Back to Journals » Nanotechnology, Science and Applications » Volume 13
Development and Study of Biocompatible Polyurethane-Based Polymer-Metallic Nanocomposites
Authors Csarnovics I , Burunkova J, Sviazhina D, Oskolkov E , Alkhalil G, Orishak E, Nilova L , Szabó I , Rutka P, Bene K , Bácsi A, Kökényesi S
Received 7 January 2020
Accepted for publication 19 February 2020
Published 31 March 2020 Volume 2020:13 Pages 11—22
DOI https://doi.org/10.2147/NSA.S245071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Israel (Rudi) Rubinstein
István Csarnovics,1 Julia Burunkova,2 Danara Sviazhina,2 Evgeniy Oskolkov,2 George Alkhalil,2 Elena Orishak,3 Ludmila Nilova,3 István Szabó,1 Péter Rutka,1 Krisztián Bene,4 Attila Bácsi,4 Sándor Kökényesi1
1Institute of Physics, Faculty of Science and Technology, University of Debrecen, Debrecen, Hungary; 2International Scientific and Research Institute of Bioengineering, School of Photonics, ITMO University, St., Petersburg, Russian Federation; 3Department of Medical Microbiology, Faculty of Preventive Medicine, North-Western State Medical University Named After I.I. Mechnikov, St., Petersburg, Russian Federation; 4Department of Immunology, Faculty of Health, University of Debrecen, Debrecen, Hungary
Correspondence: István Csarnovics
University of Debrecen, Faculty of Science and Technology, Institute of Physics, Bem sq 18/a, Debrecen 4026, Hungary
Email [email protected]
Introduction: In this work we selected components, developed technology and studied a number of parameters of polymer nanocomposite materials, remembering that the material would have high optical and good mechanical characteristics, good sorption ability in order to ensure high value of the optical signal for a short time while maintaining the initial geometric shape. In addition, if this nanocomposite is used for medicine and biology (biocompatible or biocidal materials or the creation of a sensor based on it), the material must be non-toxic and/or biocompatible. We study the creation of polymer nanocomposites which may be applied as biocompatible materials with new functional parameters.
Material and Methods: A number of polymer nanocomposites based on various urethane-acrylate monomers and nanoparticles of gold, silicon oxides, zinc and/or titanium oxides are obtained, their mechanical (microhardness) properties and wettability (contact angle) are studied. The set of required, biology-related properties of these materials, such as toxicity and sorption of microorganisms are also investigated in order to prove their possible applicability.
Results and Discussion: The composition of the samples influences their microhardness and the value of contact angle, which means that varying with the monomer and the metallic, oxide nanoparticles composition, we could change these parameters. Besides it, the set of required, biology-related properties of these materials, such as toxicity and sorption of microorganisms were also investigated in order to prove their possible applicability. It was shown that the materials are non-toxic, the adhesion of microorganisms on their surface also could be varied by changing their composition.
Conclusion: The presented polymer nanocomposites with different compositions of monomer and the presence of nanoparticles in them are prospective material for a possible bio-application as it is biocompatible, not toxic. The sorption of microorganism could be varied depending on the type of bacterias, the monomer composition, and nanoparticles.
Keywords: polymer nanocomposites, metallic nanoparticles, oxide nanoparticles, biocompatible materials, microhardness
Background
The development of biology, medicine and related branches of science in recent years requires an increasing number of modern materials and technologies. In medical practice, various devices that are implanted in the body are actively used, such as pacemakers, bone prostheses, artificial blood vessels, etc. Much attention is paid to the use of biodegradable polymers that can safely remain in the body for some time, for example, to restore tissues and organs after damage.1,2 Another very attractive field of study polymers is biosensing. Among modern research methods, a separate group is made up of sensor devices (micro- and nanosystems) for analyzing liquid media or gases, which provide quick detection of substances with minimal sample preparation, automation of control processes, and quite accurate and reproducible measurement under various conditions. The development of optical sensors is especially active due to the frequent advantages of optical detection methods in biology, medicine, and environmental protection. To date, various types of sensors have been proposed both for determining different pathogenic microorganisms and viruses,3,4 and for controlling heavy metal ions in juicy waters,5 however, the need for universal and inexpensive devices remains high. In this regard, one of the key tasks of modern materials science is the creation and study of biocompatible materials with a variety of properties, applicable in modern prostheses and implants,6 artificial bio-tissues,7 and in optical biocompatible sensors.8 One of the most promising types of such materials is a polymer nanocomposite (for example poly(lactic-coglycolic) acid, gums, polyaniline, poly (aniline-co-pyrrole)@Fe3O4@alginic acid, polyxanthone triazole, etc).9–17 based on polymers and nanoparticles, which have properties that are individually not achievable for each of the components.18,19 An important feature of such materials is the combination of positive properties of the polymer matrix, such as lightness, flexibility, and easiness of the production, as well as the ability to radically modify the properties of the material by changing the monomer composition and/or by introducing functional fillers, for example, various nanoparticles.
