Back to Journals » International Journal of Nanomedicine » Volume 15
Development of an Experimental Dentifrice with Hydroxyapatite Nanoparticles and High Fluoride Concentration to Manage Root Dentin Demineralization
Authors Leal AMC, Beserra dos Santos MV , da Silva Filho EC , Menezes de Carvalho AL , Tabchoury CPM , Vale GC
Received 5 June 2020
Accepted for publication 31 August 2020
Published 5 October 2020 Volume 2020:15 Pages 7469—7479
DOI https://doi.org/10.2147/IJN.S264754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Aila Maria Cipriano Leal,1 Marcus Vinícius Beserra dos Santos,2 Edson Cavalcanti da Silva Filho,2 André Luis Menezes de Carvalho,3 Cinthia Pereira Machado Tabchoury,4 Glauber Campos Vale1
1Department of Restorative Dentistry, Federal University of Piaui, Teresina, Piauí, Brazil; 2Interdisciplinary Laboratory of Advanced Materials, Federal University of Piaui, Teresina, Piauí, Brazil; 3Dermopharmacy Laboratory, Federal University of Piaui, Teresina, Piauí, Brazil; 4Departament of Physiological Sciences, Campinas State University, Piracicaba, São Paulo, Brazil
Correspondence: Glauber Campos Vale
Department of Restorative Dentistry, Federal University of Piaui, Campus Universitário Ministro Petrônio Portella – SG 10 Bairro Ininga, CEP: 64049-550, Teresina, Piauí, Brazil
Tel +55 (86) 999912-9200
Email [email protected]
Background: High-fluoride dentifrice is used to manage root caries, but there is no evidence whether its association with nanohydroxyapatite could provide an additional protection for root caries. Therefore, this study aimed to develop and evaluate the effect of an experimental dentifrice with high fluoride (F−) concentration and nanohydroxyapatite (nano-HA) on root dentin demineralization.
Materials and Methods: After formulation of dentifrices, root dentin specimens were randomly assigned to six groups (n = 10) using different dentifrice treatments: placebo; nano-HA without F−; 1,100 μg F−/g; 1,100 μg F−/g + nano-HA; 5,000 μg F−/g; and 5,000 μg F−/g + nano-HA. A pH cycling model was performed for 10 days, in which treatments were performed twice a day. After that period, the longitudinal hardness was evaluated and the area of demineralization (ΔS) was calculated. The formulated dentifrices were evaluated for primary stability, cytotoxicity, and other technical parameters. Two-way ANOVA and Tukey’s test with p set at 5% were used for data analysis.
Results: The experimental dentifrices were stable and had no cytotoxicity. Regarding dentin demineralization, the placebo group significantly increased ΔS compared to all other treatment groups (p< 0.001). The dentifrices containing 5,000 μg F−/g, regardless of the presence of nano-HA, led to a smaller lesion area in relation to the other treatments (p< 0.001).
Conclusion: The findings of this study suggest that nano-HA reduced dentin demineralization, and dentifrice with 5,000 μg F−/g dentifrices, regardless of the presence of nano-HA, showed a greater reduction in root dentin demineralization.
Keywords: material development, dentin, dentifrice, fluoride, nanohydroxyapatite
Introduction
Root caries is a global burden especially in the older population.1 Therefore, several preventive methods have been studied to manage root caries and since root dentin is a more soluble tissue than enamel and would benefit more with Fluoride (F−),2 one of the most used strategies is the use of products with high F− concentration.3 Indeed, evidence suggests that the use of high-F− dentifrice is more effective than the use of conventional dentifrice, especially for root caries and patients with high caries risk.4,5 Moreover, it is important to address that F–dentifrices is the most rational mode of F− use since it associates the mechanical disorganization of biofilm with the preventive and therapeutic action of F−.6
In addition to F−, new materials have been developed to interfere in the development of dental caries, including those containing hydroxyapatite (HA). HA is the main constituent of the inorganic matrix of teeth and bones and this mineral would be able to provide calcium and phosphate ions for dealing with tooth demineralization and remineralization.7 Studies have evaluated the effect of HA in nanoparticles (nano-HA) on the tooth surface and showed positive results as a remineralizing and desensitizing material.8,9 These nano-HA have a high surface area and proportion of atomicity due to decrease of the particle, which makes this product able to act in the reorganization of calcium and phosphate ions lost during the demineralization process.10–12
The main advantages of nano-HA are similar morphology, size, crystal structure, solubility, and biocompatibility compared to dental apatite.13,14 Furthermore, nano-HA could penetrate on tooth porosities and produce a protective layer on the tooth surface similar to the natural tooth.11,14 Therefore, we hypothesized that the association of high-F− dentifrice and nano-HA could have a synergetic effect on root dentin demineralization, however, this product is not available in the market. Thus, the aim of this study was to develop and evaluate the effect of an experimental dentifrice containing high-F− concentration associated with nano-HA on root dentin demineralization in vitro.
