquid per well. The OD changes of the samples were measured by UV-visible spectrophotometry (Synergy 2, Biotek) set at 37°C under the kinetic mode and monitored over 1,440 min at 5-min intervals. The gelation time was defined as the time span from mixing to the point when OD values plateaued. Four samples were tested in order to obtain average OD values and standard deviations.
Viscosity
The effect of temperature on the viscosity of different concentrations of 8% DEX PEG-silk was determined byRheolabQC. The shear rate was 50 rpm and the temperature was 25–40°C. The sample solution (4 mL) was placed in a tube, heated at a speed of 1 °C/min, and the viscosity was recorded.
Total reflection Fourier transform IR spectroscopy (ATR-FTIR)
The secondary structure of the hydrogel was determined by FTIR spectroscopy with a MIRacleTM attenuated total reflection (ATR) Ge crystal cell in reflection mode. All spectra were obtained between 4,000 and 400 cm−1 at a 2 cm−1 resolution with 32 scans (Nicolet 5,700, Thermo Electron Corp, Waltham, MA, USA).
In vitro release
To evaluate the releasing property of the DEX-SILK hydrogel in vitro, 15% Silk-PEG hydrogels loaded with 8% DEX were prepared as described above. Prior to the hydrogel transfer, 10 μL of the mixture was added to a 1.5 ml Eppendorf tube with a pipette.
A total of 1 mL of PBS (pH 7.4) was subsequently added to the tubes. The tubes were incubated for 7 days at 37°C. The release medium (900 μL) was transferred into a sterile empty tube and stored at −20°C for high-pressure liquid chromatography combined with mass spectrometry (LC/MS/MS) analysis. The LC/MS/MS analysis was conducted at the following time points: 1, 3, 6, 12, 24, 48, 72, 96, 120, 144, and 168 hrs. The same volume of artificial perilymph buffer was added into each tube as a supplement. The concentrations of DEX were determined by LC/MS/MS, as previously described.13
In vivo release
Water-soluble DEX and lipid-soluble DEX loaded SILK-PEG hydrogel were both prepared as described above.
Guinea pigs were anesthetized with 60 mg/kg ketamine hydrochloride and 10 mg/kg xylazine. During the surgery, the animals were placed on a thermostatic plate (37.5°C) to maintain body temperature. The skin behind the left ear was shaved and disinfected with alcohol. Auditory bulla was fully exposed by the retroauricular approach. A hole of 3 mm diameter was drilled in order to ensure a round window niche that was visualized by a surgical microscope. A total of 10 μL of the mixture was injected onto the RWM directly by a pipette. Following injection, the defect of bulla was sealed by dental cement and sternocleidomastoid muscle was replaced to the normal anatomical position. The incision was closed with suture. The animals remained anesthetized in the right lateral decubitus for 30 min in order to prevent leakage. The animals were returned to the cage after regaining consciousness.
The perilymph (3–5 samples) was collected ex vivo at different time points of 1, 3, 6, and 12 hrs in the water-soluble DEX group, and 1, 3, 6, 12, 24, 48, 96, 144, 192, 240, 336, and 504 hrs in the lipid-soluble DEX group. The cochleae were harvested from the animals after an anesthesia overdose. The perilymph (5–7 μL) was subsequently collected by inserting a sharp glass pipette (World Precision Instruments, Sarasota, FL, USA) into the scala tympani from the RWM. All samples were stored at −80°C for subsequent LC/MS/MS analysis.
Cell experiments
Cell viability assay
The HEI-OC1 cell line was kindly provided by Yiqing Zheng(Sun Yat-sen University, Guangzhou, China). Use of HEI-OC1 cell line was approved by the Ethics Committee of Shanghai Jiaotong University, School of Medicine.
The cell counting kit (CCK-8) was used to evaluate the cytoprotective effect of DEX-SILK against cisplatin ototoxicity. The in vitro protective effects of DEX-SILK against HEI-OC1 cisplatin-induced cytotoxicity were examined after 4 hrs of pretreatment with DEX or DEX-SILK, followed by 24 hrs of co-incubation with 60 μM cisplatin. Briefly, HEI-OC1 cells (5,000 cells/well) were seeded on 96-well plates and incubated overnight for attachment. Following pretreatment of the cells with DEX or DEX-SILK for 4 hrs, the optimum cisplatin concentration was selected and co-incubated with DEX or DEX-SILK for 24 hrs to induce cytotoxicity. Subsequently, 10 μL of CCK-8 was added for 2 hrs. The OD at 450 nm was measured using a microplate reader (Model 550, Bio Rad). The control wells had cells without drug treatment, whereas the wells that did not contain cells were used as the blank. The cell viability was calculated by the following equation: Cell viability (%) = [(ODsample-ODblank)/(ODcontrol-ODblank)]×100%.