This is especially true for gold nanoparticles in various materials of sensor devices, due to the ability to vary the spectral position and amplitude of their plasmon resonance by changing the size, shape, structure of the particles and of the surroundings. High electron density, the ability to scatter and emit secondary electrons, the characteristic absorption, and scattering in the visible region of the spectrum of electromagnetic radiation, intense red color makes it easy to detect gold particles. To record changes in the physicochemical parameters of biochips and biosensors that occur during a specific reaction, visual observations are used, as well as methods of light scattering, vibrational spectroscopy, and others. Biosensors based on gold nanoparticles are already used in immunoassays.20 The use of such nanocomposites opens possibilities for controlling the rate of development of bacteria, suppressing their vital functions, controlling their sorption on the surface, and also creating antimicrobial surfaces.
Polyurethane (PU) is one of the main groups of polymeric materials used in the manufacturing of various implants, as well as many other products. Most of them have new medical applications as biphasic block copolymers or, as they are highly hemocompatible, they became one of the preferred types of polymers for products, contact with blood. Another possible area is to use this polymer material in reconstructive surgery (allows reconstructing defects of various tissues and organs and improving the quality of life for patients without the use of transplants and biosynthetic organs), reconstruction of bone defects (great interest of using polyurethanes, because of their good compatibility with growing cells and very durability, to obtain biodegradable materials) and the reconstructive orthopedics (filling with this materials the large cavities in bone tissue).21,22 All of the above-listed polymer-based materials are well-researched as biocompatible ones and they are relatively expensive. However, the acrylate-based nanocomposites are practically not used in such quality and are little studied before. It is known similar mixtures of monomers with silicon dioxide, barium, titanium, and zinc oxides, which are used by dentists; however, these materials are not nanocomposites. As a rule, these are opaque mixtures in which the inorganic components are micrometer size. Nanoparticles can improve the homogeneity of the mixture, prevent phase separation — inorganic components are released in the organic environment of the polymers by separate islands. Nanocomposites medium homogeneity at the nanoscale is important to improve the bioactive properties of the medium. Besides of it, it is necessary to obtain optical material, which we propose to use, for example, for the preparation of fluorescent sensors. In comparison to other polymer biocompatible materials, to which metallic nanoparticles could be added as well, on the surface of polyurethane-based nanocomposites surface patterns could be created in a step optical writing process, so it enhances the possibility of using this material for biological, sensing applications, like creation of waveguide-based sensors with in-situ, created coupling gratings, etc.
The antimicrobial properties of silver,19,23 gold19,24 and some other materials19,25 and nanoparticles of these metals are well known. Silicon oxide nanoparticles are thermally stable and highly bioactive. There are works on the analysis of their size- and dose-dependent cytotoxicity, an increase in the active forms of oxygen and anti-inflammatory stimulation.26,27 It is also known, that data obtained in in vitro studies demonstrate nanoparticle-induced inflammation and pulmonary fibrosis, the formation of granulomas and emphysema.28 Medical and biotechnological applications of silicon dioxide nanoparticles include the production of sorbents and molecular sieves, DNA delivery vehicles, proteins, and anti-cancer drugs. The high photocatalytic activity of ZnO and TiO2 nanoparticles determines their antibacterial and antiseptic properties, which in turn are important for applications.29 The main mechanism of the toxic effect of titanium oxide and zinc oxide nanoparticles was the induction of reactive oxygen species, and the reactivity depends not only on the size of the nanoparticles but also on the type of structure.30
Based on the above-mentioned data, in this work we selected components, developed technology and studied a number of parameters of polymer nanocomposite materials, remembering that the material would have high optical and good mechanical characteristics, good sorption ability in order to ensure high value of the optical signal for a short time while maintaining the initial geometric shape. In addition, if this nanocomposite is used for medicine and biology (biocompatible or biocidal materials or the creation of a sensor based on it), the material must be non-toxic and/or biocompatible.
