Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Development of a liposome-based formulation for vitamin K1 nebulization on the skin
Authors Campani V, Marchese D, Pitaro MT, Pitaro M, Grieco P, De Rosa G
Received 27 November 2013
Accepted for publication 22 January 2014
Published 10 April 2014 Volume 2014:9(1) Pages 1823—1832
DOI https://doi.org/10.2147/IJN.S58365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Virginia Campani,1,2 Dario Marchese,1 Maria Teresa Pitaro,2 Michele Pitaro,2 Paolo Grieco,1 Giuseppe De Rosa1
1Department of Pharmacy, Università degli Studi di Napoli Federico II, Naples, Italy; 2Xenus Srl, Rome, Italy
Abstract: Vitamin K1 (VK1) is a very lipophilic and photosensitive molecule contained in some vegetables. Recently, the use of VK1 on the skin has been proposed for different pharmaceutical or cosmeceutical applications. In this study, an innovative strategy for the administration of VK1 on the skin was proposed. In particular, to overcome the drawbacks associated with a VK1-containing fatty ointment available on the market, an aqueous formulation suitable to be administered by nebulization was developed. The use of liposomes encapsulating VK1 enabled issues due to the lipophilicity of VK1 to be overcome. Thus, different liposomal formulations, with different VK1 concentrations, were prepared and characterized in terms of size, zeta potential, VK1 encapsulation into liposomes, and stability of the formulations during storage. After a first phase of screening, the selected formulation was tested by a portable device for nebulization. No alteration of the vesicle characteristics following the liposome supply through the nebulizer was found. Finally, permeation studies were carried out on pig-excised skin in Franz cells and the newly developed formulation was compared to a marketed VK1-containing ointment. In this test, an enhanced VK1 accumulation into the skin was found when using nebulized liposomes. In conclusion, in order to administer VK1 on the skin, the newly developed formulation could be a valid alternative to the products available on the market today. In particular, the use of liposomes could facilitate the multiple administrations per day by aerosol, but also increase, compared to a semi-solid preparation, the accumulation of VK1 into the epidermis and dermis.
Keywords: liposomes, liposome stability, liposome nebulization, Franz cells
Introduction
Vitamin K1 (VK1) or 2-methyl-3-phytyl-1,4-naphthoquinone (or phylloquinone) is a vitamin contained in different plants, especially in leaf vegetables and, in smaller quantities, also in fruits, tubers, and seeds.1 Although VK1 is well known as an anti-hemorrhagic factor, other pharmacological activities and potential therapeutic or cosmeceutical uses have emerged in recent years. Due to its chemical structure, which is similar to that of ubiquinone, VK1 was found to act as an antioxidant agent.2 VK1 has been proposed to prevent vascular events due to aging, by suppressing the skin pigmentation and leading to resolution of bruising, as well as countering changes in the skin after irradiation with laser beams.3–5 Finally, it has been shown that the topical application of VK1 is able to facilitate the removal of extravascular blood from the skin.6 In recent years, creams containing VK1 have been proposed to prevent side effects on the skin in patients with metastatic tumors of the colon and rectum, treated with cetuximab, a monoclonal antibody directed against the epidermal growth factor receptor (EGFR).7 Indeed, the use of EGFR skin inhibitors involves a set of reactions in the skin (has been reported in approximately 80% of patients), among which the commonest are acneiform reactions that frequently appear at the level of the head, neck, and trunk.8,9 Recent studies have shown that the degree of these acneiform reactions is strongly reduced in those regions of the body that are prophylactically treated with a cream containing VK1. This effect has been attributed to the capability of VK1 to activate the EGFR by upregulation of EGFR phosphorylation, thus counteracting the cetuximab-induced inhibition of this receptor.10,11 The topical application of VK1 is, for this reason, effective in ensuring a better quality of life of the patient, with no dose reduction or discontinuation of the therapy.7,12
However, VK1 is yellow viscous oil, soluble in organic solvent and insoluble in water. The high lipophilicity of the VK1 requires it to be administered in fat-based ointments (eg, Reconval® K1; Pharmadab, Ljubljana, Slovenia; VigorSkin K1®, Merck Serono, Bergamo, Italy), making multiple daily administrations on the skin necessary, which can lead to poor compliance, especially in summer. In light of these observations, the development of a formulation designed for a more practical and more frequent administration of the VK1 on the skin could be very useful in allowing prophylactic use of this vitamin with higher patient compliance.