Materials and Methods
Ethics Statement
This study was approved by the Research Ethics Committee of the Federal University of Piauí (Opinion no. 1962181).
Experimental Dentifrice Development
Synthesis and Characterization of Nano-HA
Nano-HA synthesis was performed using the precipitation method, by the chemical reaction between dibasic ammonium phosphate (NH4)2HPO4 and calcium hydroxide Ca(OH)2. Quantities of each reagent were weighed on a precision analytical balance and separately stirred with distilled water until obtaining a homogeneous solution. This solution was centrifuged at 4000 rpm for 3 min and to the concentrated powder obtained in the tube, distilled water was added and mixed. This process was performed four times to remove impurities from the powder. The solution was placed on a Petri dish and kept in an oven at 100 °C for 16 h. After drying, the obtained powder was macerated and stored in plastic tubes.
X-Ray diffraction (XRD) was performed on a LabX-XDR 600, Shimadzu, CuKα (λ=1.5406 Å), with 2θ in the range of 5°–75°, a scanning rate of 2 °. min,−1 and 40 min of exposure time. The size of crystallites were calculated using the following Debye-Scherrer equation: 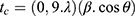 , where tc is the size of the crystallite (nm), λ is the wavelength for the radiation CuKα, β is the width at half height of the diffraction peak under consideration (rad) and θ is the angle of diffraction (degree).
, where tc is the size of the crystallite (nm), λ is the wavelength for the radiation CuKα, β is the width at half height of the diffraction peak under consideration (rad) and θ is the angle of diffraction (degree).
The powder was analyzed by Attenuated total reflectance/Fourier transform infrared spectroscopy (ATR/FTIR) using a Spectrometer (Brucker Optics—Vertex 70, Brucker, Billerica, MA, USA) with 64 scans for each sample, with a resolution of 4 cm−1.
To determine the shape and size of particles obtained, the powder was analyzed by transmission electron microscope. For this analysis, the samples were diluted 1:100 in isopropyl alcohol, sonicated and a 25-µL aliquot of the supernatant was placed on a thin carbon film previously deposited on a copper grid. A JEO JEM 1400 microscope (Akishima, Tokyo, Japan) was used with an acceleration voltage of 200 kV and a field emission gun (FEG).
Experimental Dentifrice Formulation
Six experimental dentifrices were produced and its composition is shown in Table 1. The source of F− in dentifrices was Sodium Fluoride (NaF, Sigma-Aldrich, Saint Louis, MO, USA). Considering the dentifrices preparation technique, the NaF was previously added to heated distilled water to allow total solubilization. Subsequently, this fluoride solution was added to the other components of the formulation, such as saccharin, sodium lauryl sulfate, xanthan gum, titanium dioxide, and sorbitol, until it had a homogeneous appearance. Then, silica and nano-HAp (if present in the composition) were added previously levigated with glycerin. Finally, the flavoring agents were incorporated into the formulation. After dentifrices formulation, they were placed in stability for 48 h, protected from light and heat. F− was quantified in formulations using an ion-specific F− electrode (Orion model 96–09, Orion Research, Cambridge, MA, USA) coupled to an ion analyzer (Orion EA – 740). The calibration curve was performed prior to sample analysis, both performed in triplicate using F− standards ranging from 0.5 to 64 µg F−/mL. F− concentration was calculated by linear regression of the calibration curve and expressed in µg F−/mL.
 |
Table 1 Composition of Formulated Experimental Dentifrices |
Primary Stability of Dentifrice
All tests described above were performed according to the quality control guide for cosmetic products.15 Organoleptic (macroscopic analysis) and physico-chemical tests (pH and spreadability determinations) were performed before and after the freeze-thaw cycle, which consisted of 12 days of cycles of 24 h at 45 ± 2 °C and 24 h at - 5 ± 2°C to accelerate possible reactions with the alternance of extreme temperature conditions.
After 48 h of initial formulation and after the freeze-thaw cycle the dentifrices were analyzed for organoleptic characteristics to identify any signs of instability, color change, or phase separation. Also, samples of the initial formulated dentifrices were subjected to a thermal stress test, which consisted to gradual and significant temperature increases (30 min at 40 °C, 30 min at 50 °C, 30 min at 60 °C, 30 min at 70 °C, 30 min at 80 °C) to verify possible macroscopic changes.