Estimation of intracellular ROS levels
The intracellular ROS levels were determined using 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, Abcam, MA, USA), a fluorogenic dye that measures intracellular hydroxyl and peroxyl, activities. Briefly, HEI-OC1 cells (5×105 cells/well) were seeded in 24-well plates for 24 h to achieve a minimum of 80% confluency. Subsequently, the medium was replaced by DEX and DEX-SILK at an equivalent DEX concentration of 1, 5, 10, 30, and 60 ng/mL. After 4 hrs of incubation, 60 μM cisplatin was added into the medium containing DEX or DEX-SILK and incubated for an additional 24 hrs. Finally, the cells were incubated with 20 mM of DCFH-DA in serum-free medium for 30 min. The residual reagent was removed by washing with PBS (pH 7.4) three times. After fixing the cells with 4% paraformaldehyde for 20 min at room temperature, the ROS levels were detected by confocal microscopy (Zeiss, Germany) at excitation and emission wavelengths of 480 and 525 nm, respectively.
Animal experiments
Organ of corti explants
All mice pups (P0-2) were decapitated and cochlea was dissected from the skull. The specimens were washed in cold PBS (Gibco). The organs of Corti were separated from the spiral ligament and attached to coverslips that were previously coated with Matrigel (1:10, Invitrogen, CA, USA). The organ explants were maintained in culture medium (DMEM/F12, Gibco by Invitrogen, CA, USA) supplemented with 2% B-27™ supplement (Gibco) and 1% N-2 supplement (Gibco) at 37°C and 5% CO2, followed by recovery for 24 hrs. The organs of Corti explants were then used as controls or were treated with cisplatin, DEX+cisplatin or DEX-SILK+cisplati,n, respectively. The preservation of the hair cells was observed by myosin 7a staining.
Establishment of a hearing loss mouse model
C57/BL6 mice were treated with a single dose of cisplatin (12 mg/kg) or multiple doses (4 mg/kg/day, 3 days) in order to induce hearing loss. Specifically, 15 C57/BL6 mice were randomly assigned to the control, 12 mg/kg single dose, and 4 mg/kg multi-dose groups (n=5 per group). Auditory brainstem response testing (ABR) thresholds were measured at frequencies of 4, 8, 16, and 22 kHz on day 0 and day 5 after cisplatin treatment as previously described.13 The optimal ototoxic dose of cisplatin exhibited desired ototoxicity and low mortality rates. ABR tests were conducted using the ABR workstation from Tucker–Davis Technologies Inc (Alachua, FL, USA).
DEX-SILK protects hearing ability from cisplatin-induced ototoxicity
Based on the optimized cisplatin-induced ototoxic mouse model, we evaluated the otoprotective effects of DEX-SILK against cisplatin-mediated toxicity. Briefly, 32 mice were divided into four groups: (1) cisplatin (n=8); (2) DEX (n=8); (3) drug-free SILK (n=8); (4) DEX-SILK (n=8). In all these groups, the mice were intraperitoneally injected with 12 mg/kg of cisplatin. Water-soluble DEX powder was dissolved in deionized water to obtain 8% DEX solution, which was absorbed by gelfoam to prepare the DEX-gelfoam. DEX-Silk hydrogel was prepared as described above. The DEX and DEX-SILK groups were administered by RWM 2 hrs prior to the cisplatin injection. The surgical procedure was the same as the previous experiment in guinea pigs, with differences of anesthetizing with intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg), and a smaller hole of 2 mm diameter. A total of 1 μL of DEX-SILK mixture and DEX-gelfoam were injected or placed onto the RWM.The ABR threshold was measured prior to DEX or DEX-SILK RWM administration and 5 days after cisplatin injection in order to evaluate the changes in hearing ability, as previously described (2.6.2).
Results
Optimized preparation of silk-PEG hydrogel
In the present study, the preparation of the Silk-PEG hydrogel was simplified from two steps into one step. In order to obtain a lyophilized mixture of Silk fibroin and PEG (3:8, m/m), PEG-8000 was selected, instead of PEG-400. The Silk-PEG hydrogel was prepared by adding deionized water to the lyophilized mixture (Figure 1A). After incubation at 37°C for a certain period of time, the colloidal particles were formed. Different formulations were used to prepare hydrogels from silk fibroin solution by a solution–gelation transition in the presence of various silk fibroin and drug concentrations. The gelation time periods required for different formulations are listed in Table 1. The gelation time was prolonged with the increase of drug concentration following incubation of SILK-PEG at 37°C. In addition, the gelation time was reduced with the increase of silk concentration at the same drug concentration. The results indicated that the increase in gelation time of DEX-SILK was majorly influenced by the concentration of Silk, while the effect of DEX was not apparent. However, the gelation time was significantly reduced in 20% Silk-PEG containing 8% DEX.
 | Table 1 Gelation time after mix dexamethasone under 37°C |
The formulation that included the highest drug concentration (8%) was used to evaluate the morphology alteration by SEM following treatment with different SILK-PEG concentrations. Five percent Silk-PEG exhibited poor mechanical properties such as softness and fragility, and neither pores nor silk nanospheres were observed (Figure 1B). As the concentration of the silk increased, the mechanical properties of the hydrogel were effectively improved. Ten percent Silk-PEG hydrogel exhibited homogeneous pores with silk nanospheres, and drug molecules could be embedded in it. However, only a small number of nanospheres was observed.15% and 20% DEX-SILK hydrogels exhibited more dense pores and nanospheres.With the increase of concentration from 10% to 20%, the size of silk nanospheres formed varied from 1 to 100 µm. The pores were elongated in shape and surrounded by laminar silk layers, which were consistent with a previous report.17 The presence of silk nanospheres could improve the mechanical properties of the hydrogel and sustained release of drugs. These results indicated that increased concentration of silk had a positive effect on the sustained release of the drug.