In the last 2 decades, a growing number of studies have been dedicated to the use of nanotechnology-based approaches for topical administration of drugs. The use of colloidal systems, such as liposomes, can allow increased drug accumulation into the skin, depending on the type of drug, as well as on the characteristics of the nanocarrier.13–18
The aim of this study was to develop a new aqueous formulation containing VK1 suitable to be administered by aerosol (with a portable device) on the skin. In particular, we investigated if a formulation based on liposomes could be used to overcome the low water solubility of VK1. Thus, different formulations were prepared and characterized. Liposomes with the optimal technological characteristics, in terms of size, VK1 encapsulation, and stability during storage in different conditions, were selected for the following studies. Then, the possibility to nebulize the liposomal suspension, without alteration of the vesicles, was evaluated. Finally, VK1 accumulation and permeation into and through the skin were investigated in Franz diffusion cells (Microglass Heim, Napoli, Italy), using ear porcine skin.
Materials and methods
Materials
VK1, α-tocopherol (αTOC), and iron thiocyanate (FeSCN3) were purchased from Sigma-Aldrich (St Louis, MO, USA). High-performance liquid chromatography (HPLC)-grade methanol (CH3OH) and acetonitrile (CH3CN), analytical grade chloroform (CHCl3) and ethanol (CH3CH2OH) were obtained from Carlo Erba Reagents (Cornaredo, Italy). Benzalkonium chloride was provided by Farmalabor (Canosa di Puglia, Italy); soy phosphatidylcholine (SPC) was kindly gifted by Lipoid GmbH (Steinhausen, Switzerland).
Preparation of liposomes
The preparation of liposomes was performed by hydration of a lipid film followed by extrusion. Briefly, an organic solution (CHCl3:CH3OH [2:1]) containing SPC was dried in a round-bottom glass flask by a rotary evaporator (4010 Laborota digital; Heidolph, Schwabach, Germany) under a nitrogen atmosphere for about 20 minutes at 110 rpm and at a temperature of 30°C. VK1 and αTOC were added to the organic solution at different concentrations. The obtained lipid films were then rehydrated, in the presence of glass beads, with phosphate-buffered solution (PBS) with a pH of 7.4 or an aqueous solution 0.01% w/v of benzalkonium chloride. The resulting suspension was repeatedly passed through polycarbonate membranes (five times per membrane) of decreasing porosity (0.4, 0.2, and 0.1 μm) by using a thermobarrel extruder (Northern Lipids Inc., Burnaby, BC, Canada). Finally, the liposomes were purified by molecular exclusion chromatography with a Sephadex G-50-50 (Sigma-Aldrich) to remove the non-encapsulated VK1. For each preparation, three batches were prepared.
Liposome size and zeta potential
Dimensional analysis was performed by photon correlation spectroscopy (PCS). For each sample, an aliquot of approximately 20 μL was diluted in filtered water and analyzed by PCS (N5; Beckman Coulter, Brea, CA, USA). The average diameter and the size distribution of each formulation were determined. The results were expressed as liposome mean diameter in nanometers and polydispersity index (PI).
The zeta potential of liposomes was performed by the Zetasizer Nano Z (Malvern Instruments, Worcestershire, UK). Briefly, an aliquot of each sample (20 μL) was diluted in filtered water and analyzed. The results were calculated by the average of the measurements obtained from three batches of the same formulation.
Determination of lipid concentration
The concentration of lipids present in the liposome suspension after preparation was determined using the Stewart assay.19 Briefly, an aliquot of the liposome suspension was added to a two-phase system consisting of an aqueous ammonium ferrothiocyanate solution or iron thiocyanate solution (0.1 N) and CHCl3. The concentration of SPC was obtained by measuring the absorbance at 485 nm into the organic layer with an ultraviolet–visible spectrophotometer (UV VIS 1204; Shimadzu Corporation, Kyoto, Japan). Quantification of SPC was carried out by means of a calibration curve (r2=0.999) with standard SPC samples at concentrations ranging from 0.005–0.04 mg/mL.
