Back to Journals » Cancer Management and Research » Volume 11
Development and validation of a prognostic score predicting recurrence in resected combined hepatocellular cholangiocarcinoma
Authors Tian MX , Luo LP, Liu WR, Deng W, Yin JC, Jin L, Jiang XF, Zhou YF, Qu WF, Tang Z, Wang H, Tao CY, Fang Y, Qiu SJ, Zhou J, Liu JF , Fan J, Shi YH
Received 26 November 2018
Accepted for publication 19 March 2019
Published 5 June 2019 Volume 2019:11 Pages 5187—5195
DOI https://doi.org/10.2147/CMAR.S195964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Meng-Xin Tian,1,2,* Liu-Ping Luo,3,4,* Wei-Ren Liu,1,2,* Wei Deng,5 Jia-Cheng Yin,1,2 Lei Jin,1,2 Xi-Fei Jiang,1,2 Yu-Fu Zhou,1,2 Wei-Feng Qu,1,2 Zheng Tang,1,2 Han Wang,1,2 Chen-Yang Tao,1,2 Yuan Fang,1,2 Shuang-Jian Qiu,1,2 Jian Zhou,1–2,6 Jing-Feng Liu,3,4 Jia Fan,1–2,6 Ying-Hong Shi1,2
1Department of Liver Surgery, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, Shanghai, People’s Republic of China; 3Department of Hepatobiliary Surgery, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 4The United Innovation of Mengchao Hepatobiliary Technology Key Laboratory of Fujian Province, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 5Department of Health Statistics and Social Medicine, Key Laboratory of Public Health Safety, Ministry of Education, School of Public Health, Fudan University, Shanghai, People‘s Republic of China; 6Institutes of Biomedical Sciences, Fudan University, Shanghai, People‘s Republic of China
*These authors contributed equally to this work
Purpose: To develop and validate a decision aid to help make individualized estimates of tumor recurrence for patients with resected combined hepatocellular cholangiocarcinoma (CHC).
Patients and methods: Risk factors of recurrence were identified in the derivation cohort of 208 patients who underwent liver resection between 1995 and 2014 at Zhongshan Hospital to develop a prediction score. The model was subsequently validated in an external cohort of 101 CHC patients using the C concordance statistic and net reclassification index (NRI).
Results: On multivariate analysis, five independent predictors associated with tumor recurrence were identified, including sex, γ-glutamyl transferase, macrovascular invasion, hilar lymphoid metastasis and adjuvant transcatheter arterial chemoembolization. The prediction score was constructed using these 5 variables, with scores ranging from 0 to 5. A patient with a score of 0 had a predicted 1- and 5-year recurrence risk of 11.1% and 22.2%, respectively. In the validation cohort, the NRIs of prediction score vs American Joint Committee on Cancer 7th, TNM staging system at 1-year and 5-year were 0.185 (95% CI, 0.090–0.279, P<0.001) and 0.425 (95% CI, 0.044–0.806, P=0.03), respectively.
Conclusion: Our developed and validated prediction score might be a simple and reliable method in postoperative CHC patients and help clinicians identify candidates who may benefit from future adjuvant therapies.
Keywords: combined hepatocellular cholangiocarcinoma, recurrence prediction, prognosis, liver resection
Introduction
Combined hepatocellular cholangiocarcinoma (CHC) is a rare liver malignancy, accounting for 0.4~14.2% of all primary liver cancers.1 It is comprised of dual histologic features: hepatocellular and biliary epithelial differentiation. Since the first report of Allen and Lisa in 1949,2 there have been an increasing number of clinical studies describing both the demographic and clinical features of CHC.3–5 Due to the limitations of small study populations in previous reports, the demographic, clinical characteristics, and prognostic factors are far from clearly understood.6
Over the past decades, many staging systems have been developed to guide the prognosis and treatment of patients with hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (ICC), including the Barcelona Clinic Liver Cancer,7 Hong Kong Liver Cancer,8 American Joint Committee on Cancer (AJCC) 7th edition,9 Nathan10 and Liver Cancer Study Group of Japan staging systems,11 but none for CHC patients. Previously, we established a novel risk prediction model that could be applied to facilitate the diagnosis of CHC patients in contrast to HCC or ICC patients.12 Further, we observed that surgical resection could provide the same outcome between elderly and younger patients.13 Though CHCs share similar clinical and pathological characteristics (mean age, positive viral hepatitis and solitary tumors) with HCC patients, the features of CHC are genetically closer to that of ICC.5,14 Considering the vastly different mechanisms of carcinogenesis and biological behavior, the current cancer classification for HCC or ICC may not be suitable for CHC patients. Thus, developing an accurate recurrence prediction model would make a contribution to treatment for CHC patients postoperatively.