The pH was measured directly on the formulations using a pH meter (PHS – 3B digital meter) calibrated with specific solutions. The pH measurement was performed in triplicate for each sample at 25.6 °C.
A base plate of known weight was placed on 1 g of sample. After 1 min, the diameters covered by the sample were read in two opposite directions, using the graph paper scale. Subsequently, the mean diameter was calculated. Results were expressed according to the following equation: Ei = d2 x π/4, where: Ei = sample spreadability for a given weight (mm2); d = mean diameter (mm).
Cytotoxicity of Dentifrices
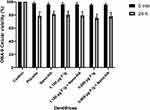
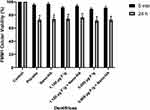
To test cell viability after treatment with dentifrices, two cell types were used: immortalized gingival epithelial cells (OBA-9)16 and human gingival fibroblasts (FMM1).17 The use of the cell lines was approved by the review board of Institute of Biomedical Sciences, University of São Paulo (approval no. 2556792). OBA-9 was cultured in serum-free keratinocyte medium (K-SFM GIBCO™, Life Technologies, Carlsbad, CA, USA) supplied with human PenStrep GIBCO™ recombinant epidermal growth factor (EGF) (10,000 units.mL−1 penicillin; 10,000 µg.mL−1 de streptomycin) and 25 µg.mL−1 amphotericin B at 37 °C in 5% CO2. FMM1 were routinely cultured in DMEM supplemented with 10% fetal bovine serum, penicillin, and streptomycin at 37 °C and 5% CO2. Cells were seeded at 6x105 cells/in 96-well plates and incubated for 24 h at 37 °C. Next, the cells were exposed to 200 µL of the two dentifrices tested, diluted 1:3 in the cell-specific medium. After 5 min and 24 h, the medium was removed. Cell survival was determined using the MTT assay. A 10 µL volume of MTT solution was added to each well, and cells were incubated for 1 h. The resulting formazan crystals were dissolved by removing the culture medium and adding 100 µL of dimethyl sulfoxide solvent to each well. The plates were stirred at room temperature for 10 min to dissolve the crystals and were then analyzed using a microplate reader. Quantitation of enzyme inhibition was performed using a spectrophotometer at 570 nm. Four replicated cell cultures were exposed to each of the dentifrices tested in two independent experiments.18 Absorbance value readings were normalized to untreated control cultures (100%).
Experimental Design of the in vitro Model
Dentin specimens were obtained from bovine incisors previously sterilized in 0.5% thymol solution.8 Diamond disks separated by a 4-mm spacer were used to cut the cementoenamel junction in order to separate crown and root. The roots obtained were sectioned in the mesiodistal direction, and the dentin specimens were sanded and polished, with approximately 4 x 4 x 4 mm in the end.19 To calculate initial hardness, three indentations in the center of the blocks’ surface were made with a Knoop tip under a load of 5 g for 5 s with 100 µm spacing between them. The average of the three values of each block was calculated, and samples with variations up to 15% were selected. After selection, the blocks were randomized into six treatment groups. One-way ANOVA was performed to assess whether there was a statistically significant difference between the hardness of the allocated blocks in each group.
Sixty dentin specimens were randomly distributed in the following treatments (n = 10): (1) placebo; (2) nano-HA dentifrice; (3) 1,100 µg F−/g dentifrice; (4) 1,100 µg F−/g + nano-HA dentifrice; (5) 5,000 µg F−/g dentifrice; (6) 5,000 µg F−/g + nano-HA dentifrice.
The selected blocks were isolated with acid-resistant varnish. Double-sided tape was placed on the central surface of each block, and the other surfaces were protected with varnish. After using the varnish, the tape was removed, and an area of 8 mm2 was left for the treatment.