VK1 encapsulation into the liposomes
The amount of VK1 entrapped in liposomes was determined by HPLC. For the analysis, a Shimadzu HPLC system, consisting of an LC-10AD pump and a Rheodyne injection valve 7725i and equipped with an SPV-10A ultraviolet–visible detector (λ set at 333 nm), was used; a SCL-10AVP system controller (Shimadzu) connected to a computer was also used previously. The analysis was performed on a Luna C8 column (250×4.6 mm, 5 mm; Phenomenex, Torrance, CA, USA) under isocratic conditions and using CH3OH as mobile phase at a flow of 1 mL/minute. The acquisition of the chromatograms was carried out using Class VP Client/Server software (v 7.2.1; Shimadzu Scientific Instruments, Columbia, MD, USA). The analysis in HPLC showed a chromatographic peak associated with the VK1 at a retention time of about 7 minutes. The amount of VK1 was calculated by means of a calibration curve (r2=0.999) with standard VK1 samples at concentrations ranging from 50–0.5 μg/mL. To determine the amount of VK1 encapsulated in liposomes, the suspension was diluted (1:100) with CH3OH to allow the dissolution of the vesicles and the consequent release of VK1. The samples were then centrifuged for 30 minutes at 13,000 rpm (MIKRO 20; Hettich, Tuttlingen, Germany) and then the supernatant was analyzed by HPLC. The results are expressed as actual loading, calculated as μg of VK1 per mg of SPC. The results are the mean of measures made on three different batches.
Stability studies
Physical stability of liposomes was evaluated after different time frames on the formulations prepared using increasing VK1 concentrations. Briefly, after preparation, each batch was stored at 4°C and, at predetermined intervals, approximately 10 μL of the suspension was diluted in filtered distilled water and analyzed by PCS, as reported above.
The VK1 release from the liposomes at 4°C was determined in the different VK1 formulations. Briefly, at predetermined intervals, an aliquot of approximately 1 mL of the suspension was purified by molecular exclusion chromatography. Then, the purified liposomes were analyzed in terms of VK1 content by HPLC, as reported previously. The formulations of liposomes encapsulating VK1 were also tested in different storage conditions. In particular, liposomes were incubated at different temperatures, namely 4°C, 25°C, and 40°C, in a refrigerator (Indesit Company SpA, Fabriano, Italy) or in a laboratory oven (STF-F52Lt; Falc Instruments, Treviglio, Italy). All samples were kept in a nitrogen atmosphere and protected from light. In the case of samples stored at 25°C, vials exposed to sunlight were also prepared. At predetermined intervals, the samples were analyzed in terms of physical appearance, odor, liposome size, and VK1 content. Liposome size and VK1 content were determined by PCS and HPLC, respectively, as described previously. For each formulation, the results were obtained as the means of three different batches (n=3). The liposomes were also characterized before and after nebulization. To nebulize the liposome suspension, a portable nebulizer (Eauté, Xenus, Rome, Italy), kindly provided by Xenus (Rome, Italy), was used. Briefly, approximately 1 mL of the liposome suspension, previously characterized for size and VK1 encapsulation, was loaded into the device and nebulized by collecting the aerosol in a 20 mL glass vial. The collected suspension was then analyzed in terms of size and VK1 encapsulation.