Methods
Initial Materials
The following materials and chemicals were used for sample preparation:
- Diurethane dimethacrylate, a mixture of isomers (Aldrich 436909, UDMA)
- Isodecyl acrylate (Aldrich 408956, IDA)
- A 2-Carboxyethyl acrylate (Aldrich 552348, 2Car)
- Initiator - 2.2-Dimethoxy-2-phenylacetophenone (Aldrich 19611–8, In2)
- Dodecanethiol functionalized gold nanoparticles with size 5 nm (ALDRICH 660434, AuNP)
- SiO2 nanoparticles with size 14 nm (Aldrich 066K0110, SiO2NP)
- Zinc oxide nanoparticles (ALDRICH 205532, ZnONP)
- Titanium dioxide nanoparticles (ALDRICH 637254, TiO2NP)
Preparation of Polymer Nanocomposites
The polymer nanocomposites are mixtures of different monomers with SiO2NP, AuNP/TiO2NP/ZnONP, and photo-initiator. Three different monomer mixtures were created: two on the basis of UDMA/IDA (with different concentrations of UDMA and IDA in it) and one - UDMA/IDA/2Car. The nanoparticles and photo-initiator were added to the monomer mixture and the nanocomposite was formed.
The synthesis method of creation of monomer nanocomposites consisted of the following steps:
Sample 1:
The SiO2NPs were added by small portions into the mixture of UDMA and IDA (26.99 wt% and 62.99 wt % so the proportion was 3/7). Adding toluene to the mixture to decrease the viscosity of it. After mixing the composition, the necessary amount of AuNP in toluene is added. Further, the photoinitiator In2 is added (0.02 wt% of total mass). It was mixed for 3 hours in a magnetic mixer. Afterward, the toluene is evaporated at 35 ºС for 24 hours till the constant weight. The composite is stored at 25 ºС.
Sample 2:
The preparation was the same as for Sample 1, but the ratio of UDMA and IDA in the mixture was different (71.99 wt% and 18.99 wt % so the proportion was 8/2) (see Table 1).
 |
Table 1 The Compositions of the Prepared Polymer Nanocomposites |
Sample 3:
The SiO2NPs were added by small portions into the mixture of UDMA, IDA and 2Car (67.48 wt%, 16.20 wt% and 6.30 wt % so the proportion was 75/18/7). In this case, toluene was added as well. After mixing the composition, the necessary amount of AuNP in toluene, TiO2NP and ZnONP were added separately to the monomer mixture. Further, the photoinitiator In2 is added (0.02 wt% of total mass). It was mixed for 3 hours in a magnetic mixer. Afterward, the toluene is evaporated at 35 ºС for 24 hours till the constant weight. The composite is stored at 25 ºС.
To create polymer nanocomposite samples layers with thicknesses 200–300 μm were formed on a glass substrate in the gap surrounded by a spacer between the glass and cover polyester film. For the photopolymerization, the layers were cured by a UV lamp for 20 minutes.
The compositions of the prepared polymer nanocomposites are presented in Table 1. There are samples 1a, 2a, and 3a, which will be the basic monomer composites, containing just a mixture of monomers, SiO2NP, and a photoinitiator. While the samples 1Au, 2Au, 3Au, 3ZnO and 3TiO, besides the basic samples (1a, 2a and 3a) contain the indexed metallic nanoparticles as well.
The novelty of the synthesis of these samples was using a mixture of monomer composites, their fabrication routs, a combination of different metallic, oxide nanoparticles, which increased the multifunctionality of the nanocomposite, including optical applications. Another novelty of the prepared polymer nanocomposites in the present work was the improvement of the compatibility of the mixture of the monomers with the inorganic nanoparticles, not only by using silicon dioxide nanoparticles but also by modification with silane The introduction of 10 wt% of modified SiO2NP allows to obtain a homogeneous solution of organic monomers with inorganic particles (oxides), thus, phase separation can be prevented in solutions and in films after polymerization, as a result, a uniform film could be obtained.
Characterization of the Polymer Nanocomposites
Vickers hardness of the prepared polymer nanocomposites was established by a Buehler hardness tester with PMT-ZM “Lomo”. Five microhardness indentations were made on each prepared specimen. Vickers microhardness measurements were made with 50 g load for 20 seconds in a micro-hardness testing machine. The advantage of the Vickers hardness method is that deformation due to the indentation can reveal structural characteristics of the test material in a quick measurement. The errors of the measurements were ± 5–10%.