In the present study, our aim was to establish a prognostic estimation of CHCs after resection (PECAR) score predicting recurrence on the basis of the clinicopathological data from Zhongshan Hospital and validate with an external cohort from Mengchao Hepatobiliary Hospital in China.
Materials and methods
Patients
The retrospective analysis obtained ethical approval and complied with the standards of the Declaration of Helsinki and current ethical guidelines. Informed consent was obtained from each patient before surgery for using their data for research. The CHC was histologically defined according to the WHO criteria,15 and two independent pathologists reassessed all these samples. The standard technique was adopted for hepatic resection.16 The probability of hilar lymph node metastasis was evaluated with preoperative contrast-enhanced computed tomography (CT) and (or) magnetic resonance imaging (MRI). The dissected lymphoid metastasis was routinely confirmed by pathology. Patients with no previous antitumor therapy, confirmed with CHC pathologically, no other malignancies and Child–Pugh class A or B were included in this study. The exclusion criteria were as follows: ICC or HCC proved by histopathology, tumors of uncertain origin, metastatic liver tumors, perioperative mortality and distant or intrahepatic metastasis. In the derivation cohort, 208 CHC patients who underwent liver resection, between April 1999 and December 2014, at Zhongshan Hospital of Fudan University were enrolled. Further, we included an external validation cohort with 101 patients, from September 2003 to January 2016, at Mengchao Hepatobiliary Hospital of Fujian Medical University. The study complied with the standards of the Declaration of Helsinki. The institutional review board of Zhongshan Hospital approved this study. All patients gave written informed consent.
Laboratory test and data collection
The serum chemistries, blood cell count and tumor biomarkers (serum alpha-fetoprotein [AFP], carcinoembryonic antigen [CEA], carbohydrate antigen 19-9 [CA19-9]) were measured in routine examination according to standard laboratory procedures.17 Hepatitis B surface antigen (HBsAg) and antibodies to hepatitis C virus (HCV) were detected using standard test systems. To evaluate the potential risk predictors, all the data associated with demographic and pathological information were collected at the time of initial diagnosis.
Follow-up and detection of recurrence
All patients underwent follow-up every 3 months in the first year and every 6 months thereafter until death or dropout. Abdominal ultrasound, liver function tests, serum AFP, CEA and CA19-9 levels were analyzed every 3 months, and abdominal MRI or CT scanning was performed every 6 months. According to standard guidelines for HCC,18 ICC19 or radiologic features of CHC described previously,20 recurrence was confirmed by contrast-enhanced imaging studies and tumor biomarkers.
Recurrent patients were managed with different therapeutic modalities, including radiofrequency ablation, repeated resection, transcatheter arterial chemoembolization (TACE) and supportive therapy. Overall survival (OS) was defined as the time period between the date of surgery and death and disease-free survival (DFS) was defined as the interval between the date of surgery and recurrence.
Statistical analysis
Continuous variables were reported as medians with IQR and categorical variables were as percentages. Pearson’s χ2 test and Fisher’s exact test were employed to compare categorical variables, whereas Wilcoxon test was used to evaluate continuous variables. Survival curves were computed with Kaplan–Meier methods and compared by using log-rank tests. Cox proportional hazards regression models were performed to determine univariate and multivariable HRs for predicting factors of CHC recurrence or survival. Predictors (P<0.10 in univariate analysis) were selected in the multivariate analysis. The final multivariate model was performed using a backward stepwise procedure for variable selection with a liberal P<0.05 as the retention criteria. According to the final multivariable model coefficients,21 the novel prognostic score was developed, assigning ordinal scores (0 or 1) to each of the selected factors. This simplified point scale could reflect the relative impact of risk covariables in the new model. And then, the relative value of each model component was summed to calculate the PECAR score. The PECAR score was tested and compared with AJCC 7th TNM system in the validation cohort. We used the C-index22 to assess model discrimination and absolute net reclassification index (NRI)23,24 to evaluate the improvement of model performance for CHC recurrence. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R software version 3.30 (R Foundation for Statistical Computing, Vienna, Austria). A P-value <0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 presents the baseline characteristics of the derivation cohort (n=208) and the validation cohort (n=101). There were no differences in the baseline characteristics of the derivation and validation cohort. Patients were younger, male predominant and more likely to have a single tumor in both groups. Hepatitis B virus (HBV)-positive patients made up 64.9% of the derivation cohort and 69.3% of the validation cohort, respectively. Compared with the validation set, the derivation set tended to have a high proportion of adjuvant TACE after liver resection.