For this study, a pH cycling model developed by Queiroz20 with modifications was used. The pH cycling used consisted of 10 cycles. The blocks were kept for 6 h in demineralizing solution (1.4 mM Ca, 0.91 mM P, 0.06 µg F−/mL, 0.05 M acetate buffer, pH 5.0) and 18 h in remineralizing solution (1.5 mM Ca, 0.9 mM P, 150 mM KCl, 0.05 µg F−/mL, 0.1 M Tris buffer, pH 7.0) each day. Phosphorus, calcium, and F− concentrations were determined in these solutions to ensure the desired concentration. The volume of demineralizing and remineralizing solutions used per block area was 6.25 mL/mm2 and 3.12 mL/mm2, respectively. Twice a day, before and after immersion in the demineralizing solution, the dental specimens were kept for 5 min under agitation (60 rpm) in the slurry with treatment solution, produced with one part of the dentifrice to three parts of water. This corresponds to the dilution caused by saliva during a regular toothbrushing. After the 10-day cycling period ended, the dentin specimens remained for an additional 24 h in the remineralizing solution.21
After pH cycling, bovine root dentin specimens were fixed to acrylic plates and cut longitudinally. For this, a diamond disk coupled to the electric cutter was used. After cutting, the obtained hemiblock was embedded (Arotec Pre 30 inlayer) in colorless acrylic resin, so that the hardness of the inner part of the block could be evaluated. The sectioned surface of the dentin was sanded and polished with 320, 600, and 1200 grit sandpaper under cooling with purified water. Felt disks were also used for final polishing, and longitudinal hardness was determined. The indentations were made in the center of the block, at distances of 10, 20, 30, 40, 50, 60, 80, 100, 120, 140, 160, 180, and 200 µm from the dentin surface. Two more print lines were repeated 100 µm above and 100 µm below, all with 5 g loading and 5 s time22 and the demineralization area was then calculated. Lesion area (ΔS) was calculated by the difference between the area under the curve (kg/mm2 x µm) of sound and demineralized areas.23,24 The hardness was chosen as analysis because there is a correlation between hardness and mineral loss and gain.25,26
The response variables were submitted to statistical analysis using the Statistical Analysis System Software (SAS, Cary, USA) version 9.0 for Windows, with a significance level of 5%. The Shapiro–Wilk and Levene's test were performed to confirm the normal distribution and homoscedasticity of data, respectively, which were analyzed by two-way ANOVA, considering the factors F concentration in dentifrices (0, 1100, or 5000 µg F−/g) and nano-HA (presence or absence), followed by Tukey’s post hoc test. A paired t-test was performed to compare dentifrice formulation variables before and after the freeze–thaw cycle. The effect of F− concentration in dentifrices on demineralization reduction was evaluated by linear regression analysis and Pearson correlation.
Results
Table 2 presents the summary of two-way ANOVA of the studied variables. For ΔS, pH and spreadability, there was statistical significance both for the isolated factors and for the interaction between them (p<0.001), but not for cytotoxicity (p> 0.05).
 |
Table 2 ANOVA Statistical Analysis (p values) for the Variables Used |
Characterization of Nano-HA
XRD Analysis
Through XRD analysis, the atoms of a crystal build an interference pattern on the waves present in an X-ray beam. The analysis of the obtained pattern confirmed the formation of nano-HA crystals (Figure 1). The material produced was analyzed before and after sonication, and the comparison between the HA diffractogram synthesized with the corresponding crystallographic file showed that the desired phase of HA synthesis occurred. The Debye-Scherrer’s equation was used to determine the size of hydroxyapatite crystallites that showed a nanometric size, ranging from 0.83 to 3.85 nm.
 |
Figure 1 XRD analysis of the synthesized nano-HA. |
Fourier Transform Infrared Spectroscopy – FTIR
FTIR spectrum analysis of both nano-HA and nano-HAs (Figure 2) showed the presence of a band in 3400 cm−1 referring to the stretching vibrations of OH groups present in the HA. A band at 1649 cm−1 was also observed, resulting from a deformation of the OH groups. The band present in the region of 1050 cm−1 corresponded to the asymmetric deformation of the phosphate (PO43−) groups. The phosphate groups also appeared at 611 cm−1. Therefore, FTIR analysis characterizes the successfully synthesized nano-HA
 |
Figure 2 FTIR spectra of the synthesized nano-HA. |
Transmission Electron Microscope (TEM) Analysis
The TEM images showed that the biomaterial had cylindrical morphological particles with sizes varying in the order of nanometers confirming the proper format and size of the material (Figure 3)
 |
Figure 3 Image of synthesized nano-HA by transmission electron microscope (TEM). |
Experimental Dentifrice Formulation
Determination of the Amount of F−
Dentifrices containing nano-HA and F−, irrespective of the F− concentration, showed a slightly less total soluble F− (FST) than the expected value, however without statistical significance and within of 15% variation accepted (Figure 4).
 |
Figure 4 F− concentration (µ F−/g) found in each dentifrice in relation to that expected (n=6). |
Macroscopic Analysis
There was no macroscopic alteration of the formulated dentifrices regarding color, smell, taste, segregation, compound precipitation, or phase separation neither before nor after the freeze–thaw cycle. Also, after the gradual increase in temperature (thermal stress test), there was no macroscopic alteration of the initial formulations.
pH Determination
Overall nano-HA containing dentifrices had a higher (more basic) pH than non-nano-HA dentifrices, and all showed statistical difference (p < 0.001). After the freeze–thaw cycle, all formulations reduced the pH value, except the 5000 µg F−/g + nano-HA group (Table 3).
 |
Table 3 pH and Spreadbility (Average ± SD) of Dentifrices Before and After the Freeze-Thaw Cycle |
Determination of Spreadability
The spreadability results suggest the resistance of the material to the movement induced by the weight of the overlapping plates. As can be seen in Table 3, nano-HA-containing toothpastes have lower spreadability compared to non-biomaterial (p< 0.001), and the freeze–thaw cycle did not change dentifrices’ spreadability according to the paired t-test (p>0.05).