The contact angle of wetting for the samples was measured by the equipment, which is shown in Figure 1. It consists of a high-resolution USB camera, a sample holder and a diffuse light source. On the surface of prepared samples, the same amount of water was used to create a drop on it. The picture of the drop on the surface was recorded and analyzed by a Fiji software (http://fiji.sc/), with Drop analysis-Drop Snake and Drop analysis-LB-ADSA plugins (http://bigwww.epfl.ch/demo/dropanalysis). As a result, the contact angle of the water on the surface of the prepared polymer nanocomposites was established. The errors of the measurements were ± 5%.
 |
Figure 1 Contact angle measurement: (A) the measuring system, (B) the image of the drop on the surface of polymer nanocomposite. |
Biocompatibility of the Polymer Nanocomposites
Human Monocyte-Derived Dendritic Cell Cultures
Leukocyte-enriched buffy coats were obtained from healthy blood donors drawn at the Regional Blood Center of the Hungarian National Blood Transfusion Service (Debrecen, Hungary) in accordance with the written approval of the Director of the National Blood Transfusion Service of the University of Debrecen, Faculty of Medicine (Hungary) and from the Regional and Institutional Research Ethical Committee of the University of Debrecen (DEOEC RKEB/IKEB 3855–2013). Written, informed consent was obtained from the blood donors prior to blood donation, their data were processed and stored according to the directives of the European Union. Peripheral blood mononuclear cells (PBMCs) were separated by a standard density gradient centrifugation with Ficoll-Paque Plus (Amersham Biosciences, Uppsala, Sweden). Monocytes were purified from PBMCs by positive selection using immunomagnetic cell separation and anti-CD14 microbeads, according to the manufacturer’s instruction (Miltenyi Biotec, Bergisch Gladbach, Germany). After separation on a VarioMACS magnet, 96–99% of the cells were shown to be CD14+ monocytes, as measured by flow cytometry. Isolated monocytes were cultured for three days in 12-well tissue culture plates at a density of 5.0 x 105 cells/mL in Gibco’s serum-free AIM-V medium (Thermo Fischer Scientific, Waltham, MA, USA), supplemented with 80 ng/mL granulocyte-monocyte stimulating factor (GM-CSF) (Gentaur Molecular Products, Brussels, Belgium) and 100 ng/mL interleukin (IL)-4 (PeproTech EC, London, UK) into monocyte-derived dendritic cells (moDC) at 37°C atmosphere containing 5% CO2.
Measuring the Effects of Polymer Nanocomposites on the Activation Level and Viability of moDC
Resting human monocyte-derived dendritic cells (moDCs) were cultured for 24 hours on the surface of the prepared, sterilized by UV light, polymer nanocomposites in the presence or absence of the specific Toll-like receptor (TLR) ligand bacterial lipopolysaccharide (LPS) (250 ng/mL ultrapure LPS, InvivoGen, San Diego, CA, USA) for 24 hours. As human moDCs are highly sensitive for LPS the reagents and lab equipment are endotoxin-free. Some samples were cultured with both – polymer nanocomposites and LPS (nanocomposites + LPS) to detect the effect of nanocomposites on moDC viability and activation under microbial stimuli of LPS, while untreated cells (ctrl) were served as negative controls.
Flow Cytometry
Resting human monocyte-derived dendritic cells (moDCs) were cultured for 24 hours on the surface of the prepared, sterilized by UV light, polymer nanocomposites in the presence or absence of LPS. At the end of incubation, cells were harvested. Phenotyping of resting and activated moDCs cultured with or without polymer nanocomposites was performed by flow cytometry using anti-human CD83-fluorescein-isothiocyanate (FITC) (R&D Systems, Minneapolis, MN, USA), The viability of moDCs was determined with 1 µg/mL 7-amino-actinomycin D (7-AAD) (LKT Laboratories Inc., St. Paul, MN, USA) dye followed by a 24 h activation period with LPS. 7-AAD is internalized in the cell if the cell membrane is disrupted (dead cells). The 7-AAD dye binds to the cellular DNA. Fluorescence intensities were measured by FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA) and data were analyzed by the FlowJo software (Tree Star, Ashland, OR, USA).
Growth of Lactobacteria for Monitoring Biocompatibility with Polymer Nanocomposites
L. reuteri ATCC 6475 strain was grown in DifcoTM de Man, Rogosa and Sharpe (MRS) broth medium for 18 h stationary at 37°C (MRS, BD BioSciences, Franklin Lakes, NJ, USA) in the presence or absence of polymer nanocomposites for 18 h. Bacterial suspensions were measured by spectrophotometry and converted to CFU/mL following OD600 nm x 2.5 x 108 CFU/mL. L. reuteri ATCC 6475 was kindly provided by Nathalie Juge (Institute of Food Research, Norwich, UK).
