Back to Journals » Drug Design, Development and Therapy » Volume 14
Development and Validation of UHPLC-MS/MS Assay for Therapeutic Drug Monitoring of High-dose Methotrexate in Children with Acute Lymphoblastic Leukemia
Authors Lian LJ , Lin B , Cui X, He J, Wang Z , Lin XD, Ye WJ, Chen RJ, Sun W
Received 9 July 2020
Accepted for publication 7 October 2020
Published 10 November 2020 Volume 2020:14 Pages 4835—4843
DOI https://doi.org/10.2147/DDDT.S271568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Le-jing Lian,1 Bin Lin,2 Xiao Cui,1 Jie He,1 Zhe Wang,1 Xiao-dong Lin,1 Wei-jian Ye,1 Rui-jie Chen,1 Wei Sun1
1Department of Pharmacy, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou 325027, People’s Republic of China; 2Department of Clinical Pharmacy, Changxing People’s Hospital, Changxing 313100, People’s Republic of China
Correspondence: Wei Sun; Rui-jie Chen Tel +8613968803162
; +8613806890233
Email [email protected]; [email protected]
Purpose: Precise and timely detection of methotrexate (MTX) concentration played a key role in high-dose MTX individualization therapy in acute lymphoblastic leukemia (ALL) children to avoid serious adverse effects or nonresponse. This report described a sensibility and validation of ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) method for therapeutic drug monitoring (TDM) of methotrexate concentration in children’s plasma.
Methods: One-step protein precipitation of samples was accomplished by adding 200 μL of acetonitrile to 100 μL of plasma sample. The separation of plasma samples was carried out on a ZORBAX Eclipse Plus C18 Rapid Resolution HD column with gradient elution using a mobile phase constituted of acetonitrile and 1% formic acid. The detection was executed by electrospray ionization (ESI) of triple quadrupole tandem mass spectrometer (TQMS) in the multiple reaction monitoring (MRM) mode with the transitions m/z 455.2 → 307.9 for methotrexate and m/z 458.2 → 311.2 for IS, separately. Linear concentration range of the calibration curve was 44– 11,000 nmol/L and 44 nmol/L was the lower limit of quantification.
Results: The methotrexate elution time was at 1.577 min, and the overall running time was only 3.3 min. The intra- and interday precision for all the analysis results was within 11.24%, and mean recoveries rate of methotrexate exceeded 87.98%.
Conclusion: The described and fully validated UHPLC-MS/MS method was successfully applied in clinical TDM after infusion of high-dose methotrexate 1– 5 g/m2 to 41 childpatients.
Keywords: high-dose methotrexate, UHPLC-MS/MS, therapeutic drug monitoring, children, acute lymphoblastic leukemia
Introduction
Methotrexate (MTX) was a vital medication in treatment regimens for acute lymphoblastic leukemia (ALL) in children.1–4 There were evidently individual differences in MTX plasma concentrations owing to large inter-individual variation in the absorption, distribution, metabolism, and excretion of MTX in the body. Therefore, individualized therapies were important for therapeutic dose adjustments. The high-dose MTX (HD-MTX) (1–5 g/m2) as a 24-h infusion, 1/6 of the high-dose MTX was infused in initial one hour and the rest of the dose at a constant rate in the remaining 23 h, based on HD-MTX treatment regimen.1,2,5 MTX was mainly eliminated via the kidneys in vivo, and renal clearance was influenced by many prerequisites, especially dosage, individual differences, and urinary pH.6,7 During HD-MTX administration, patients could achieve the cytotoxic benefits by avoiding overtreatment which may lead to acute renal function injury and tubular toxicity.8–10 Folinic acid (CF) was a drug used for decreasing the toxic effects of methotrexate.11–13 CF rescue treatment was initiated at 36 h after the beginning of HD-MTX infusion, and CF at an initial dose of 15 mg/m2 was repeated intravenously every six hours. However, the action of CF not only reduced the adverse reactions of HD-MTX, but also, to some extent, rescued cancer cells.14 Thus, concentration of MTX was monitored at 24, 48, and 72 h, after the start of the HD-MTX individualization therapy in children with ALL. In routine therapy, the appropriate dose adjustments of CF were according to protocol instructions.13 CF dosage was increased while concentration of MTX at 48 h was above 1000 nmol/L and was stopped if the concentration of MTX was below 100 nmol/L. Meanwhile, it was very valuable to develop a selective, sensitive, rapid and simple method to determine the MTX concentration in children’s plasma. There were several analytical methods for the determination of MTX plasma including fluorescence polarization immunoassay,15,16 HPLC,17 and LC-MS/MS.18
To some extent, these previous methods were not going to deal with emergency situations (an abnormally high concentration of MTX plasma and large sample size measurement) the reason why these existed had their own drawbacks, including time-consuming requirement for plasma sample preparation, long-term chromatographic run, and low sensitivity. Fortunately, the combination of UHPLC and MS/MS techniques were more suitable for the detection of compounds in complicated mixtures, such as MTX in plasma. There were various advantages, such as high sensitivity, fast analyzing rate, and low consumption of solvent. Thus, the highly sensitive, time-saving, specific UHPLC-MS/MS method in the present study that was validated and developed could determine concentrations of MTX and methotrexate-d3 (MTX-d3), which was considered as an internal standard (IS). Currently, the new method was fully validated in lower limit of quantification (LLOQ), linearity, accuracy, recovery, precision, selectivity, matrix effect, and stability as well. It was successfully applied in TDM in ALL children.