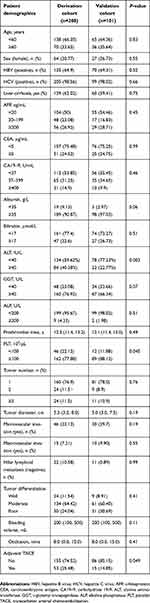 | Table 1 Demographic, clinical and tumor characteristics of patients with combined hepatocellular cholangiocarcinoma |
Outcomes of CHC patients
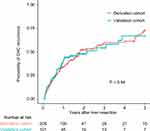
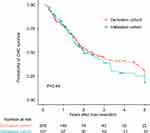
Among the entire cohort, the median OS was 18.1 months (range, 1.6–192.5 months). The 1-, 3- and 5-year OS probability was 76.2%, 41.2% and 28.3%, respectively (Figure S1). The median RFS was 11.0 months (range, 1.0 to 192.5). The 1-, 3- and 5-year RFS probability was 48.0%, 37.8% and 28.0%, respectively. Till to the date of last follow-up, 171 (55.3%) patients died and 149 (48.2%) patients encountered tumor relapse. There was no significant difference in the CHC recurrence between two groups (Figure 1).
Predictor selection and construction of the PECAR score
By using univariate analysis, male sex, elevated γ-glutamyl transferase (GGT), bleeding volume, tumor diameter and presence of macrovascular invasion, confirmed with hilar lymphoid metastasis and adjuvant TACE, at the first month after surgery were associated with subsequent CHC recurrence in the derivation cohort (Table 2). On multivariate analysis, the predictors that independently correlated with CHC recurrence included (1) sex, (2) GGT (at the following cutoffs: 0–39.9 and ≥40), (3) macrovascular invasion, (4) hilar lymphoid metastasis and (5) adjuvant TACE. The multivariate model coefficients for these 5 significant variables were transformed into relative points, and then summed to calculate the PECAR score as listed in Table 3. By adding total points for these 5 variables, the individualized PECAR score is calculated (Table 3).
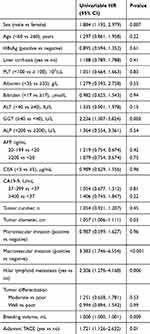 | Table 2 Univariate analysis of CHC recurrence in the derivation cohort (n=208) |
 | Table 3 Multivariate analysis of risk factors of CHC recurrence and creation of the PECAR score (n=208) |
Predictive performance and discrimination
The PECAR score ranged from 0 to 4, with the most common score being 1 and 2 (Table 4). A female patient with CHC after liver resection, no macrovascular invasion, no hilar lymphoid metastasis and the GGT level lower than 40 U/L would have a PECAR score of 0, predicting 1- and 5-year recurrence risk of only 11.1% and 22.2%, respectively. Predicted risk of 1- and 5-year CHC recurrence rose with each point score (Figure 2), such as a patient with a PECAR score of 4 or more, had a predicted 1- and 5-year recurrence risk of 72.7% and 81.8%, respectively. The C-index of PECAR score was 0.651 (95% CI, 0.593–0.710) in the derivation cohort for predicting CHC recurrence compared with AJCC 7th TNM staging system 0.520 (95% CI, 0.465–0.575). By using the NRI, we found that the PECAR score improved prediction of CHC recurrence after liver resection compared with AJCC 7th TNM staging system at 1 year (0.194, P=0.002) and 5 years (0.407, P<001) after liver resection.