Adhesion of Different Microorganisms on the Surface of Polymer Nanocomposites
To analyze the adherence of different microbial organisms on the surface of polymer nanocomposites three species of the colonizing microbes of the human body were selected including Escherichia coli, Staphylococcus aureus, and Candida albicans. For all species, 3 different strains were used for this measurement. The results of the species were averaged the strain results. The investigation was done in the following way:
- 24 hours cultured microorganism has created a suspension with saline solution in concentration 1.5*108 cells/mL.
- The samples of polymer nanocomposites were sterilized by UV light and were put to the sterile Petri dish. The suspension of the microorganism was put to this dish and totally covered the samples for 30 minutes at room temperature.
- After the incubation, the suspension was recovered by a pipette. The samples were dried in normal air.
- The fixation of the microorganism was done by the flame of alcohol.
- In the next step, the staining of the samples was done by methylene blue, the remaining part of the stain was removed by water.
- The determination of the sorption of the microbes was done by the calculation of the average number of stuck bacterias on the surface of the investigated samples. It was done by optical microscope Nikon Eclipse E-200 at 1000 resolution. For each sample, 10 images were done and analyzed. The optical image of the microorganisms is shown in Figure 2.
- For the calculation of the bacteria’s from the images, an open-access software was used: PO OpenCFU (http://opencfu.sourceforge.net/).
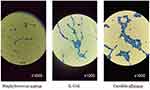 |
Figure 2 The optical microscopy images of the studied microorganisms. |
Results
Sample Characterization
Polymer nanocomposites based on urethane-acrylate monomer structure were created for the investigation of possible bioapplications. As it was shown earlier, this monomer material could be used as a photosensitive material, doped with different nanoparticles and as a result enhance some optical properties. In this work, different monomers (UDMA, IDA, 2Car) and nanoparticles (SiO2NP, AuNP, ZnONP, TiO2NP) were used. As a result of using different materials, some properties of the polymer nanocomposite could be modified, such as optical parameters, microhardness, sorption, surface energy (contact angle) and even toxicity. It could be connected with different functional properties of these materials.
It was shown in our previous paper,31 that the prepared polymer materials with SiO2NPs are highly transparent from 400 to 1100 nm. Adding ZnO NPs did not affect the transmission of the initial polymer samples, while TiO2NPs decrease it. However, the addition of AuNPs results in the appearance of well-known absorption, localized surface plasmon resonance peak near 530 nm. The refractive index of the created polymer nanocomposites was between 1.4 and 1.6. The reflection was not larger in comparison to another optical polymer material like PMMA.
The parameters of the prepared polymer nanocomposites were studied with different techniques. For analyzing the influence of different monomers and added nanoparticles on the mechanical properties of the created polymer nanocomposites, Vickers microhardness was measured. The results are shown in Table 2. It could be seen, that introducing IDA and 2Car to the UDMA basic monomer mixture decreases the microhardness while adding AuNPs increases it. Although, the ZnONPs and TiO2NPs have not influenced much on the measured value. It was shown previously31 that the modification of the composition could result in changes in the structure. In this way, the mechanical properties, such as microhardness could be increased by adding nanoparticles to the monomer mixture. It could be explained by the photopolymerization process, structuring of polymers, formation of the different absorption layers, changing the polymer matrix and also by the influence of added nanoparticles on the microstructure of the polymer matrix.32–35
 |
Table 2 Microhardness and Contact Angle Values of the Created Polymer Nanocomposites |
It is important to check materials of possible bio-related applications for reaction with water, their surface energy, and hydrophobic/hydrophilic character as well. For these reasons, the contact angle of water was measured on the created polymer nanocomposites. It gives us information about the character of the surface – is it hydrophilic or hydrophobic one. For producing biosensing devices, it is more effective to have a hydrophilic character of the surface.36–38 While for antibacterial surfaces – both types of the surface could be used: depending on the effect on the bacteria, it is an anti-bactericidal surface or bacteria-release surface.39 The results are presented in Table 2. It could be seen that, as in the case of microhardness, the composition has an influence on the measured data. Adding monomers IDA and 2Car to the basic mixture decreased the contact angle, which resulted in a more hydrophilic character of the surfaces. However, adding AuNPs to the monomer mixture essentially increases the angle, so the character of its’ surface became more hydrophobic. Using ZnONP and TiO2NP in the composition increases the value of the contact angle, but not essentially.