The characteristic absorption peaks of the silk structure exhibited approximate wavelengths of 1,652 cm−1(amide), 1,543 cm−1 (amide), 1,242 cm−1 (amide), and 669 cm−1 (amide), respectively (Figure 1C). The silk structure exhibited wave numbers of approximately 1,626 cm−1 (amide), 1,532 cm−1(amide), 1,236 cm−1 (amide), and 696 cm−1 (amide). With the increase in concentrations of silk, the absorption peak at 1,626 cm−1 was gradually increased, which indicated the formation of additional β-sheet structures.
Temperature effects on rheological properties of 5%–20% Silk-PEG with 8% DEX were shown in Figure 1D. With the increase of temperature from 20.6°C to 38.7°C, the viscosity of 20% Silk-PEG with 8% DEX significantly increased from 0.110 to 1.380 Pa.s, while such changes were not observed in the other groups.
In vitro release
The only difference between these two DEX is the presence of (2-hydroxypropyl)-β-cyclodextrin in water-soluble DEX. (2-Hydroxypropyl)-β-cyclodextrin is commonly used to get lipophilic drugs into aqueous solutions, while its influence on the sustained release of DEX from DEX-SILK is unknown. Hence, it is necessary to determine the release profile of two different DEX-loaded Silk-PEG hydrogels.The in vitro release of DEX from the hydrogel and the cumulative release percentage (artificial perilymph, pH=7.4) noted at different time points are shown in Figure 2A and B. The sample encapsulating water-soluble DEX indicated a burst release at 1 hr with the highest concentration of 24.395±4.473 µg/mL. DEX concentration levels rapidly dropped below the limit of detection in the first 24 hrs. However, the lipid-soluble DEX exhibited a burst release within 1 hr with higher concentration levels of 75.251±5.373 µg/mL, followed by a sustained release over 144 hrs, and finally dropped to 1.272±0.717 µg/mL at 168 hrs. The cumulative release graph indicated complete DEX release from the lipid-soluble DEX-SILK in 168 hrs. The Silk-PEG hydrogel loaded with lipid-soluble DEX showed considerably longer release time and higher concentration levels. These data indicated that hydrogel encapsulated lipid-soluble DEX exhibited a stronger sustained-release capability. In addition, the failure to achieve 100% DEX release in both formulations may be due to incomplete degradation of the hydrogel and presence of PEG that inhibited burst release.
In vivo release
DEX concentration in the perilymph was tested at different time points following hydrogel injection on the RWM niche (Figure 2C and D). Following a burst release at 1 hr, the concentration of the water-soluble DEX sample rapidly decreased from 121.388±20.479 μg/mL to the baseline during 24 hrs of incubation. A burst release at 1 hr,followed by a slow release in the following 14 days were noted for the Silk-PEG formulation in the presence of lipid-soluble DEX. The concentration range was 11–111 μg/mL. Hydrogel encapsulated lipid-soluble DEX released DEX slowly and steadily, which could be detected at 14 days. According to a previous report,17 silk nanospheres were not detected in silk-hydrogel obtained by mixing silk and PEG-400, which indicated that PEG-400 cannot induce the formation of nanospheres. Meanwhile, homogeneous and spherical nanospheres were reported to be formed when the molecular weight of PEG ranged from 4000 to 10,000. Such nanospheres were also observed by SEM in the present study with PEG-8000. DEX molecules could be embedded in nanospheres, which can change the sustained release property of the Silk-PEG hydrogel. These results indicated that replacement of PEG-400 by PEG-8000 could change the sustained release activity of the Silk-PEG hydrogel. Longer release time was observed in the present study.
Cellular experiments
DEX-SILK protects against cisplatin-induced HEI-OC1 injury
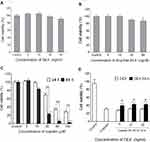
The viability of HEI-OC1 cells treated with DEX was examined in vitro after 24 hrs of incubation. The results indicated that DEX formulations were non-cytotoxic at the experimental concentrations used (0, 5, 10, 30, 60 ng/mL) (Figure 3A). In addition, we evaluated the toxicity of drug-free SILK hydrogel on HEI-OC1 cells. The hydrogel concentration was adjusted to be the same in all drug-free SILK hydrogel formulations. HEI-OC1 cells were treated with different DEX concentrations of drug-free SILK hydrogel, namely, 0, 5, 10, 30, and 60 ng/mL for 24 hrs, and the cell viability was estimated to be 100.39±9.50%, 100.44±9.80%, 90.49±8.96%, and 87.59±7.53%, respectively (Figure 3B). The control cell viability was 100%, while 60 ng/mL of drug-free SILK hydrogel exhibited significantly decreased cell viability as compared to the control cells (p<0.05). The other concentrations did not cause significant toxicity, indicating that the silk fibroin was biocompatible and the SILK-PEG hydrogel was non-toxic to HEI-OC1 cells at a concentration below 60 ng/mL. Therefore, 5, 10, and 30 ng/mL of DEX-SILK were used for cytoprotective experiments.