 | Table 4 The percentage of PECAR score in the derivation and the validation cohort |
Validation of the PECAR score
To validate whether the PECAR score would be applicable to other datasets, we conducted an external validation study with 101 CHC patients in the validation group. In the validation cohort, median OS and RFS were 17.0 (1.8, 152.0) and 10.8 (1.0, 152.0) months, respectively. The C-index of the PECAR score for predicting postoperative recurrence was 0.610 (95% CI, 0.524–0.697), while the C-index of AJCC 7th TNM staging system was 0.598 (95% CI, 0.519–0.678). Similarly, the PECAR score improved the performance of recurrence prediction on CHC compared with AJCC 7th TNM staging system in the validation cohort (1 year: 0.185, P<0.001; 5 years: 0.425, P=0.03).
Discussion
CHC is an uncommon liver tumor with distinctive biological behavior and clinicopathological features. Due to its rarity, the clinical information is limited, especially for the patients’ survival and recurrence. In the present study, we developed and externally validated a novel prognostic score for postoperative CHC patients. This final model was based on 5 independent predictors with C-index of 0.651 (95%CI, 0.593–0.710) and presented better performance in recurrence prediction than AJCC 7th TNM staging system.
Growing evidences document CHC as an aggressive cancer with dismal prognosis, and the tumor recurred frequently at the liver.16,25,26 Previously, we demonstrated that CHC had a median prognosis between HCC and ICC.4 Further, we observed that the CHC patients had a short OS (18.1 months) and DFS (11.0 months) similar to previous studies.5,27 CHC was thought to be derived from hepatic progenitor cells with the biopotential to differentiate into both hepatocytes and cholangiocytes.6,28 Aoki et al20 reported that the prognosis of CHC might be like that of mass-forming ICC, though the clinical characteristics of CHC are similar to those of HCC. However, several studies indicated that there was no relationship between poor outcome and the predominance of ICC cells (or HCC cells).29,30 Further studies are needed to investigate the intrinsic mechanism of CHC.
Definitive evaluation of recurrent predictors in resected CHC patients has not been well established. Previous studies found that tumor number, vascular invasion, radical resection, lymphoid metastasis and tumor stage were prognostic factors for CHC patients.1,3,4,25 Kim et al also reported that CA19-9 was a risk predictor for CHC patients.1 In our present study, five risk factors (sex, GGT, macrovascular invasion, lymphoid metastasis and adjuvant TACE) were identified as independent factors associated with recurrence. Interestingly, sex was selected as an independent risk factor related to CHC recurrence in our model, unlike tumor markers (serum AFP, CA19-9 or CEA). Previous studies indicated that CHC was more prevalent in male population, particularly in the endemic area of chronic hepatitis.25,31,32 The causative association between sex and recurrence of CHC patients is required to be confirmed in future studies. Consistent with previous studies,5,33 GGT, a risk factor of liver cancer, was found to be associated with RFS in our study. Macroscopic vascular invasion, the essential causes of intrahepatic recurrence and long-term survival, was also identified as an independent predictor of CHC recurrence in accordance with previous studies in HCC34,35 and ICC.36 In liver cancer, Wang et al37 revealed that the prognosis of patients with lymph node metastasis or direct invasion and local metastasis was significantly poor. Also, lymphoid metastasis was found to be an independent predictor of recurrence with a HR of 2.443 (95% CI, 1.341–4.450) in the present study. Extrahepatic recurrence was reported mainly in lymph nodes of CHC patients,5 suggesting that regional nodal groups need to be resected during operation.
Different from HCC, the pathological pattern of CHC is less vascular and much more fibrotic tissues, resulting in poor uptake of chemotherapeutic agents. Retrospective studies have shown that TACE could improve survival outcomes in recurrent or unresectable CHC patients,38,39 especially for hypervascular lesions. However, in patients with peripherally enhancing lesions, the prognosis was worse than HCC or globally enhancing CHC patients.39 In our study, we found that adjuvant TACE did not have a preventive effect, but may increase the risk of recurrence for resected CHC patients. The explanations may be as follows: first, patients benefiting from adjuvant TACE were mainly combined with an intermediate or high risk of recurrence;17 second, CHC is an uncommon tumor with significant heterogeneity and aggressive biological behavior, patients with residual tumor may not respond to postoperative TACE; third, in our study, nearly 60% of patients combined with chronic HBV infection, the injury derived from TACE may promote local recurrence of CHC. Further investigation will be needed to verify the clinical efficacy of adjuvant TACE in CHC patients after hepatectomy.