It was shown that the composition and the presence of different nanoparticles have an influence on the microhardness and on the value of the contact angle, so on the surface character of the samples. The addition of IDA and 2Car monomers to the basic UDMA mixture led to the decrease of microhardness and contact angle. This tendency can be connected with the process of photopolymerization, which was studied in some previous papers.40–42 The added monomers (IDA, 2Car) led to a lower rate of photopolymerization process, which resulted in a softer and more hydrophilic surface. The addition of AuNP to the mixture of monomers in all cases leads to higher microhardness and contact angle value. It was also shown previously that the AuNP enhances the process of photopolymerization, increasing/decreasing the time of it, and increasing the polymerization rate. It leads to a harder and more hydrophobic surface than the initial one.40–42 The addition of ZnONPs and TiO2NPs has not influenced essentially the measured values. However, as there was a difference in composition, in structure and in the parameters, it may influence the interaction with the microorganism.
Biocompatibility of the Polymer Nanocomposites
To test the biocompatibility of the non-water reacting polymer nanocomposites, we set up a model to monitor the effects of polymer nanocomposites on the viability of eukaryotic and prokaryotic cells, respectively.
The biocompatibility and toxicity were studied by measuring the viability and activation level of human monocyte-derived dendritic cells (moDC). MoDCs are an essential part of the human immune system and regulate defense mechanisms against microbes in the body, which can be affected by the presence of nanocomposites. We found that the majority of polymerized nanocomposites did not affect the number of dead cells and did not decrease the viability of human dendritic cells (Figure 3A). The stimulation of moDCs by LPS and nanocomposites together slightly, but not significantly elevated the number of dead cells.
To test further the biocompatibility of the nanocomposites, we monitored the proliferation of a lactic acid bacteria L. reuteri (Figure 3B) and we showed that that polymer nanocomposites with different nanoparticles did not affect the proliferation of the bacteria.
In conclusion, the created polymer nanocomposites did not modify the viability of human dendritic cells in the presence or absence of LPS and did not inhibit the proliferation of probiotic lactic acid bacteria.
Adhesion of the Microorganism on the Surface of Polymer Nanocomposites
Adhesion of two bacterial and one fungal species on the surface of created polymer nanocomposites with different composition was studied using Gram-negative E. coli, the Gram-positive S. aureus and C. Albicans. In Table 3, the number of microorganisms is presented. It was shown that S. aureus and C. Albicans have a higher value of sorption in comparison to E. coli, which could be connected with the structure of the created polymer nanocomposites. It was found that the composition and addition of the nanoparticles in the polymer nanocomposites influenced the sorption of different microorganisms, see Tables 1 and 3. However, the adhering capacity was higher for S. aureus and C. Albicans as compared to E. coli. Moreover, S. aureus showed the highest ability to adhere on the surface of different nanocomposites.
 |
Table 3 The Sorption of Microorganisms on the Surface of Different Polymer Nanocomposites |
It could be seen from Table 3 than the S. aureus and C. Albicans have higher sorption ability on the surface of polymer nanocomposites then E. coli. There are some papers dealing with it and based on them, accordingly, it could be shown that the sorption of bacteria on different surfaces could depend on its hydrophobic/hydrophilic character, surface roughness, surface charge, chemical potential and etc.43 The surface charge of the microorganism could be estimated from the chemical composition of its’ cell structure. The interaction between the cells and the surface depends on the surface charge, while the character of the sorption forces could be determined by the chemical composition of the cells and of the surface (in our case by the polymer nanocomposites).44
Discussions
Thus, the chemical composition of the microbial cell wall has an important role in studies of sorption on different surfaces. These microbial characteristics were diverse for the tested microbes. The lipid content of the Gram-negative bacterial cell wall is within 19–20% and in the case of Gram-positive bacteria, it is 1–4%. For Gram-positive S. aureus – cell walls include peptidoglycans modified by polysaccharides, for gram-negative E. coli – it consists of LPS modified by carbohydrates, for candida’s – it contains polysaccharides in a multilayer structure.45–49 As it is known, peptides are molecules built on more than two amino acids, bonded by amino bonds -C(O)NH-, while polysaccharides are long chains of monosaccharide bonded by glycoside bonds and the lipids.45–52 So the cell walls of these microorganisms are rather different, which could influence their sorption on the surface of the investigated polymer nanocomposites. As a result, the sorption of S. aureus was essentially higher in comparison to E. coli, which effect partly could be connected with the structure of these bacteria. The structure of the monomers have different functional groups and their chemical structure is closer to Gram-positive bacteria as compared to the Gram-negative ones.