The cells were treated with 0, 5, 10, 30, 60, and120 μM cisplatin for 24 hrs and 48 hrs in order to select the optimal concentration required for inducing HEI-OC1 cytotoxicity. Cisplatin exhibited cytotoxicity in a dose- and time-dependent manner (Figure 3C). When HEI-OC1 cells were incubated with cisplatin for 24 hrs, the cell viability was estimated to 103.10±8.34%, 83.25±7.17%, 23.19±3.18%, 5.98±1.27%, and 2.80±0.69%, respectively. The viability of the control cells was set at 100%. After incubation of the cells with cisplatin for 48 hrs, cell viability was estimated to be 102.50±4.6%, 99.27±3.74%, 68.90±7.0%, 34.03±2.53%, and 27.89±1.57%, respectively, as compared to the control cells (100%). Sixty μM of cisplatin treatment for 24 hr was selected as the optimal concentration since cell viability was decreased to approximately 50% as compared to the control.
In order to examine the cytoprotective effects of DEX-SILK in cisplatin-induced cytotoxicity, we divided the experimental samples into the following groups: Control, cisplatin, DEX + cisplatin, and DEX-SILK + cisplatin. The DEX+cisplatin and DEX-SILK+cisplatin groups comprised three different subgroups that corresponded to 5, 10, and 30 ng/mL of DEX. As shown in Figure 3D, the cell viability in the cisplatin group was 30.79±5.64%, whereas the DEX (5, 10, 30 ng/mL) + cisplatin subgroups did not show protective effects against cisplatin (p>0.05 as compared to cisplatin). Conversely, the cell viabilities of DEX-SILK (5, 10, 30 ng/mL) + cisplatin subgroups were estimated to be 39.34±4.38%, 42.74±4.40%, and 42.29±4.03%,respectively, as compared to the control cells. These results indicated that DEX-SILK could partially protect HEI-OC1 cells from cisplatin-induced cell injury (***p<0.001). Furthermore, the cell viabilities of the DEX-SILK (5, 10, 30 ng/mL) + cisplatin subgroups were significantly increased as compared to those of the DEX (5, 10, 30 ng/mL) + cisplatin subgroups (## p<0.01).
Taken together, these results showed that Silk hydrogels exhibited enhanced cytoprotective effects by long-term sustained release of DEX in the HEI-OC1 cells that were pretreated with cisplatin.
DEX-SILK inhibits cisplatin-induced ROS production
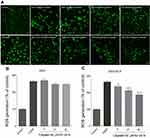
ROS production is a secondary effect caused during cisplatin-induced hair cell damage.18 The DCFH-DA agent was used for the measurement of hydroxyl, peroxyl, and other types of intracellular ROS activity, notably the cisplatin-treated HEI-OC1 cells that were treated with DEX-SILK. The confocal images and the representative histograms of ROS fluorescence are shown in Figure 4A. The results indicated that ROS levels were increased approximately 2.5-fold following cisplatin treatment as compared to the control cells, and the levels of ROS were partially reduced by DEX-SILK pretreatment (5, 10, 30 ng/mL) as compared to the cisplatin group. In addition, the ROS levels did not exhibit a significant change in the DEX pretreatment group (Figure 4B and C). These results demonstrated that DEX-SILK could attenuate the ROS levels in HEI-OC1 cells following cisplatin treatment.Cisplatin increased ROS generation in a dose-dependent manner, and cisplatin toxicity was closely associated with the increased production of ROS and intracellular oxidative stress, which could activate the apoptotic pathway causing the death of cochlear cells. DEX alone was rapidly eliminated from the cells, while the sustained release of DEX from DEX-SIlK hydrogel could down-regulate the production of ROS.
DEX-SILK hydrogels protect from cisplatin-induced hearing loss in cochlear organotypic cultures
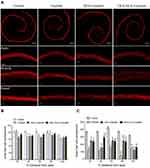
Hair cell damage was observed in whole organ inner ear explants under simulated culture conditions following exposure to cisplatin for 24 hrs. Hair cell morphology and structure were evaluated by immunohistochemical labeling with the hair cell marker myosin 7a. As shown in Figure 5A, the three rows of the outer hair cells (OHCs) and the single row of the inner hair cells (IHCs) were arranged in orderly rows. The spiral region from the base to the apex of the cochlea was treated with saline. The number of IHCs did not decrease when the cochlea were cultured with 60 µM of cisplatin for 24 hrs. However, the number of OHCs was significantly reduced in all other regions.
Figure 5B and C indicate the mean numbers of OHCs and IHCs per 1 mm following 24 hrs of treatment with cisplatin, DEX+cisplatin, and DEX-SILK + cisplatin. Pretreatment with DEX resulted in a statistically significant survival in the number of OHCs that were located in an area covering 20% and 80% distance from apex (p<0.05; p<0.001, respectively, as compared to those noted in the cisplatin group), whereas pretreatment with DEX-SILK hydrogel exhibited significant survival in the number of OHCs in an area covering 20%, 40%, and 80% distance from apex (p<0.05; p<0.001; p<0.001, respectively, as compared to that of the cisplatin group). Moreover, pretreatment with DEX-SILK hydrogels showed enhanced protective effects against cisplatin treatment at 40% of the region from the apex as compared to DEX pretreatment (p<0.01).