Experimental
Materials and Reagents
Methotrexate (purity >98%), methotrexate-d3 (purity >98%), and formic acid (purity >98%) were obtained from Sigma Chemical Co., USA. Methanol (HPLC grade) and acetonitrile (HPLC grade) were provided by Merck Co., Germany. Ultrapure water was purified by using a Milli-Q system (Millipore Co., USA). In this study, blank children’s plasma used was collected from the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (China).
UHPLC-MS/MS Conditions
Chromatographic separation was complied with ZORBAX Eclipse Plus C18 Rapid Resolution HD column (2.1×50 mm, 1.8 μm) and inline stainless steel frit filter (0.2 μm). The temperatures of C18 column and autosampler were kept at 30°C and 4°C respectively. Mobile phase which was consisted of solvent A (acetonitrile) and solvent B (0.1% formic acid) was used in the study. A gradient elution was divided into four steps: 0–0.4 min, 10% B; 0.4–1.8 min, 10–95% B; 1.8–2.3 min, maintained at 95% B; one minute for re-equilibration time. The whole running time was 3.3 min with 6 μL of pre-injection with a flow rate keeping at 0.4 mL/min. UHPLC-MS/MS with Agilent 1290 UHPLC system and 6420 series Triple-Quadrupole Tandem Mass Spectrometer (Agilent, CA, USA) coupled to ESI source operating was applied for MS detection and analyses in positive ion mode.
The main working parameters of MS and multiple reaction monitoring (MRM) fragmentation transitions were summarized in Table 1. The other conditional parameters were set as follows: desolvation gas (nitrogen) flow: 12 L/h, nebulizing gas and drying gas (both nitrogen): 45 psi and 350°C, source temperature: 150°C, capillary voltage: 4000 V. Data acquisition and analysis were executed by MassHunter Agilent Software (version B.07.00).
 |
Table 1 Summary of MS Parameters for Methotrexate and Methotrexate-d3 (MTX-d3) (IS) |
Calibration Standards and Quality Control (QC) Samples, and IS Solutions
A concentration of 1.00 mg/mL MTX stock solution was obtained by dissolving 10 mg MTX in 10 mL methanol, and was frozen at −80°C. Stock solution was thawed to ambient temperature and then was diluted with methanol to prepare several concentration levels of working solution. The corresponding working solutions were further diluted with blank children’s plasma to obtain QC and calibration standards samples. The three different levels of QC samples for MTX in the plasma were 110, 440, and 4400 nmol/L, respectively. Final concentration levels of the calibration samples for MTX in the plasma were 44, 110, 220, 440, 2200, 4400, and 11,000 nmol/L, separately. Dissolving 5 mg MTX-d3 into 5 mL methanol was applied to obtain an initial concentration of 1.00 mg/mL IS stock solution. The IS stock solution was further diluted with methanol to prepare IS working solution at concentration 5000 nmol/L.The aliquots of 100 μL of calibration samples, QC samples, and working solutions were frozen at below 0°C (−20°C).
Sample Preparation
The frozen plasma samples were placed at room temperature (23–25°C) for 0.5 h and they were vortexed for one minute. Aliquots of 100 μL of plasma samples and 5 μL IS solution were transferred to a 1.5 mL centrifuge tube and were vortex for one minute. The protein was precipitated by adding 200 μL of acetonitrile and vortexed for one minute, then centrifuged at 13,000 rpm for 10 min. At the end, 6 μL of supernatant was injected into the UHPLC-MS/MS system for further analysis.