The sorption of microorganisms depends on their composition, structure, and character as well. As it was shown during the analysis of sorption, the composition and the existence of nanoparticles had an essential effect on the adhering ability of S. aureus. There is a connection between the sorption and the contact angle value of the investigated samples. Higher sorption of bacteria and hydrophilicity was established for samples without metallic nanoparticles (1a, 2a, and 3a) in comparison with the samples with them. So it could be concluded that the sorption of the bacteria was higher on the surface of more hydrophilic samples. At the same time for samples with metallic nanoparticles (1Au, 2Au, 3Au, 3ZnO, 3TiO) the sorption of the bacteria and hydrophilicity was lower, while the hydrophobicity of these samples was higher in comparison to the samples without nanoparticles. In fact of that, the sorption of the bacteria was lower for samples with lower hydrophilicity. The structure of polymer nanocomposites which contains metallic nanoparticles has an essential role in sorption and hydrophilic/hydrophobic character of them. On the other hand, the composition of polymer mixture has an influence on these parameters as well, since a higher amount of IDA (1a) results in higher sorption of bacteria and hydrophobicity. Besides the surface character of the samples, the surface charge could play an important role as well. For the sample 3TiO, the contact angle was not lower, however, the sorption was really low – the surface charge could play an important role and leads to a lower amount of bacteria on it.
As a result of these investigations, it could be established that the sorption of different microorganisms on the surface of polymer nanocomposites can be changed, controlled by the composition of the polymer mixture and by the existence of metallic, oxide nanoparticles in them.
For the fabrication of possible biosensor devices based on the created polymer nanocomposites, high sorption of the possible detected objects is needed. For example, for the detection of S. aureus, a monomer mixture of UDMA, IDA and 2Car are needed, which has a lower contact angle and high sorption. One way to create an optical sensing device is using rare earth elements, as nanoparticles and their luminescence properties, which were studied in.53 We tried to create a model of optical sensing device based on ErO nanoparticles, which were added to the mixture of UDMA/IDA/2Car to enhance its’ luminescent character and to optimize the measurement. First of all sorption of CuSO4 water solution on the surface of samples with and without ErO nanoparticles was studied. Shimadzu UV-1800 spectrophotometer was used for the sorption measurements on thin layers of polymer nanocomposites. The luminescence of the samples was studied by LSM 710 Zeiss confocal optical microscope at excitation wavelength 514 nm. The luminescence spectra were taken before and after the absorption of CuSO4 solution on the samples with and without ErO nanoparticles. The results on luminescence are shown in Figure 4.
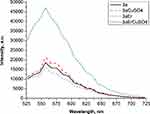 |
Figure 4 Luminescence spectra of the investigated samples with and without ErO nanoparticles. |
It could be seen that adding ErO nanoparticles the sorption can be increased from 8 to even 17%, so the signal of luminescence can be increased by 2.5 times. It was shown that for samples containing rare earth nanoparticles, the luminescence increased due to the sorption of copper. However, in other cases, the copper decreases the luminescence, as earlier was shown in.54 The luminescence in the presence of the copper-containing solution in the region of 500–650 nm could be connected with ligand transition in their structure, which could be enhanced by blue light.55 So it was shown that adding ErO nanoparticles may enhance the detection of heavy metals by this composite, and it a good model material to create optical sensors based on their luminescence properties.
On the other hand, the nanocomposites containing AuNP, have an absorption peak near 550 nm, connected with localized surface plasmon resonance, as it was shown in our previous paper.40 As the position of this peak is sensitive to the refractive index of the surrounding media, the changes could be detected optically in transmission mode. This is supported by the fact, that our samples are highly transparent in the visible range of light.56 The photopolymerization process, which leads to an increase in the refractive index, shifts the position of the peak.40 This phenomenon can be effectively utilized for a large variety of sensing purposes (chemical sensors, gas sensors, biosensors, etc.) by measuring the changes in the refractive indices. Such an optical sensor element can be used for monitoring of molecular-scale interactions. However, it should be analyzed more carefully further.