How does our constructed model potentially influence clinical practice for CHC patients? First, it could help clinicians predict the likelihood of recurrence in postoperative CHC patients. In the present study, the probability of recurrence in patients with score 0, 1, 2, 3 and 4 at 1 year was 11.1%, 20.0%, 29.9%, 45.9% and 72.7%, respectively. Second, the PECAR score will help us to determine whether CHC surveillance after resection is warranted. Previous studies indicated that the prognosis of CHC may be worse than HCC or ICC patients, implying that the surveillance should be modified and fitted for CHC patients in clinical practice. Currently, the clinical efficacy of therapeutic strategies on CHC after surgery remains unclear, and our PECAR score may facilitate the improvement of postoperative management of patients with intermediate or high risk of recurrence in the future.
This study is not devoid of limitations. First, the study is based on a retrospective cohort in China, with over 60% of patients having HBV infection; prospective studies in different populations are required to further validate our model. Second, our predictive model is used for postoperative decision-making. Third, genomic analyses were not performed for resected tumor specimens. Genomic classification has been shown to provide unique prognostic information, except for clinical parameters, and may help to identify patients at higher risk for subsequent metastatic tumor formation.
Conclusions
In conclusion, we constructed and validated a novel PECAR score predicting the recurrence of HBV-related CHC patients in stage I/II. The PECAR score may help predict the per-patient recurrence risk and facilitate clinicians manage patients with stage I/II CHC patients, and further studies are also needed to improve clinical strategy.
Abbreviations list
CHC, combined hepatocellular cholangiocarcinoma; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; OS, overall survival; DFS, disease-free survival; BCLC, Barcelona Clinic Liver Cancer; HKLC, Hong Kong Liver Cancer; AJCC, American Joint Committee on Cancer; LCSGJ, Liver Cancer Study Group of Japan; HBV, hepatitis B virus; MRI, magnetic resonance imaging; CT, computed tomography; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; PEI, percutaneous ethanol injection; C-index, concordance index; VI, vascular invasion.
Acknowledgments
We all express our sincere thanks to Zun-Song Hu for statistical analysis. We would like to thank the participants and staff of the Zhongshan Hospital for their valuable contributions; the staff included Zhen-Bin Ding, Yuan-Fei Peng, Zhi Dai, and Pei-Yun Zhou. This work was supported by grants from the National Natural Science Foundation of China (81472674, 81773067, 81800790 and 81502486), the National Key Sci-Tech Project (2012ZX10002011-002), the Shanghai Committee of Science and Technology, China (No. 16JC1404000), the Shu Guang Project of Shanghai Municipal Education Commission and Shanghai Education Development Foundation (13SG04), and the China Postdoctoral Science Foundation Funded Project (2018M640343).
Disclosure
The authors declared no conflicts of interest in this work.
References
1. Kim KH, Lee SG, Park EH, et al. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann Surg Oncol. 2009;16(3):623–629. doi:10.1245/s10434-008-0278-3
2. Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25(4):647–655.
3. Jarnagin WR, Weber S, Tickoo SK, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94(7):2040–2046.
4. Yin X, Zhang BH, Qiu SJ, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012;19(9):2869–2876. doi:10.1245/s10434-012-2328-0
5. Lee SD, Park SJ, Han SS, et al. Clinicopathological features and prognosis of combined hepatocellular carcinoma and cholangiocarcinoma after surgery. Hepatobiliary Pancreatic Dis Int. 2014;13(6):594–601. doi:10.1016/S1499-3872(14)60275-7
6. Brunt E, Aishima S, Clavien PA, et al. cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology (Baltimore, Md). 2018;68:113–126. doi:10.1002/hep.29789
7. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi:10.1055/s-2007-1007122
8. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–1700.e1693. doi:10.1053/j.gastro.2014.02.032
9. Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC-IHCC-2009 study group. Cancer. 2011;117(10):2170–2177. doi:10.1002/cncr.25712
10. Nathan H, Aloia TA, Vauthey JN, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16(1):14–22. doi:10.1245/s10434-008-0180-z
11. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10(4):288–291. doi:10.1007/s00534-002-0732-8
12. Tian MX, He WJ, Liu WR, et al. A novel risk prediction model for patients with combined hepatocellular-cholangiocarcinoma. J Cancer. 2018;9(6):1025–1032. doi:10.7150/jca.23229
13. Tao CY, Liu WR, Jin L, et al. Surgical treatment of combined hepatocellular-cholangiocarcinoma is as effective in elderly patients as it is in younger patients: a propensity score matching analysis. J Cancer. 2018;9(6):1106–1112. doi:10.7150/jca.23921
14. Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, et al. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol. 2004;41(2):292–298. doi:10.1016/j.jhep.2004.04.030
15. Hurlimann J, Gardiol D. Immunohistochemistry in the differential diagnosis of liver carcinomas. Am J Surg Pathol. 1991;15(3):280–288.