DEX-SILK hydrogels protect from cisplatin-induced hearing loss in a mouse model
Establishment of a cisplatin-induced hearing loss mouse model
Auditory function was assessed by ABR measurements. The hearing function of the sham group and drug-free SILK group were evaluated, and no significant differences were observed as compared to the control (Figure S1).The mice treated with multiple doses of cisplatin (4 mg/kg/day, 3 days, IP) exhibited a significant threshold elevation at 4 kHz, whereas the mean threshold shift was below 20 dB. In addition, no distinct threshold increase was noted at 8, 16, and 22 kHz (Figure 6A). Thus, multiple-dose administration of cisplatin failed to establish ototoxicity in this mouse model.
 | Figure S1 Evaluation of surgery and drug-free SILK on hearing function. Mean ABR threshold of mice treated with saline and drug-free SILK. The values are expressed as mean ± SEM, n=6. |
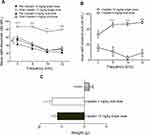
In contrast, a single-dose administration of cisplatin (12 mg/kg, IP) indicated a threshold elevation with an average threshold shift of 33±3.74 dB, 46±2.92 dB, 47±2.00 dB, and 47±3.39 dB at the corresponding frequencies of 4, 8, 16, and 22 kHz, respectively. In addition, the average threshold shifts for the aforementioned frequencies induced by single-dose administration were notably higher than those induced by multiple-dose administration (Figure 6B, p<0.05 for 4 kHz, p<0.001 for 8, 16, and 22 kHz).
The lethality rates of mice at day 5 following treatment with multiple doses and single dose of cisplatin were approximately 25%. In addition, a significant reduction in body weight (p<0.01) was observed in both groups. Meanwhile, no significant weight loss was observed between the single dose and multi-dose treatment groups. Therefore, the single dose of cisplatin treatment (12 mg/kg, IP) was selected for subsequent experiments (Figure 6C, p<0.001 for both single-dose and multi-dose treatment groups, p>0.05 between the single-dose and multi-dose treatment groups).
Otoprotective performance of DEX-SILK by RWM administration
The schematic diagram of the RWM administration with DEX-SILK is shown in Figure 7A. As mentioned above, lethality rates of mice at day 5 following treatment with single-dose cisplatin was approximately 25%. Ten mice died due to cisplatin toxicity and 22 micesurvivedin different groups: (1) cisplatin (n=6); (2) DEX (n=4); (3) drug-free SILK (n=6); (4) DEX-SILK (n=6). DEX and/or DEX-SILK hydrogel was administrated 1 hr prior to cisplatin injection. Gelfoam can absorb 45 times its weight in water or blood and is widely used in the clinic as a drug carrier placed onto RWM to prolong drug release time. In contrast, free DEX could be rapidly eliminated from perilymph. In order to prove that DEX-SILK hydrogel has a stronger sustained release ability, which has a potential clinical application, we chose DEX-gelfoam as the control group.DEX-SILK could rapidly and firmly attach to RWM. ABR was tested following cisplatin administration on day 0 and day 5. No significant threshold shift was observed for the Silk-PEG treated group at a frequency of 4, 8, 16 and 22 kHz on days 0 and 5 (data were shown in Figure S1). This result indicated that Silk-PEG hydrogel exhibited no effect on the auditory function of C57/BL6 mice, which was consistent with our previous study.13
On day 0, ABR thresholds of cisplatin, DEX+cisplatin, and DEX-SILK+cisplatin showed no significant difference (Figure 7B). However, on day 5 after cisplatin injection, the mean ABR threshold tests indicated that the auditory function was partially protected by the DEX-SILK hydrogel. The mean ABR thresholds of the DEX-SILK+cisplatin group (69.17±3.76 dB at 4 kHz, 59.17±4.92 dB at 8 kHz, and 64.17±7.36 dB at 16 kHz) were significantly lower as compared to those of the cisplatin group (87.50±4.18 dB at 4 kHz, 85.83±4.92 dB at 8 kHz; and 75.83±5.85 dB at 16 kHz; p<0.001, p<0.001, p<0.01, respectively). The mean ABR threshold of the DEX+cisplatin group exhibited no significant difference as compared to the cisplatin group.
The ABR threshold shifts of deaf mice that were pretreated with DEX-SILK were significantly lower at the frequencies of 4 kHz (13.33±8.76 dB), 8 kHz (25.00±6.32 dB), and 16 kHz (41.67±6.06 dB) as compared to those of the cisplatin group (33.33±7.53 dB at 4 kHz; 46.67±6.06 dB at 8 kHz; 50.50±9.53 dB at 16 kHz). Nevertheless, the DEX+cisplatin group indicated no significant differences in the ABR threshold shifts as compared to the cisplatin group at all detected frequencies (Figure 7B and C).