Method Validation
According to the US FDA bioanalytical method validation guidelines, the method was validated in some specific aspects, including selectivity, linearity, precision, accuracy, stability, matrix effect, carry-over effect, and recovery.19
Selectivity
Selectivity was evaluated by comparing the chromatograms of blank children's plasma from six different volunteers with the corresponding MTX and IS spiked blank children's plasma samples. Selectivity investigated whether endogenous substances in plasma interfered with the analyte and the IS.
Linearity, LLOQ and Carry-over Effect
Standard curves was acquired by peak area ratios of MTX to IS plotting against analyte concentrations and fitted to the equations by weighted (1/x2) least-squares linear regression at seven concentration levels (44–11,000 ng/mL). The LLOQ was defined as the lower limit concentration of MTX in the calibration curve. Acceptable accuracy (±20%) and intraday and interday precision (<20%) were set here. The carry-over effect was estimated by subsequently injecting an upper limit of quantification (ULOQ) sample and an blank plasma sample into UHPLC-MS/MS system.
Precision and Accuracy
The accuracy and precision of the method were validated by detecting six replicate QC samples in children’s plasma at three levels on three consecutive days. Accuracy was presented as percentage relative error (RE, %), and precision was expressed as the percentage relative standard deviations (RSD, %). The relative error (RE, %) for QC samples was accepted to be with ±15%, and relative standard deviations (RSD, %) was required to be <15%.
Extraction Recovery and Matrix Effect
The recovery of the MTX was assessed by comparing the peak areas of the MTX acquired from QC samples with the postextraction spiked plasmas at the same concentration. The detection method of recovery rate of IS was the same as above. Matrix effect which might be caused by endogenous substances of plasma samples could lead to increase of the signal or ion suppression. Matrix effects were evaluated by the ratio of peak areas of MTX spiked into blank plasma extraction at three concentrations to parallel standard samples. The detection method of matrix effect of IS (250 nmol/L) was the same as above.
Stability
The stabilities of MTX in plasma were assessed by analyzing three QC levels (110, 440, and 4400 nmol/L, n=5). The short-term stability was performed by analyzing spiked samples exposed at temperature 23–25°C for two hours and supernatant samples switched to the autosampler of UHPLC-MS/MS system for 24 h after centrifugation. After samples had been experienced three freeze–thaw cycles (−20 to 25°C) on consecutive days, the freeze–thaw stability was assessed. The long-term stability was detected by analyzing spiked plasma samples which werefrozen at −20°C for 42 days.
Dilution Integrity
The dilution integrity was used to validate the extent which plasma sample higher than the ULOQ of the standard curve could be extended with acceptable accuracy and precision. Six replicates were processed in blank plasma at a concentration level of about 2 times of the uppermost calibration standard, and then were diluted (10-fold) with the same blank plasma before analysis.
Application to TDM of High-Dose Methotrexate in Children with Acute Lymphoblastic Leukemia
The advanced and validated method was applied in clinic MTX TDM. After written informed consent from legal guardian and the local Institutional Review Board (IRB) approval, 41 children (aged 2–15 years) received HD-MTX-CF chemotherapy regimen. The MTX plasma concentrations were medically examined at Yuying Children’s Hospital of Wenzhou Medical University in Zhejiang Province, China. The data of our study were collected during the period from January 1, 2017 to December 31,2018. The subjects were required blood collection after HD-MTX treatment. A dose of 1/6 MTX as a loading dose was infused in the initial first hour, and the remaining dose was intravenously infused at a uniform rate within 23 h . Then 0.3 mL of venous blood samples were collected into EDTA tubes at 24 h, 36 h, 72 h, 96 h, 120 h, 144 h, 168 h, after the end of MTX infusion. The EDTA tubes were centrifuged at 13,000 rpm at 4°C for 10 min and the collected plasma samples were separated into glass vials and frozen at −20°C.