Cytotoxicity of Dentifrices
None of the dentifrices showed considerable cytotoxicity, and there was no statistically significant difference between the formulations analyzed, nor was there any significant difference between the placebo and other formulations. The viability of OBA-9 and FMM1 was lower after 24 h of contact with the dentifrices (Figures 5 and 6).
pH Cycling
The placebo treatment group presented a significant larger lesion when compared to the other treatments (p<0.001), as can be observed in the Figure 7. There was no difference among the nano-HA, 1,100 µg F−/g, and 11,00 µ F−/g + nano-HA groups (p>0.05). These presented a larger lesion area than the groups with 5,000 µg F−/g, regardless of the presence of nano-HA (p<0.001), which did not present a significant difference between them (p>0.05). There was a strong negative correlation between F− concentration in dentifrices and lesion area, regardless of the presence of nano-HA, showing that increased F− concentration leads to decreased lesion area (Figure 8).
Discussion
Despite advances in studies on restorative techniques and dental materials, effective and less invasive therapies remain a challenge in dentistry. Nanotechnology is indicated as a means of effective alternatives for prevention and less invasive treatments.27 The use of nanotechnology is investigated in several biomaterials to repair biological structures and functions, including HA-based materials, which possess biocompatibility and biofunctionality as important properties.28
In the present study, it was possible to observe that nano-HA decreased dentin demineralization, even in the absence of F− in the dentifrice. Evidence shows great similarity in morphology and crystalline structure of nanometer-sized particles to dental apatite.29 The similarity of the particles and their deposition in the demineralized structure may justify the effect promoted by the nano-HA dentifrice. Furthermore, the nanometer size of the particle, which promotes an increase in their contact surface with the dental surface and may promote the reorganization of HA-calcium and phosphate ions on demineralized tissue, is also responsible for this result. Indeed, XRD and FIRT techniques confirmed the synthesis of nano-HA, while TEM revealed the morphology and the reduction of particles to a nanometer scale. Furthermore, particle size also influences the intrinsic properties of this material, such as solubility and biocompatibility.13,28
In dentistry, nano-HA is currently used for the treatment of dentin hypersensitivity,9,12,30 remineralization of carious lesion,11,31 repair and prevention of initial erosion lesions, and formulation of resin adhesives.7,32 In vitro and in situ studies have also been performed on the effect of nano-HA on biofilm formation,27 dentin demineralization,14 and obliteration of dentinal tubules.31 The applicability of nano-HA and the positive results of its use may be related to the effect of the material on the dental demineralization and remineralization process. The demineralization process occurs with dental substrate solubilization, and there are indications that calcium and phosphate ions present in nano-HA can replace lost ions and form a protective layer of HA in the dental structure,29 which may justify the results of the present study, in which dentifrices containing nano-HA had lowest scores of dentin demineralization.
Several studies associate the increase of F− concentration in dentifrices with the effectiveness in the dental demineralization.33–35 F− acts by incorporating the ion into hydroxyapatite, which results in the formation of fluorapatite during the demineralization and remineralization process. The critical pH, which is the pH at which tooth dissolution begins to occur, is lower for fluorapatite. Therefore, this compound is more resistant to acid dissolution. The constant presence of F− ions promotes inhibition of demineralization and remineralization activation.36 In the present study, apart from the better results of high-fluoride dentifrice in reducing dentin demineralization, it was possible to observe a dose-response effect on F− dentifrices, regardless of the presence of nano-HA. Therefore, the higher the F concentration in dentifrice the lower dentin demineralization, which corroborates with a study showing that dentin benefits from higher F concentrations since is a more soluble substrate than enamel.2,5
After the dentifrices formulation, a primary stability test was performed through the freeze-thaw cycle, and parameters such as pH and spreadability were evaluated. The rise and fall in temperature may have altered the chemical properties of the formulations, which caused changes in the pH and the spreadability. Nevertheless, the pH of dentifrices containing nano-HA was overall higher. According to international norms, the dentifrices pH should be within a range considered safe (5.5–10.5),37 which was observed in all formulated dentifrices. The enamel and dentin solubilization process is caused by a drop in pH beyond the critical value for the solubilization of these structures. In the oral environment, saliva (pH = 7.0) acts as a buffer and helps to resist tooth decay, as there is a neutralization of acids. Dentifrices with more basic pH may have a similar effect, which is considered another factor associated with the effect of nano-HA on demineralization38 and could help to explain the lower dentin demineralization in nano-HA dentifrices found in this study. On other hand, the spreading capacity reflects the consistency of a dentifrice, as a large spreading area is equivalent to better consistency.39 Regarding spreadability, the dentifrices containing nano-HA showed a higher consistency, meaning that the material is more viscous and therefore more difficult to apply to the toothbrush for instance.