Discussion
Inner ear has a unique anatomy of the temporal bone and blood-inner ear barriers, which limit the access of therapeutic agents to the inner ear. Nanomedicine, including advanced drug delivery systems and hydrogels, have gained immense importance due to their ability to overcome the disadvantages of the conventional drugs. This can be achieved by delivering agents to specific cells, by protecting its therapeutic contents and controlling its release.19–21 OTO-104, a poloxamer hydrogel containing DEX, has been reported to alleviate the effects of multiple intratympanic injections. Longer half-life of a drug protects against cisplatin ototoxicity by intratympanic injection a day before cisplatin treatment.22 DEX/poloxamer 407 hydrogel can reduce cochlear implantation-induced hearing threshold shifts at low, middle, and high frequencies.23 We developed an in-situ-forming silk-PEG-mDEX hydrogel to readily attach on RWM for the delivery and sustained release of DEX without inflammatory reaction.13 In the present study, we aimed to optimize the preparation of silk-PEG-mDEX hydrogel in order to extend its release in vitro and in vivo, and further evaluate the therapeutic applications of this formulation in the cisplatin-induced hearing loss mouse model.
PEG is a highly hydrophilic molecule that binds to water. In silk fibroin solution, PEG can cause hydrophobic domains assembly on the silk molecules, which in turn forms crystalline β-sheets and gel networks.24 In the present study, PEG-8000 was used with silk fibroin to prepare lyophilized powder. The SILK-PEG hydrogel preparation was simplified from two steps into one step. With the increase in the concentration of Silk fibroin, additional homogeneous pores could be observed (Figure 1B). The presence of the microspheres may largely improve the mechanical properties of the hydrogel and play an important role in the sustained drug release.25 Furthermore, PEG-8000 increases the softness of the hydrogel, which is suitable for RWM attachment. Following incubation at 37 °C for 1 h, the mixture could be easily aspirated by a pipette while maintaining its formation in an artificial perilymph buffer (pH 7.4) solution. The data of the gelation time indicated that the concentration of silk played a more important role as compared to DEX. However, a significantly shorter gelation time was observed after mixing 8% DEX with 20% Silk-PEG (Table I). This difference was likely due to the effect of DEX on the silk protein structure. Moreover, the absorption peaks at 1,626 cm−1 (amide) were sharper in the 20% DEX-SILK sample with the addition of 8% DEX, whereas the changes of the absorption peak in other samples were not apparent (Figure 1C).
Previous studies had reported a protective effect of DEX against cisplatin-induced cytotoxicity, while such an effect was not observed in a clinical trial by transtympanic injection.This discrepancy noted between in vitro and clinical studies may be due to the lack of sufficient DEX concentration in the inner ear.26–28 In the present study, we utilized a drug-loaded silk hydrogel as a drug storage system in order to improve the drug concentration in the inner ear. Silk-PEG with water-soluble DEX showed poor sustained release properties in vitro and in vivo. This might be associated with the poor drug loading in the hydrogel used for water-soluble DEX. In terms of the hydrogel with lipid-soluble DEX, the release of this compound was sustained for at least 168 h in vitro and 21 d in vivo, which indicated that the release property was better than that demonstrated in our previous study.13 Lower PEG concentration and higher amount of DEX loading in the silk hydrogel matrix may account for enhanced sustained release as compared to that noted in our previous study.13 Thermosensitive hydrogels that have been used for inner ear drug delivery exhibit lower sustained release than that noted for the aforementioned silk hydrogel formulation of DEX.29,30 In this study, PEG concentration was reduced from 84% to 72%, while the concentration of DEX was increased from 2.5% to 8% as compared to our previous study.13 In addition, other factors, such as the degradation of Silk-PEG hydrogel and the interaction between DEX and the crystalline β-sheet structure of silk, may also account for the enhanced and longer release of DEX. Zhong et al reported that aspirin, which is a hydrophilic drug, could exhibit controlled release from the composite hydrogel at a constant rate for 5 days.31 However, the hydrophobic molecule indomethacin could last for up to two weeks. Therefore, silk fibroin hydrogel may have optimal potential as a useful composite system with wide applications for controlled and sustained release of hydrophobic drugs. These sustained drug release properties and high concentration levels of DEX were maintained in the inner ear and could guarantee maximum efficacy of DEX.
The molecular mechanism of DEX otoprotective effect against cisplatin-induced ototoxicity is not fully understood. However, it has been reported that the predominant cause of this protection is the reduction in the production of ROS.18 In the present study, DEX-SILK exhibited a partial cytoprotective effect against cisplatin-induced cytotoxicity by reducing intracellular ROS production at 5, 10, 30, and 60 ng/ml. This result is consistent with our previous study that utilized a nanoparticle drug delivery system to prevent cisplatin-induced hearing loss.32 Therefore, partial protection against cisplatin-induced ototoxicity in cochlear organ culture and reduction of intracellular ROS production in vitro implied that this DEX-loaded silk hydrogel formulation might alleviate the ototoxicity in an animal model.
Suitable cisplatin-induced hearing loss animal models are crucial for evaluating the otoprotective effect of candidate drugs. A single large dose33 and/or multiple small doses of compound34 are the two main dosing models in previously reported cisplatin-induced ototoxicity studies. Although the multiple small doses approach is more analogous to clinical cisplatin regimens, it has low feasibility and high toxicity. In the first cycle (three cycles in total), hearing loss (mean ABR threshold shift) is usually less than 10 dB (Figure 6). In the present study, more than 80% of the mice tested did not survive after three cycles of cisplatin administration (data not shown). Therefore, we selected the single, large dose model because it was more practical and the resulting data were easier to interpret. The study demonstrated that DEX-loaded SILK-PEG hydrogels partially protected HEI-OC1 cells, cultured cochleae, and mouse model from cisplatin-induced cytotoxicity, organ of corti damage, and hearing loss. However, DEX only exhibited partial protection against cisplatin-induced organ of corti damage in 20% and 80% regions from the apex. Our findings illustrate the limitations of the intratympanic injection of DEX as a treatment application, since the solution is eliminated rapidly from the middle ear and provides limited temporal exposure to the inner ear. Hence, a sustained-exposure approach, such as Silk-PEG hydrogel, represents an optimal strategy, providing higher and prolonged drug levels in the inner ear.