Skin penetration experiments
The penetration of VK1 into the skin and its transdermal delivery were assessed using porcine ear skin. The porcine ears were kindly provided by a local slaughterhouse (Vendor Carni, Montefredane, Italy). Full-thickness skin was removed from the dorsal side of the freshly excised pig ear, stored at –20°C and thawed within 6 months for the experiments. On the day of the experiment, punches were cut out and hairs cut with scissors, as reported previously by other authors.20 The outer skin surface (stratum corneum) and the inner region (dermis) were used for the experiments. After drying, the skin was cut into circles of 3 cm diameter. For permeation experiments, the skin was mounted in the Microglass Heim Franz diffusion cells. Briefly, the porcine skin was mounted on the receptor compartment of a Franz diffusion cell assembly with the stratum corneum side facing upwards into the donor compartment. Seven milliliters of 3:7 (v/v) CH3CH2OH:pH 7.4 PBS was used as the receptor medium. A measured amount of liposomes containing VK1 was poured or nebulized into the donor compartment. Alternatively, a weighted amount of VigorSkin K1® cream was added into the donor compartment in contact with the excised skin. The concentration of all samples was adjusted to achieve the same VK1 amount (1 mg) in the donor compartment. The available diffusion area between compartments was 0.6 cm2. The Franz cells were mounted on a H+P Labortechnik Variomag Telesystem (Munchen, Germany) and placed in a thermostatic bath (Haake DC30; Thermo Fisher Scientific, Waltham, MA, USA). The experiments were carried out at a stirring rate of 600 rpm and temperature of 37°C. At predetermined time frames, 700 μL of the receptor phase were withdrawn and replaced with the same amount of fresh medium. The amount of VK1 in the withdrawn samples was determined by HPLC. At the end of the experiments, skin surfaces were thoroughly washed with distilled water to remove the excess formulation. Epidermis and dermis were then separated by heating and then placed in separate eppendorfs. Finally, the VK1 accumulated in epidermis or dermis was extracted with 1 mL of CH3CN by bath sonication (Branson 3510) five times for 30 minutes. The CH3CN phase was filtered using 0.45 μm membranes, and the resulting filtrate was analyzed by HPLC to determine the VK1 content. The amount of VK1 accumulated in the different layers of the skin was calculated as the ratio of amount of VK1 (ng) to weight of epidermis or dermis (mg).2,21
Results and discussion
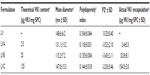
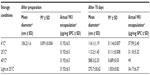
This study was focused on the development of a new formulation for the topical application of VK1. In particular, the aim of the work was to design and develop an aqueous formulation able to overcome the unpleasant feeling of a fatty ointment on the skin, especially when application is required several times per day. Moreover, to facilitate the administration of the VK1 on the skin, a formulation with a low viscosity, suitable to be nebulized in a portable device, was developed. In order to meet all these requirements, a formulation based on nano-sized lipid vesicles, ie, liposomes, was designed. Liposomes were proposed for their capability to encapsulate hydrophobic molecules, such as VK1, and to form homogeneous colloidal dispersion in water. In the first phase of the work, three different liposomal formulations with increasing initial concentrations of VK1 were prepared and characterized. The characteristics of the prepared formulations are summarized in Table 1. The aim of this first part of the study was to obtain liposomes with low mean diameter (in the range of 100–150 nm), a narrow size distribution, high VK1 encapsulation, and good physical stability during storage. All formulations had an average diameter ranging from approximately 115–150 nm, with a narrow size distribution, as shown by a PI lower than 0.2. The presence of VK1 in the preparation did not significantly influence the mean diameter or the size distribution of the liposomes. LV (Table 1) had a neutral zeta potential, which was slightly lower in the case of the formulation containing VK1. No significant difference in zeta potential were found when encapsulating different amounts of VK1 (Table 1). The amount of VK1 encapsulated into liposomes was determined by HPLC analysis. For each formulation, the actual VK1 encapsulation was higher than the theoretical one. These findings could be explained by lipid loss during the preparation that was higher than the vitamin loss, especially if we hypothesize that the inclusion of VK1 into the lipid bilayer results in the formation of vitamin-rich domains immiscible with the phospholipid bulk, as suggested by previously reported study.22 It is worth noting that, in all three preparations, HPLC analysis of the liposome content showed only one chromatographic peak, attributed to the VK1, suggesting that the phylloquinone was not altered during the preparation (data not shown).
In the second step of the study, the stability of the developed formulations during storage in different conditions was evaluated following storage at 4°C in the absence of light. These results are summarized in Table 2. Blank liposomes did not show significant alteration in mean size or PI following 150 days of storage at 4°C. LVA (Table 1) did not show significant change in mean size during storage, while VK1 loading was gradually reduced during storage. In particular, about 10% of the VK1 initially loaded into liposomes was lost during the first week of storage. In the following 2 months, VK1 loading of LVA did not significantly change, but a further and significant decrease of VK1 loading (to about 57% of the VK1 initial loading) was observed following 150 days of storage. In the case of LVB (Table 1), about 5% of the VK1 was leaked from liposomes after the first 7 days of storage; a further and significant VK1 release from liposomes was also observed following 70 days of storage with a total VK1 release of about 30%. Interestingly, the VK1 content of LVB did not significantly change following further storage until 150 days. Indeed, the formulations LVA and LVB did not present any organoleptic alteration during storage. Different results were found for the formulation LVC, for which agglomerates were visible in the suspension before 70 days of storage; moreover, a characteristic odor was reported for this formulation.