Results and Discussion
Method Development and Optimization
Comparing to ESI negative ion mode, a more stable and intense signal for the MTX was observed in positive ion mode. MS/MS parameters (eg, ESI source temperature, flow rate of desolvation gas and cone gas) were optimized to display the optimal response in multiple reaction monitoring (MRM) mode. Then, the observed scan mass spectra showed that the MTX parent ion and the daughter ion kept at m/z 455.2 and m/z 307.9, respectively (Table 1, Figure 1). The concentration of MTX was evaluated under the MRM mode according to the MRM data acquisitions. Sample preparation played a critical step when drugs in biological samples needed the quantitative analysis. The effective and simple protein precipitation was applied in our present study. In the present work, adding 200 μL acetonitrile into plasma sample was applied for the sample preparation. The recovery of this one-step precipitation was sufficiently consistent and satisfactory.
 |
Figure 1 The mass spectrum and chemical structures of methotrexate and IS in the present study: (A) MTX; (B) MTX-d3 (IS). |
Several various mobile phases were used to evaluate the ionization response and chromatographic behavior of MTX. The best response was performed by specific mobile phases consisting of acetonitrile and water fortified with 0.1% formic acid. A mobile phase which consisted of 0.1% formic acid and 99.9% water could enhance the ionization and sensitivity of MTX. The gradient elution mode which was used in chromatographic separation obtained an ideal peak shape. Meanwhile, the available UHPLC-MS/MS system with ZORBAX Eclipse Plus C18 Rapid Resolution HD column was applied for providing satisfactory peak shapes with short retention time. A clean chromatogram for blank plasma samples was produced by this simple procedure. The whole running time of MTX and IS was only 3.3 min per sample. The retention time of MTX was eluted at about 1.577 min, and IS was eluted at 1.558 min.
Selectivity
Figure 2 showed MRM chromatogram of blank plasma; blank plasma spiked with MTX and IS, and children's plasma sample 24 h after intravenous infusion of high-dose methotrexate (HD-MTX 2 g/m2). The clean UHPLC–MS/MS chromatogram was produced by a blank plasma sample, and there was no obvious interference of endogenous peaks at the retention time of the MTX and IS. The ideal peak shape chromatogram was produced by a blank plasma sample spiked with MTX and IS.
LLOQ and Calibration Curve
The LLOQ was 44 nmol/L for MTX in children's plasma, with the precision and accuracy of 9.40 and −0.54%, separately. A regression equation for the calibration curve is: y=1.118478x+0.001234, r2=0.9965. Calibration curves was used to analysis linear regressions of the peak area ratios of MTX/IS vs linear concentration (44–11,000 nmol/L) for MTX in children’s plasma sample.
Carry-over Effect
After injection of the ULOQ sample, injection of blank sample showed no clear visible peak at the retention time of the MTX and IS and the carry-over effect was excluded. The results indicated that determining a sample with low concentration after injecting a high concentration sample was achievable.
Precision and Accuracy
Table 2 collected all the analysis results of intra- and interday precision and accuracy of three QCs and the analytes at the LLOQ concentrations. The method was repeatable and reliable since RSD% was <11.24%, and RE% was ranged from −2.50% to 0.69% for all investigated MTX concentrations in children’s plasma.
 |
Table 2 Precision, Accuracy, Recovery and Matrix Effect for Methotrexate or IS of Quality Control Sample in Children's Plasma (n=6) |
Matrix Effect and Recovery
As shown in Table 2, the matrix effect for MTX concentrations at three QCs was from 95.84% to 99.15% in children’s plasma. The matrix effect for IS (250 nmol/L) was 96.86±4.91%. These results indicated that there was no obvious difference in matrix effect in present study. Meanwhile, the matrix effects for children’s plasma sample were also acceptable and complying with the guidance of USFDA bioanalytical method validation guidelines. Mean recoveries for MTX in children’s plasma were ≥87.98% (n=6), and the recovery of the IS (250 nmol/L) was 85.15±3.57% (n=6).
Dilution Integrity
In dilution integrity study, the precisions (RSD, %) were 1.83%, and the accuracies (RE) ranged from −2.63% to 2.03% for the analytes. The results concluded that a plasma sample with a concentration higher than the ULOQ of the standard curve could be analyzed.