Conclusion
The DEX-SILK hydrogel was successfully developed with a sustained release profile (inner ear release for 21 days). The sustained release from the DEX-SILK hydrogel and the long-term retention of DEX in cells exhibited statistically enhanced cytoprotective effects against cisplatin exposure via the inhibition of ROS regeneration. The round window membrane administration of DEX-SILK hydrogel provided significant protection against cisplatin-induced hearing loss in mice at 4, 8, and 16 kHz in contrast to single DEX administration.
Acknowledgments
This work was supported by the Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (No. 20171919), the National Natural Science Foundation of China (No. 81700899), Shanghai Health and Family Planning Commission (No. 201740005), the State Key Program of National Natural Science Foundation of China (No. 81330023), Shanghai Key Laboratory of Translational Medicine on Ear and Nose Diseases (14DZ2260300), and Fundamental research program funding of Ninth People's Hospital affiliated to Shanghai Jiao Tong University School of Medicine (No. JYZZ025). Yuming Chen and Jiayi Gu share first authorship.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Robertson MS, Hayashi SS, Camet ML, Trinkaus K, Henry J, Hayashi RJ. Asymmetric sensorineural hearing loss is a risk factor for late-onset hearing loss in pediatric cancer survivors following cisplatin treatment. Pediatr Blood Cancer. 2018;18:e27494. doi:10.1002/pbc.2749.
2. Kalyanam B, Sarala N, Azeem Mohiyuddin SM, Diwakar R. Auditory function and quality of life in patients receiving cisplatin chemotherapy in head and neck cancer: a case series follow-up study. J Cancer Res Ther. 2018;14(5):1099–1104. doi:10.4103/0973-1482.188426
3. Biswas B, Ganguly S, Ghosh J, Batra A. Cisplatin-induced hearing loss in children with cancer. Natl Med J India. 2017;30(6):327–328. doi:10.4103/0970-258X.239073
4. Waissbluth S, Del Valle Á, Chuang A, Becker A. Incidence and associated risk factors for platinum-induced ototoxicity in pediatric patients. Int J Pediatr Otorhinolaryngol. 2018;111:174–179. doi:10.1016/j.ijporl.2018.06.021
5. Brock PR, Maibach R, Childs M, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018;378(25):2376–2385. doi:10.1056/NEJMoa1801109
6. Hazlitt RA, Min J, Zuo J. Progress in the development of preventative drugs for cisplatin-induced hearing loss. J Med Chem. 2018;61(13):5512–5524. doi:10.1021/acs.jmedchem.7b01653
7. Marshak T, Steiner M, Kaminer M, Levy L, Shupak A. Prevention of cisplatin-induced hearing loss by intratympanic dexamethasone: a randomized controlled study. Otolaryngol Head Neck Surg. 2014;150(6):983–990. doi:10.1177/0194599814524894
8. Waissbluth S, Salehi P, He X, Daniel SJ. Systemic dexamethasone for the prevention of cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013;270(5):1597–1605. doi:10.1007/s00405-012-2150-0
9. Murphy D, Daniel SJ. Intratympanic dexamethasone to prevent cisplatin ototoxicity: a guinea pig model. Otolaryngol Head Neck Surg. 2011;145(3):452–457. doi:10.1177/0194599811406673
10. Lyu A-R, Kim DH, Lee SH, Shin D-S, Shin S-A, Park Y-H. Effects of dexamethasone on intracochlear inflammation and residual hearing after cochleostomy: a comparison of administration routes. PLoS One. 2018;13(3):e0195230. doi:10.1371/journal.pone.0195230
11. Özel HE, Özdoğan F, Gürgen SG, Esen E, Genç S, Selçuk A. Comparison of the protective effects of intratympanic dexamethasone and methylprednisolone against cisplatin-induced ototoxicity. J Laryngol Otol. 2016;130(3):225–234. doi:10.1017/S0022215115003473
12. Hayashi Y, Minamiyama S, Ohya T, et al. Daily cisplatin and weekly docetaxel versus weekly cisplatin intra-arterial chemoradiotherapy for late T2-3 tongue cancer: a pilot and feasibility trial. Medicina (Kaunas). 2018;54(4):52.