Taking these results into account, it is possible to conclude that VK1 influences the physical stability of liposomes, as well as the release of VK1 during storage, depending on the concentration. It has been reported that VK1 can influence the properties of phospholipid bilayers by broadening and shifting the lipid transitions to lower temperature. Moreover, the formation of vitamin-rich domains, immiscible with the bulk phospholipids, has been reported.22 This report is in agreement with our observations. In particular, the formation of these vitamin-rich domains could be favored when using high VK1 loading (formulation LVC). VK1 can be released from these domains and, consequently, be altered in the aqueous medium, as testified by the development of unpleasant odor probably caused by vitamin alteration.
Thus, we considered that the formulation LVB had the best characteristics in terms of VK1 encapsulation and stability during storage and was therefore to be used in the following step of the work.
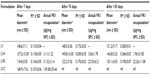
The stability of the formulation LVB was investigated in different conditions of storage according to ICH guidelines (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use). The aim of these stability tests was to evaluate how environmental factors, such as temperature and light exposure, can affect the product quality, thus establishing a shelf life for the drug product and recommending storage conditions. Visual inspections to check the presence of agglomerate, coloring of the suspension, and organoleptic evaluation of the samples were carried out at different time frames. At the same intervals, samples were characterized in terms of vesicle size and VK1 actual loading. The results of the visual and organoleptic analysis are reported in Table 3. Liposome incubation at 40°C resulted in development of a pronounced characteristic odor after 30 days, while the presence of aggregation in the suspension was also observed at longer time frames (ie, 70 days). The development of a characteristic odor was significantly delayed and attenuated in samples stored at 25°C (see Table 3). Finally, in samples stored at 25°C under light, a marked odor was reported after the first 30 days, although not associated to the presence of aggregates. For all the samples, the storage under nitrogen atmosphere did not result in any influence of the suspension stability (data not shown). The same samples were also characterized in terms of liposome size and VK1 leakage during storage (see Table 4). In agreement with the visual and organoleptic analysis, the samples stored at 40°C showed a marked increase of mean diameter and PI, suggesting the presence of aggregates into the suspension. For this reason, samples stored at 40°C did not undergo further characterization. In the case of liposomes stored in presence of light, only a moderate increase of the vesicle size was found, once more in agreement with previous observations (see Table 3). It is worth noting that, in the samples exposed to light, a reduction of the initial actual loading of VK1 of about 50% was found. Moreover, HPLC analysis revealed an additional chromatographic peak (Figure 1), with a retention time that was shorter than that of native VK1. The additional compound found in the HPLC analysis could be reasonably attributed to the transformation of the VK1 into its derivatives. This additional chromatographic peak was not found in the case of samples stored at 40°C in absence of light.
  | Table 3 Visual and organoleptic analysis of the formulation LVB stored in different conditions |
  | Figure 1 Chromatogram of sample extracted by LVB following storage under light. |
From our results, we can hypothesize that, depending on the storage conditions, ie, 40°C or light exposure, different mechanisms could be responsible for the sample alteration observed in LVB. In detail, light exposure could result in the VK1 alteration, as evidenced by the appearance of an additional chromatographic peak. On the other hand, in samples stored at high temperature, liposome aggregation and enhanced VK1 release could occur. This physical instability of vesicles is likely due to the presence of VK1 that could affect the bilayer characteristics, as hypothesized previously. In fact, blank liposomes were found to be stable at the same temperature and for the same time frames. Thus, VK1 encapsulation into the liposomes can affect physical characteristics of the bilayer, making vesicles more unstable and enhancing VK1 release, following incubation at high temperature. Finally, it is worth noting that a higher VK1 actual loading could be observed after storage of liposomes, especially at 40°C. Changes in the SPC and VK1 concentrations during storage in a liposome suspension may be due to different reasons. Actually, in the case of the VK1, as hypothesized above, a reduction of the concentration into the liposomes could be due to its release from the vesicle or to chemical alteration of the vitamin. In the case of SPC, reduced content into the liposome suspension could be mainly ascribed to lipid hydrolysis. It is well known that high temperature favors lipid degradation, which can be minimized at low temperature.23,24 In agreement with this, we found that a decrease of the lipid concentration, in terms of liposomes, was found in samples stored at high temperature, with a consequent increase of the vitamin/lipid ratio (actual loading).