Stability
The results of stabilities of MTX under various conditions, such as room temperature (two hours), auto-sampler (24 h), short-term, freeze–thaw cycles (−20 to 25°C), and long-term (−20°C for 42 days) suggested that the stabilities of the MTX and IS were successfully validated under the processing and storage conditions. The bias in concentration when compared with nominal values was between 85% and 115%, and the related date was shown in Table 3.
 |
Table 3 Summary of stability of Methotrexate Under Various Storage Conditions (n=5) |
Method application
The validated method was suitable for TDM of high-dose methotrexate in 41 children’s plasma. The mean plasma concentration time curve was shown in Figure 3 and Table 4, respectively. As shown in Table 4, the plasma concentration of 41 children patients were detected by UHPLC/MS-MS and expressed as mean ±SD, with n=41 for 24 h, n=40 for 48 h, n=41 for 72 h, n=22 for 96 h, n=6 for 120 h, n=4 for 144 h, n=1 for 168 h.
 |
Table 4 Plasma Concentrations at Various Time Points After Intravenous Infusion of High-dose Methotrexate (HD-MTX 1–5 g/m2) |
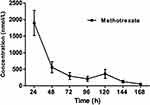 |
Figure 3 Plots of concentration versus time after intravenous infusion of high-dose methotrexate (HD-MTX 1–5 g/m2) in 41 children patients. |
Conclusions
The HD-MTX-CF treatment program was used for ALL in the clinical treatment. Precise and timely detection of MTX concentration played a critical role in HD-MTX individualization therapy in children with ALL. It was undeniable that HD-MTX treatment could cause serious III–IV adverse reactions, such as bloody diarrhea, oral mucositis, bone marrow suppression, etc. These adverse reactions might be related to delayed excretion of MTX and dose and rescue time of CF. Timely monitoring of MTX treatment was conducive to promoting the effectiveness and safety of treatment. Two definitive cases: case 7–2017, a five-year-old child, who received HD-MTX-CF treatment program (MTX 2.6 g IVGTT d1, CF 7.8 mg IV q6 h), was diagnosed with acute T lymphocytic leukemia (T-ALL). Her MTX plasma concentrations were medically examined for a total of five times, 4510 nmol/L, 618 nmol/L, 298 nmol/L, 191 nmol/L, and 57 nmol/L, respectively. During the therapy, the child’s oral mucosa suffered different degrees of damage. The various MTX plasma concentrations and the severity of adverse reactions, to some extent, determined the rescue dose of CF, from 39 mg q6 h, to 7.8 mg q6 h, respectively. Intravenous injection of CF was stopped when the concentration of MTX was 57 nmol/L. Case 8–2017, an 11-year-old child, who received HD-MTX-CF treatment program (MTX 3.9 g IVGTT d1, CF 12 mg IV q6 h), was diagnosed with acute B lymphocyte leukocytes (B-ALL). During the course of therapy, the MTX plasma concentrations were detected for a total of nine times, 7270 nmol/L, 4640 nmol/L, 2490 nmol/L, 818 nmol/L, 540 nmol/L, 249 nmol/L, 197 nmol/L, 120 nmol/L, and 84 nmol/L, respectively. The first monitoring result of MTX concentration showed 7270 nmol/L, which indicated hyper-methotrexatemia. Considering the patient might have MTX excretion disorders. Thus doctors contacted the intensive care unit, adopted continuous renal replacement treatment (CRRT), and stopped chemotherapy, in order to deduce toxicity. The ALL patient received CRRT five times. The CF dose changed from 95 mg q6 h for three days, to 60 mg q6 h for two days, to 35 mg q6 h for one day, to 12 mg q6 h for two days. CF was stopped until MTX concentration was below 100 nmol/L.
These two cases indicated that due to individual differences, the absorption, distribution, metabolism and excretion of MTX in different bodies were significantly different. Thus, clinic MTX TDM was an irreplaceable factor to optimize HD-MTX-CF rescue treatment strategy. Based on the MTX plasma concentration, clinicians could predict whether there was a delay in excretion and whether it was necessary to adjust the individualized CF rescue dose and timing of administration. We reported here, the highly sensitive, time-saving, specific UHPLC–MS/MS method was validated in the quantitative determination of MTX plasma concentrations. The whole running time of the present developed UPLC-MS/MS method was only 3.3 min. This advanced method was successfully applied for the analysis of considerable samples from ALL patients who received a therapy with HD-MTX-CF.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (Wenzhou 325027, China). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Acknowledgments
We thank Dr Jiuxiang Wang and Dr Yuan Li (the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University) for their professional answers and insightful suggestions. The study was supported by the National Natural Science Foundation of China (Grant Nos. 81600216 and 81900331), by Science and Technology Research Project of Wenzhou City (Grant Nos. Y20160534 and 2020Y0390) and by the Special Project for Significant New Drug Research and Development in the Major National Science and Technology Projects of China (2020ZX09201002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aumente D, Buelga DS, Lukas JC, et al. Garcia, population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet. 2006;45(12):1227–1238. doi:10.2165/00003088-200645120-00007
2. Cheng DH, Lu H, Liu TT, et al. Identification of risk factors in high-dose methotrexate-induced acute kidney injury in childhood acute lymphoblastic leukemia. Chemotherapy. 2018;63(2):101–107. doi:10.1159/000486823
3. Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030–1043. doi:10.1016/S0140-6736(08)60457-2
4. Wall AM, Gajjar A, Link A, et al. Individualized methotrexate dosing in children with relapsed acute lymphoblastic leukemia. Leukemia. 2000;14(2):221–225. doi:10.1038/sj.leu.2401673
5. Cheng DH, Lu H, Zou XQ. [Correlation study of blood drug concentration and nephrotoxicity on high dose methotrexate therapy in suggestion of diagnosis and treatment of childhood acute lymphoblastic leukemia in the 4th revised edition]. Zhonghua Er Ke Za Zhi. 2017;55(10):771–774. doi:10.3760/cma.j.issn.0578-1310.2017.10.012.Chinese.