13. Yu DH, Sun CL, Zheng ZZ, et al. Inner ear delivery of dexamethasone using injectable silk-polyethylene glycol (PEG) hydrogel. Int J Pharm. 2016;503(1–2):229–237. doi:10.1016/j.ijpharm.2016.02.048
14. Kim Do K, Sim BR, Khang G. Nature-derived aloe vera gel blended silk fibroin film scaffolds for cornea endothelial cell regeneration and transplantation. ACS Appl Mater Interfaces. 2016;8(24):15160–15168. doi:10.1021/acsami.6b04901
15. Fernández-García L, Marí-Buyé N, Barios JA, et al. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. Acta Biomater. 2016;45:262–275. doi:10.1016/j.actbio.2016.09.003
16. Kalinec G, Thein P, Park C, Kalinec F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear Res. 2016;335:105–117. doi:10.1016/j.heares.2016.02.019
17. Wang XQ, Partlow B, Liu J, et al. Injectable silk-polyethylene glycol hydrogels. Acta Biomater. 2015;12:51–61. doi:10.1016/j.actbio.2014.10.027
18. Sheth S, Mukherjea D, Rybak LP, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front Cell Neurosci. 2017;11:338. doi:10.3389/fncel.2017.00338
19. Yang KJ, Son J, Jung SY, et al. Optimized phospholipid-based nanoparticles for inner ear drug delivery and therapy. Biomaterials. 2018;171:133–143. doi:10.1016/j.biomaterials.2018.04.038
20. Mäder K, Lehner E, Liebau A, Plontke SK. Controlled drug release to the inner ear: concepts, materials, mechanisms, and performance. Hear Res. 2018;368:49–66. doi:10.1016/j.heares.2018.03.006
21. Kuang X, Zhou S, Guo W, Wang Z, Sun Y, Liu H. SS-31 peptide enables mitochondrial targeting drug delivery: a promising therapeutic alteration to prevent hair cell damage from aminoglycosides. Drug Deliv. 2017;24(1):1750–1761. doi:10.1080/10717544.2017.1402220
22. Fernandez R, Harrop-Jones A, Wang X, Dellamary L, LeBel C, Piu F. The sustained-exposure dexamethasone formulation OTO-104 offers effective protection against cisplatin-induced hearing loss. Audiol Neurootol. 2016;21(1):22–29. doi:10.1159/000441833
23. Honeder C, Landegger LD, Engleder E, et al. Effects of intraoperatively applied glucocorticoid hydrogels on residual hearing and foreign body reaction in a guinea pig model of cochlear implantation. Acta Otolaryngol. 2015;135(4):313–319. doi:10.3109/00016489.2014.986758
24. Wu J, Xie X, Zheng Z, Li G, Wang X, Wang Y. Effect of pH on polyethylene glycol (PEG)-induced silk microsphere formation for drug delivery. Mater Sci Eng C Mater Biol Appl. 2017;80:549–557. doi:10.1016/j.msec.2017.05.072
25. Bing G, Sun X, Papadimitrakopoulos F, Diane JB. Seeing is believing, PLGA microsphere degradation revealed in PLGA microsphere/PVA hydrogel composites. J Control Release. 2016;228:170–178. doi:10.1016/j.jconrel.2016.03.011
26. Sarafraz Z, Ahmadi A, Daneshi A. Transtympanic injections of N-acetylcysteine and dexamethasone for prevention of cisplatin-induced ototoxicity: double blind randomized clinical trial. Int Tinnitus J. 2018;22(1):40–45. doi:10.5935/0946-5448.20180007
27. Martín-Saldaña S, Palao-Suay R, Aguilar MR, et al. pH-sensitive polymeric nanoparticles with antioxidant and anti-inflammatory properties against cisplatin-induced hearing loss. J Control Release. 2018;270:53–64. doi:10.1016/j.jconrel.2017.11.032
28. Capelo IOJ, Batista AMA, Brito YNF, Diniz KB, Brito GAC, Freitas MR. Study of the protective effect of dexamethasone on cisplatin-induced ototoxicity in rats. Acta Cir Bras. 2017;32(10):873–880. doi:10.1590/s0102-865020170100000009
29. Honeder C, Engleder E, Schöpper H, et al. Sustained release of triamcinolone acetonide from an intratympanically applied hydrogel designed for the delivery of high glucocorticoid doses. Audiol Neurootol. 2014;19(3):193–202. doi:10.1159/000358165
30. El Kechai N, Bochot A, Huang N, Nguyen Y, Ferrary E, Agnely F. Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int J Pharm. 2015;487(1–2):187–196. doi:10.1016/j.ijpharm.2015.04.019
31. Zhong T, Jiang Z, Wang P, Bie S, Zhang F, Zuo B. Silk fibroin/copolymer composite hydrogels for the controlled and sustained release of hydrophobic/hydrophilic drugs. Int J Pharm. 2015;494(1):264–270. doi:10.1016/j.ijpharm.2015.08.035
32. Wang XL, Chen YM, Tao Y, Gao YG, Yu DH, Wu H. A666-conjugated nanoparticles target prestin of outer hair cells preventing cisplatin-induced hearing loss. Int J of Nanomed. 2018;13:7517–7531. doi:10.2147/IJN.S170130
33. Sockalingam R, Freeman S, Cherny TL, Sohmer H. Effect of high-dose cisplatin on auditory brainstem responses and otoacoustic emissions in laboratory animals. Am J Otol. 2000;21(4):521–527.
34. Hughes AL, Hussain N, Pafford R, Parham K. Dexamethasone otoprotection in a multidose cisplatin ototoxicity mouse model. Otolaryngol Head Neck Surg. 2014;150(1):115–120. doi:10.1177/0194599813511948
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.