In the final step of the formulative study, two other excipients were included in the formulation. In particular, αTOC (2.5 μg/mg SPC) and benzalkonium chloride (0.01%) were added to the organic solution containing the lipids and to the aqueous solution, respectively. αTOC was used to prevent the oxidation of lipids present in the bilayer.25 Benzalkonium chloride was added as antimicrobial preservative. This new formulation (LVB-TOC-BC) was developed with the same procedure used for LVB, except filtered water with benzalkonium chloride (0.01%) instead of PBS was used to hydrate the lipid film. The characteristics of LVB-TOC-BC liposomes were not significantly different to those of LVB. In detail, after preparation, liposomes had a mean diameter of about 110 nm with a PI of about 0.12. These characteristics did not significantly change following storage of the formulation (Table 5). Moreover, LVB-TOC-BC liposomes had an actual loading similar to that of LVB, ie, approximately 33 μg/mg SPC, that was reduced to approximately 25 μg/mg SPC after the first 7 days of storage at 4°C. Interestingly, further leakage of VK1 was observed following storage at 4°C for more than 2 months (Table 5). Finally, the formulation LVB-TOC-BC was also analyzed in different conditions of temperature and light exposure. The results of the visual inspection and organoleptic analysis are reported in Table 6, while, in Table 7, the liposome characteristics in terms of size and VK1 encapsulation are summarized. LVB-TOC-BC showed a similar behavior compared to LVB, with presence of aggregation at 40°C and a characteristic and pronounced odor following liposome exposure to light. Moreover, aggregation was not found in the suspension following liposome storage at 25°C for 70 days (Tables 6 and 7), suggesting that phospholipid oxidation could play a role in the suspension instability found at high temperature.
  | Table 6 Visual and organoleptic analysis of the formulation LVB-TOC-BC stored in different conditions |
Finally, to verify if the formulation LVB-TOC-BC was suitable to be administered on the skin in aerosol form, for example, with a portable device for nebulization, the physical characteristics of the liposomes were checked before and after nebulization. The aerosol produced by nebulization of LVB-TOC-BC is shown in Figure 2, while liposome characteristics before and after supply through the device are reported in Table 8. No alteration of liposome size and VK1 encapsulation was observed following nebulization, suggesting that the vesicles maintain their integrity when administered in the form of aerosol onto the skin.
  | Figure 2 Nebulization of the formulation LVB-TOC-BC by the portable device Eauté. |
The formulation LVB-TOC-BC was used for the ex vivo studies using Franz cells. LVB-TOC-BC was used as such or nebulized through the medical device Eauté. VK1 permeation through the skin or accumulation into epidermis/dermis was compared with that obtained, in the same experimental conditions, with VigorSkin K1®, a marketed cream containing VK1. Finally, to investigate if the excipients added to the formulation LVB, especially αTOC, influenced VK1 delivery into and through the skin, LVB liposomes were also investigated in the skin penetration experiments. The amount of VK1 accumulated in each of the epidermis and dermis is reported in Figure 3. In the case of skin samples treated with VigorSkin K1®, VK1 accumulation in epidermis was very low (about 6.03±4.68 ng/mg of epidermis). The amount of VK1 accumulated in the epidermis was significantly higher in the case of skin samples placed in contact with the liposomal-containing formulation (about 26.15±5.53 ng/mg of epidermis for LVB-TOC-BC), especially when the LVB-TOC-BC was applied on the skin by nebulization (35.16±10.95 ng/mg of epidermis). The difference between liposome-based formulation and the marketed cream was more evident in the case of the epidermis, where no VK1 accumulation was found in the case of skin treated with VigorSkin K1®; on the contrary, significant VK1 accumulation was found in dermis in