Cytotoxicity of dentifrices was evaluated by cell viability after contact with the formulation used. For this, the MTT trial was performed and there was no statistical difference between the different formulations. The formulations were placed in contact with OBA (gingival epithelial cells) and FMM1 (gingival fibroblasts) for 5 min and 24h. Those cells form the gingival mucosa and are exposed to the dentifrices during the tooth brushing. The formulations were produced with the constituents commonly present in dentifrices and the absence of significant difference after nano-HA addition is justified by the biocompatibility of this substance, which corroborates the safety of hydroxyapatite nanoparticles for oral care products.40
A pH cycling model was used to simulate the mineral loss and gain that occur in the oral cavity. This model is able to mimic the dynamic conditions of the oral cavity and, therefore, provides important data on in vitro models.21 When dentin is used as a substrate in pH cycling, it is important to address that the diffusion process occurs easily since this substrate is more porous and therefore the remineralization is also facilitated.2 The pH cycling model used in the present study allowed us to notice differences in dentin demineralization induced by the different dentifrice formulations. The statistically significant difference observed between 1,100 and 5,000 µg F/g dentifrices in dentin demineralization was also found in other studies.41,42
There are few studies on the effect of F dentifrice associated with nano-HA. Most of them compare nano-HA and F–free dentifrice with F− dentifrice, without investigating the possible synergistic effect of nano-HA and F−. To the best of our knowledge, this is the first study that addressed the synergistic effect of nano-HA and high-fluoride dentifrice. Although, the greater reduction in dentin demineralization in dentifrice with 5,000 µg F/g associated with nano-HA, no statistical significance was observed when compared to 5,000 µg F/g dentifrice without nano-HA. Therefore, we reject our hypotheses that the association of high-fluoride dentifrice and nano-HA would have an additional protection to root dentin caries, i.e, a synergistic effect. However, this is an in vitro study that are unable to completely simulate the complex intraoral conditions leading to caries development as the presence of saliva and plaque fluid. Furthermore, while dentifrices are typically slurried to simulate dilution during brushing, the uptake and reactivity of fluoride are consistently lower in vivo than in vitro, which could lead to an overestimation of F− treatment.43 Nevertheless, the results found in the present study highlights the importance of further investigations using in situ and clinical designs to provide more realistic data.
Conclusion
The structural characterizations, XRD, FTIR, and TEM showed the success of obtaining the nano-HA. The experimental dentifrice presented stability and no cytotoxicity. Moreover, the findings suggest that nano-HA reduces dentine demineralization and that 5,000 µg F/g dentifrices, regardless of the presence of nano-HA, showed a greater reduction in root dentin demineralization.
Acknowledgments
The cell lines used in this study were kindly provided by Dr. Marcia Mayer (Department of Microbiology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil).