16. Liu CL, Fan ST, Lo CM, et al. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg (Chicago, Ill: 1960). 2003;138(1):86–90.
17. Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24(9):2074–2081. doi:10.1158/1078-0432.CCR-17-2899
18.
19. Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289. doi:10.1016/j.jhep.2014.01.021
20. Aoki K, Takayasu K, Kawano T, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology (Baltimore, Md). 1993;18(5):1090–1095.
21. Sullivan LM, Massaro JM, D‘Agostino RB
22. Brentnall AR, Cuzick J. Use of the concordance index for predictors of censored survival data. Stat Methods Med Res. 2018;27(8):2359–2373. doi:10.1177/0962280216680245
23. Pencina MJ, Steyerberg EW, D‘Agostino RB
24. Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25(1):114–121. doi:10.1097/EDE.0000000000000018
25. Koh KC, Lee H, Choi MS, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189(1):120–125. doi:10.1016/j.amjsurg.2004.03.018
26. Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25(9):1485–1492. doi:10.1111/j.1440-1746.2010.06430.x
27. Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transplant. 2011;17(8):934–942. doi:10.1002/lt.22307
28. Cai X, Zhai J, Kaplan DE, et al. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology (Baltimore, Md). 2012;56(5):1804–1816. doi:10.1002/hep.25874
29. Ariizumi S, Kotera Y, Katagiri S, Nakano M, Yamamoto M. Combined hepatocellular-cholangiocarcinoma had poor outcomes after hepatectomy regardless of Allen and Lisa class or the predominance of intrahepatic cholangiocarcinoma cells within the tumor. Ann Surg Oncol. 2012;19(5):1628–1636. doi:10.1245/s10434-011-2150-0
30. Shin CI, Lee JM, Kim SH, et al. Recurrence patterns of combined hepatocellular-cholangiocarcinoma on enhanced computed tomography. J Comput Assist Tomogr. 2007;31(1):109–115. doi:10.1097/01.rct.0000235072.34808.9b
31. Akiba J, Nakashima O, Hattori S, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37(4):496–505. doi:10.1097/PAS.0b013e31827332b0
32. Shibahara J, Hayashi A, Misumi K, et al. Clinicopathologic characteristics of hepatocellular carcinoma with reactive ductule-like components, a subset of liver cancer currently classified as combined hepatocellular-cholangiocarcinoma with stem-cell features, typical subtype. Am J Surg Pathol. 2016;40:608–616. doi:10.1097/PAS.0000000000000579
33. Mok Y, Son DK, Yun YD, Jee SH, Samet JM. gamma-glutamyltransferase and cancer risk: the Korean cancer prevention study. Int J Cancer J Int Du Cancer. 2016;138(2):311–319. doi:10.1002/ijc.29659
34. Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261(5):939–946. doi:10.1097/SLA.0000000000000747
35. Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi:10.1097/SLA.0000000000000236
36. Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014;149(5):432–438. doi:10.1001/jamasurg.2013.5168
37. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. doi:10.1200/JCO.2012.41.5984
38. Kim JH, Yoon HK, Ko GY, et al. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 2010;255(1):270–277. doi:10.1148/radiol.09091076
39. Na SK, Choi GH, Lee HC, et al. The effectiveness of transarterial chemoembolization in recurrent hepatocellular-cholangiocarcinoma after resection. PLoS One. 2018;13(6):e0198138. doi:10.1371/journal.pone.0198138
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.