the case of skin treated with LVB-TOC-BC without nebulization (47.8±0.1 ng/mg of dermis) or nebulized (55.7±1.89 ng/mg of dermis). Finally, for all samples, VK1 permeation through the skin was not observed. It is worth noting that, in the case of formulation LVB, the amount of VK1 accumulated in the epidermis is not significantly different if compared with that observed in the case of VigorSkin K1® cream. On the other hand, the advantage of using LVB was more evident when measuring the amount of VK1 accumulated in the dermis (Figure 3B). In both epidermis and dermis, the amount of VK1 accumulated in skin with LVB-TOC-BC liposomes was significantly higher than that obtained with LVB. Different advantages concerning the use of liposomes for drug administration on the skin have been previously described.26 Enhanced VK1 accumulation in the skin by using a nanodispersed monoolein-based formulation has also been described.21 From a general point of view, liposomes can improve local drug concentration by interacting with similar lipids of the skin.26 The role of phospholipids as penetration enhancers, especially when containing unsaturated fatty acids, is well known. Phospholipids, such as SPC, can be easily incorporated within the viable cells via intercellular lipids of the outer layer of the skin; this can disrupt the lamellar structure of the stratum corneum with consequent increased fluidity and altered permeability.27,28 Thus, the use of SPC in dermatological formulations can result in enhanced diffusion of drug in the lipid domains and increased transport through the skin. While the use of a hydrophilic excipient such as benzalkonium chloride had no effect on the VK1 delivery into the skin (data not shown), the use of αTOC in the formulation dramatically increased the VK1 accumulation in epidermis and dermis. αTOC in liposomes has already been associated to SPC as a penetration enhancer.29 αTOC, when encapsulated into the liposomes, has been found not only in the stratum corneum, but also in the underlying skin.30 Once into the skin, αTOC could synergistically act with SPC by altering the structure of the stratum corneum, thus enhancing the VK1 diffusion and accumulation into the epidermis and dermis. Interestingly, including αTOC in a liposome formulation has been reported to result in increased biocompatibility of liposomes on human fibroblast.31 Finally, it is worth noting that VK1, if systemically released at high concentrations, could alter the synthesis of coagulation factors. In this study, VK1 permeation through the skin was not found, suggesting a negligible systemic effect with this formulation.
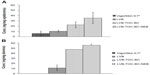  | Figure 3 Accumulation of VK1 in epidermis (A) and dermis (B). |
Conclusion
In this study, the possibility of developing a formulation for the administration of a highly lipophilic VK1 on the skin by nebulization has been investigated. Liposomes can be a valid tool by which to overcome the lipophilicity of VK1, forming a stable aqueous dispersion that can be administered on the skin in the form of an aerosol by means of a portable nebulizer without alteration of vesicle characteristics. Liposomes containing VK1 are stable if stored at 4°C in absence of light. Finally, the use of liposomes, before or after being nebulized, resulted in an enhanced VK1 accumulation in epidermis and dermis when compared with a marketed VK1-containing fatty ointment. Our results suggest that the newly developed formulation can be considered a valid alternative to fatty ointments for the administration of VK1 on the skin. This strategy for VK1 administration can be especially useful in the prevention of acneiform reactions against the skin following treatment with cetuximab in patients with metastatic tumors of the colon and rectum.
Acknowledgments
This research was supported by a grant from FILAS (Finanziaria Laziale per lo Sviluppo)–Progetti di innovazione delle micro e piccolo imprese, POR FESR Lazio 2007/2013 Asse I–Attività 2–CUP F87I12001730007.
Disclosure
The authors report no conflicts of interest in this work.