Funding
This study had financial support from FAPEPI (Piaui Research Foundation) – PPSUS Grant N ° EFP_00012129 and from Coordination of Improvement of Higher-Level Personnel (CAPES) – Procad Program (Process n. 88881.068416/2014-01).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Hayes M, Burke F, Allen PF. Incidence, prevalence and global distribution of root caries. Monogr Oral Sci. 2017;1–8. doi:10.1159/000479301
2. Hoppenbrouwers PM, Driessens FC, Borggreven JM. The demineralization of human dental roots in the presence of fluoride. J Dent Res. 1987;66(8):1370–1374. doi:10.1177/00220345870660081701
3. Wierichs RJ, Meyer-Lueckel H. Systematic review on noninvasive treatment of root caries lesions. J Dent Res. 2015;94(2):261–271. doi:10.1177/0022034514557330
4. Nordström A, Birkhed D. Preventive effect of high-fluoride dentifrice (5,000 ppm) in caries-active adolescents: a 2-year clinical trial. Caries Res. 2010;44(3):323–331. doi:10.1159/000317490
5. Fernández CE, Tenuta LMA, Del Bel Cury AA, Nóbrega DF, Cury JA. Effect of 5,000 ppm fluoride dentifrice or 1,100 ppm fluoride dentifrice combined with acidulated phosphate fluoride on caries lesion inhibition and repair. Caries Res. 2017;51(3):179–187. doi:10.1159/000453624
6. Cury JA, Tenuta LMA. How to maintain a cariostatic fluoride concentration in the oral environment. Adv Dent Res. 2008;20(1):13–16. doi:10.1177/154407370802000104
7. Souza BM, Comar LP, Vertuan M, Fernandes Neto C, Buzalaf MAR, Magalhaes AC. Effect of an experimental paste with hydroxyapatite nanoparticles and fluoride on dental demineralisation and remineralisation in situ. Caries Res. 2015;49(5):499–507. doi:10.1159/000438466
8. Yu J, Yang H, Li K, Lei J, Zhou L, Huang C. A novel application of nanohydroxyapatite/mesoporous silica biocomposite on treating dentin hypersensitivity: an in vitro study. J Dent. 2016;50:21–29. doi:10.1016/j.jdent.2016.04.005
9. Wang L, Magalhães A, Francisconi-dos-Rios L, et al. Treatment of dentin hypersensitivity using nano-hydroxyapatite pastes: a randomized three-month clinical trial. Oper Dent. 2016;41(4):E93–E101. doi:10.2341/15-145-C
10. Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4(3):034104. doi:10.1088/1748-6041/4/3/034104
11. Huang S, Gao S, Cheng L, Yu H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: an in vitro study. Caries Res. 2011;45(5):460–468. doi:10.1159/000331207
12. Leitune VCB, Collares FM, Trommer RM, Andrioli DG, Bergmann CP, Samuel SMW. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J Dent. 2013;41(4):321–327. doi:10.1016/j.jdent.2013.01.001
13. Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;27(14):2798–2805. doi:10.1016/j.biomaterials.2005.12.008
14. Cochrane NJ, Cai F, Huq NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11):1187–1197. doi:10.1177/0022034510376046
15. ANVISA. Guia de Controle de Qualidade de Produtos Cosméticos: Uma Abordagem Sobre Os Ensaios Físicos e Químicos. 2008. Available from: http://www.anvisa.gov.br/cosmeticos/material/guia_cosmetico.pdf.
16. Kusumoto Y, Hirano H, Saitoh K, et al. Human gingival epithelial cells produce chemotactic factors interleukin-8 and monocyte chemoattractant protein-1 after stimulation with Porphyromonas gingivalis via Toll-like receptor 2. J Periodontol. 2004;75(3):370–379. doi:10.1902/jop.2004.75.3.370
17. Ruano R, Jaeger RG, Jaeger MMM. Effect of a ceramic and a non-ceramic hydroxyapatite on cell growth and procollagen synthesis of cultured human gingival fibroblasts. J Periodontol. 2000;71(4):540–545. doi:10.1902/jop.2000.71.4.540
18. Camargo SEA, Jóias RP, Santana-Melo GF, Ferreira LT, El Achkar VNR, De Mello Rode S. Conventional and whitening toothpastes: cytotoxicity, genotoxicity and effect on the enamel surface. Am J Dent. 2014;27(6):307–311.