6. Bauters T, Lammens T, Belin P, et al. Delayed elimination of methotrexate by cola beverages in a pediatric acute lymphoblastic leukemia population. Leuk Lymphoma. 2013;54(5):1094–1096. doi:10.3109/10428194.2012.737918
7. Tran HX, Herrington JD. Effect of ceftriaxone and cefepime on high-dose methotrexate clearance. J Oncol Pharm Pract. 2016;22(6):801–805. doi:10.1177/1078155215608524
8. Garcia H, Leblond V, Goldwasser F, et al. [Renal toxicity of high-dose methotrexate]. Nephrol Ther. 2018;14(Suppl. 1):S103–S113. doi:10.1016/j.nephro.2018.02.015.French.
9. Howard SC, McCormick J, Pui CH, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471–1482. doi:10.1634/theoncologist.2015-0164
10. Sharbaf FG, Farhangi H, Assadi F. Prevention of chemotherapy-induced nephrotoxicity in children with cancer. Int J Prev Med. 2017;8:76. doi:10.4103/ijpvm.IJPVM_40_17
11. Cerminara Z, Duffy A, Nishioka J, et al. A single center retrospective analysis of a protocol for high-dose methotrexate and leucovorin rescue administration. J Oncol Pharm Pract. 2019;25(1):76–84. doi:10.1177/1078155217729744
12. Cohen IJ, Wolff JE. How long can folinic acid rescue be delayed after high-dose methotrexate without toxicity? Pediatr Blood Cancer. 2014;61(1):7–10. doi:10.1002/pbc.24770
13. De Moerloose B, Suciu S, Bertrand Y, et al. Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): report of the EORTC randomized Phase 3 trial 58951. Blood. 2010;116(1):36–44. doi:10.1182/blood-2009-10-247965
14. Jolivet J, Jansen G, Peters GJ, et al. Leucovorin rescue of human cancer and bone marrow cells following edatrexate or methotrexate. Biochem Pharmacol. 1994;47(4):659–665. doi:10.1016/0006-2952(94)90128-7
15. Aumente MD, López-Santamaría J, Donoso-Rengifo MC, et al. Evaluation of the novel methotrexate architect chemiluminescent immunoassay: clinical impact on pharmacokinetic monitoring. Ther Drug Monit. 2017;39(5):492–498. doi:10.1097/FTD.0000000000000434
16. Mei S, Zhu L, Li X, et al. UPLC-MS/MS analysis of methotrexate in human plasma and comparison with the fluorescence polarization immunoassay. Anal Sci. 2017;33(6):665–670. doi:10.2116/analsci.33.665
17. Brandsteterová E, Seresová O, Miertus S, et al. HPLC determination of methotrexate and its metabolite in serum. Neoplasma. 1990;37(4):395–403.
18. Al-Ghobashy MA, Hassan SA, Abdelaziz DH, et al. Development and validation of LC-MS/MS assay for the simultaneous determination of methotrexate, 6-mercaptopurine and its active metabolite 6-thioguanine in plasma of children with acute lymphoblastic leukemia: correlation with genetic polymorphism. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1038:88–94. doi:10.1016/j.jchromb.2016.10.035
19. FDA. Guidance for industry, bioanalytical method validation. U.S. Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research. Centre for Veterinary Medicine; May 2018.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