References
Pace E. Le Vitamine. Milan: Ulrico Hoepli Editore; 1949. | |
da silva al, Contri RV, Jornada DS, Pohlmann AR, Guterres SS. Vitamin K1-loaded lipid-core nanocapsules: physiochemical characterization and in vitro skin permeation. Skin Res Technol. 2012;19(1):e223–e230. | |
Lou WW, Quintana AT, Geronemus RG, Grossman MC. Effects of topical vitamin K and retinol on laser-induced purpura on nonlesional skin. Dermatol Surg. 1999;25(12):942–944. | |
Elson ML, Nacht S. Treatment of a periorbital hyperpigmentation with topical vitamin K/vitamin A. Cosmet Dermatol. 1999;12:27–127. | |
Leu S, Havey J, White LE, et al. Accelerated resolution of laser-induced bruising with topical 20% arnica: a rater-blinded randomized controlled trial. Br J Dermatol. 2010;163:557–563. | |
Shah NS, Lazarus MC, Bugdodel R, et al. The effects of topical vitamin K on bruising after laser treatment. J Am Acad Dermatol. 2002;47(2):241–244. | |
Ocvirk J. Management of cetuximab-induced skin toxicity with the prophylactic use of topical vitamin K1 cream. Radiol Oncol. 2010;44:256–266. | |
Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425–1433. | |
Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. | |
Tan EH, Chan A. Evidence-based treatment options for the management of skin toxicities associated with epidermal growth factor receptor inhibitors. Ann Pharmacother. 2009;43(10):1658–1666. | |
Li TH, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4(2):107–119. | |
Tomková H, PospíŠková M, Zábojníková M, et al. Phytomenadione pre-treatment in EGFR inhibitor-induced folliculitis. J Eur Acad Dermatol Venereol. 2013;27(4):514–519. | |
Ferreira LS, Ramaldes GA, Nunan EA, Ferreira LA. In vitro skin permeation and retention of paromomycin from liposomes for topical treatment of the cutaneous leishmaniasis. Drug Dev Ind Pharm. 2004;30(3):289–296. | |
Puglia C, Trombetta D, Venuti V, Saija A, Bonina F. Evaluation of in-vivo topical anti-inflammatory activity of indometacin from liposomal vesicles. J Pharm Pharmacol. 2004;56(10):1225–1232. | |
Ramón E, Alonso C, Coderch L, et al. Liposomes as alternative vehicles for sun filter formulations. Drug Deliv. 2005;12(2):83–88. | |
Kitagawa S, Kasamaki M. Enhanced delivery of retinoic acid to skin by cationic liposomes. Chem Pharm Bull (Tokyo). 2006;54(2):242–244. | |
Gokce EH, Korkmaz E, Tuncay-Tanriverdi S, et al. A comparative evaluation of coenzyme Q10-loaded liposomes and solid lipid nanoparticles as dermal antioxidant carriers. Int J Nanomedicine 2012;7:5109–5117. | |
Rahimpour Y, Hamishehkar H. Liposomes in cosmeceutics. Expert Opin in Drug Deliv. 2012;9(4):443–455. | |
Stewart JCM. Anal Biochem. 1959;104:10. | |
Gillet A, Lecomte F, Hubert P, Ducat E, Evrard B, Piel G. Skin penetration behaviour of liposomes as a function of their composition. Eur J Pharm Biopharm. 2011;79:43–53. | |
Lopes LB, Speretta FF, Bentley MV. Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur J Pharm Sci. 2007;32(3):209–215. | |
Ortiz A, Aranda FJ. The influence of vitamin K1 on the structure and phase behavior of model membrane systems. Biochim Biophys Acta. 1999;1418(1):206–220. | |
Pietzyk B, Henschke K. Degradation of phosphatidylcholine in liposomes containing carboplatin in dependence on composition and storage conditions. Int J Pharm. 2000;196(2):215–218. | |
Grit M, Crommelin DJ. Chemical stability of liposomes: implications for their physical stability. Chem Phys Lipids. 1993;64(1–3):3–18. | |
Fukuzawa K. Dynamics of lipid peroxidation and antioxidion of alpha-tocopherol in membranes. J Nutr Sci Vitaminol (Tokyo). 2008;54(4):273–285. | |
Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 2011;303(9):607–621. | |
Ogiso T, Niinaka N, Iwaki M. Mechanism for enhancement effect of lipid disperse system on percutaneous absorption. J Pharm Sci. 1996;85(1):57–64. | |
Yokomizo Y, Sagitani H. The effects of phospholipids on the percutaneous penetration of indomethacin through the dorsal skin of guinea pig in vitro. 2. The effects of the hydrophobic group in phospholipids and a comparison with general enhancers. J Control Release. 1996;42:37–46. | |
Lee WC, Tsai TH. Preparation and characterization of liposomal coenzyme Q10 for in vivo topical application. Int J Pharm. 2010;16; 395(1–2):78–83. | |
Baschong W, Artmann C, Hueglin D, Roeding J. Direct evidence for bioconversion of vitamin E acetate into vitamin E: an ex vivo study in viable human skin. J Cosmet Sci. 2001;52(3):155–161. | |
Berrocal MC, Bujan J, Garcia-Honduvilla N, et al. Comparison of the effects of dimyristoyl and soya phosphatidylcholine liposomes on human fibroblasts. Drug Deliv. 2000;7:37–44. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.