19. Hara AT, Queiroz CS, Paes Leme AF, Serra MC, Cury JA. Caries progression and inhibition in human and bovine root dentine in situ. Caries Res. 2003;37(5):339–344. doi:10.1159/000072165
20. Queiroz CS, Hara AT, Paes Leme AF, Cury JA. pH-Cycling models to evaluate the effect of low fluoride dentifrice on enamel De- and remineralization. Braz Dent J. 2008;19(1):21–27. doi:10.1590/S0103-64402008000100004
21. Tenuta LMA, Cury JA. Laboratory and human studies to estimate anticaries efficacy of fluoride toothpastes. Monogr Oral Sci. 2013;108–124. doi:10.1159/000350479
22. Aires CP, Tabchoury CPM, Del Bel Cury AA, Koo H, Cury JA. Effect of sucrose concentration on dental biofilm formed in situ and on enamel demineralization. Caries Res. 2006;40(1):28–32. doi:10.1159/000088902
23. Cury JA, Do Amaral RC, Tenuta LMA, Del Bel Cury AA, Tabchoury CPM. Low-fluoride toothpaste and deciduous enamel demineralization under biofilm accumulation and sucrose exposure. Eur J Oral Sci. 2010;118(4):370–375. doi:10.1111/j.1600-0722.2010.00745.x
24. Sousa RP, Zanin ICJ, Lima JPM, et al. In situ effects of restorative materials on dental biofilm and enamel demineralisation. J Dent. 2009;37(1):44–51. doi:10.1016/j.jdent.2008.08.009
25. Featherstone JDB, Ten Cate JM, Shariati M, Arends J. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17(5):385–391. doi:10.1159/000260692
26. Kielbassa AM, Wrbas K-T, Schulte-Mönting J, Hellwig E. Correlation of transversal microradiography and microhardness on in situ-induced demineralization in irradiated and nonirradiated human dental enamel. Arch Oral Biol. 1999;44(3):243–251. doi:10.1016/S0003-9969(98)00123-X
27. Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nat Nanotechnol. 2010;5(8):565–569. doi:10.1038/nnano.2010.83
28. Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6(1):41–75. doi:10.1146/annurev.bioeng.6.040803.140027
29. Buzalaf MAR, Pessan JP, Honório HM, Ten Cate JM. Mechanisms of action of fluoride for caries control. Monogr Oral Sci. 2011;22:97–114. doi:10.1159/000325151
30. Yuan P, Liu S, Lv Y, Liu W, Ma W, Xu P. Effect of a dentifrice containing different particle sizes of hydroxyapatite on dentin tubule occlusion and aqueous Cr (VI) sorption. Int J Nanomedicine. 2019;14:5243–5256. doi:10.2147/IJN.S205804
31. Farooq I, Moheet IA, AlShwaimi E. In vitro dentin tubule occlusion and remineralization competence of various toothpastes. Arch Oral Biol. 2015;60(9):1246–1253. doi:10.1016/j.archoralbio.2015.05.012
32. Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: synthesis and application. Dent Mater. 2010;26(5):471–482. doi:10.1016/j.dental.2010.01.005
33. Vale GC, Tabchoury CPM, Del Bel Cury AA, Tenuta LMA, Ten Cate JM, Cury JA. APF and dentifrice effect on root dentin demineralization and biofilm. J Dent Res. 2011;90(1):77–81. doi:10.1177/0022034510383428
34. Rolim F, Melo C, Silva M, Tabchoury C, Vale G. Root dentine demineralisation according to frequency of sucrose exposure using high‐fluoride dentifrice. Gerodontology. 2019;36(4):345–351. doi:10.1111/ger.12419
35. Ferreira RS, Ricomini-Filho AP, Tabchoury CP, Vale GC. Effect of high-fluoride dentifrice and bracket bonding composite material on enamel demineralization in situ. Clin Oral Investig. 2020;24(9):3105–3112. doi:10.1007/s00784-019-03182-7
36. Tenuta LMA, Cury JA. Fluoride: its role in dentistry. Braz Oral Res. 2010;24(suppl 1):9–17. doi:10.1590/S1806-83242010000500003
37. INMETRO. Instituto Nacional de Metrologia Q e T. Relatório de análise de pasta de dente (uso adulto e uso infantil). Published 2000. Available from: http://www.inmetro.gov.br/consumidor/produtos/pastaDente.asp.
38. Vano M, Derchi G, Barone A, Covani U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: a double-blind randomized controlled trial. Quintessence Int (Berl). 2014. doi:10.3290/j.qi.a32240
39. Resende AHM, Farias JM, Silva DDB, et al. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surf B Biointerfaces. 2019;181(February):77–84. doi:10.1016/j.colsurfb.2019.05.032
40. Ramis J, Coelho C, Córdoba A, Quadros P, Monjo M. Safety assessment of nano-hydroxyapatite as an oral care ingredient according to the EU cosmetics regulation. Cosmetics. 2018;5(3):53. doi:10.3390/cosmetics5030053
41. Nordström A, Mystikos C, Ramberg P, Birkhed D. Effect on de novo plaque formation of rinsing with toothpaste slurries and water solutions with a high fluoride concentration (5,000 ppm). Eur J Oral Sci. 2009;117(5):563–567. doi:10.1111/j.1600-0722.2009.00674.x
42. Ekstrand KR, Poulsen JE, Hede B, Twetman S, Qvist V, Ellwood RP. A randomized clinical trial of the anti-caries efficacy of 5,000 compared to 1,450 ppm fluoridated toothpaste on root caries lesions in elderly disabled nursing home residents. Caries Res. 2013;47(5):391–398. doi:10.1159/000348581
43. Buzalaf MAR, Hannas AR, Magalhães AC, Rios D, Honório HM, Delbem ACB. Ph-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: strengths and limitations. J Appl Oral Sci. 2010;18(4):316–334. doi:10.1590/S1678-77572010000400002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.