Besides the biosensing application, the polymer nanocomposites could be taken into account as materials for the creation of antibacterial surfaces. It is possible since this material is biocompatible and not toxic. For enhancing it, we should increase the contact angle of the surface, decreasing at the same time the sorption of the microorganism, which was done by changing the composition and adding nanoparticles. For this purpose, the monomer mixture of UDMA could be used with AuNPs (which increases the contact angle – decreases the sorption) and with TiO2NP (which surface charge has an influence on the microorganism – decreases their sorption). However, not only the composition and existence of nanoparticles has an influence on the sorption. The surface roughness also could be a key factor in this. This material was studied successfully for the creation of surface structures.37 As a result, holographic gratings were recorded on samples 2a, 2Au, 3a, and 3Au. The contact angle of water was measured on the created surface structures. It was shown that the presence of the structure increased the contact angle, so the character of hydrophobicity became larger in comparison to the normal thin layer from this material as it is shown in Table 4. The sorption of microorganism should be analyzed in the next step, however, for it, a lot of gratings should be created.
 |
Table 4 The Contact Angle Values of Thin Layer and Surface Structures Based on Polymer Nanocomposites |
Polymer nanocomposites based on various urethane-acrylate monomers and different nanoparticles were prepared and studied. The composition of the samples influences their microhardness and the value of contact angle, which means that varying with the monomer and the metallic, oxide nanoparticles composition, we could change these parameters. Besides it, the set of required, biology-related properties of these materials, such as toxicity and sorption of microorganisms were also investigated in order to prove their possible applicability. It was shown that the materials are non-toxic, the adhesion of microorganisms on their surface also could be varied by changing their composition. So the presented polymer nanocomposites with different compositions of monomer and the presence of nanoparticles in them are perspective material for a possible bio- application as it is biocompatible, not toxic. The sorption of microorganism could be varied depending on the type of bacterias, the monomer composition, and nanoparticles.
Acknowledgment
This work was financially supported by the GINOP-2.3.2-15-2016-00041. The project is co-financed by the European Union and the European Social Fund.
Disclosure
Professor Sándor Kökényesi report grants from University of Debrecen during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Polymer concepts in tissue engineering. J Biomed Mater Res. 1998;43:422–427. doi:10.1002/(SICI)1097-4636(199824)43:4<422::AID-JBM9>3.0.CO;2-1
2. Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477–563. doi:10.1023/B:ABME.0000017544.36001.8e
3. Yoo SM, Lee SY. Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol. 2016;34:7–25. doi:10.1016/j.tibtech.2015.09.012
4. Altintas Z, Gittens M, Guerreiro A, et al. Detection of waterborne viruses using high affinity molecularly imprinted polymers. Anal Chem. 2015;87:6801–6807. doi:10.1021/acs.analchem.5b00989
5. Zhou X, Nie J, Du B. 4-(2-Pyridylazo)-resorcinol functionalized thermosensitive ionic microgels for optical detection of heavy metal ions at nanomolar level. ACS Appl Mater Interfaces. 2015;7:21966–21974. doi:10.1021/acsami.5b06653
6. Teo AJT, Mishra A, Park I, Kim Y-J, Park W-T, Yoon Y-J. Polymeric biomaterials for medical implants and devices. ACS Biomater Sci Eng. 2016;2:454–472. doi:10.1021/acsbiomaterials.5b00429
7. Bay L, West K, Sommer-Larsen P, Skaarup S, Benslimane M. A conducting polymer artificial muscle with 12% linear strain. Adv Mater. 2003;15:310–313. doi:10.1002/adma.200390075
8. Kim HN, Zhiqian G, Weihong Z, Juyoung Y, Tian H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem Soc Rev. 2011;40:79–93. doi:10.1039/C0CS00058B
9. Zare EN, Jamaledin R, Naserzadeh P, et al. Metal-Based nanostructures/PLGA nanocomposites: antimicrobial activity, cytotoxicity, and their biomedical applications. ACS Appl Mater Interfaces. 2020;12:3279–3300. doi:10.1021/acsami.9b19435
10. Zare EN, Makvandi P, Borzacchiello A, Tay FR, Ashtari B, Padil VVT. Antimicrobial gum bio-based nanocomposites and their industrial and biomedical applications. Chem Commun. 2019;55:14871–14885.
11. Zare EN, Makvandi P, Ashtari B, Rossi F, Motahari A, Perale G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: a review. J Med Chem. 2020;63:1–22. doi:10.1021/acs.jmedchem.9b00803
12. Zare EN, Lakouraj MM, Mohseni M, Motahari A. Multilayered electromagnetic bionanocomposite based on alginic acid: characterization and biological activities. Carbohydr Polym. 2015;130:372–380. doi:10.1016/j.carbpol.2015.05.020
13. Hasantabar V, Lakouraj MM, Zare EN, Mohseni M. Innovative magnetic tri-layered nanocomposites based on polyxanthone triazole, polypyrrole and iron oxide: synthesis, characterization and investigation of the biological activities. RSC Adv. 2015;5:70186–70196.
14. Hasantabar V, Lakouraj MM, Zare EN, Mohseni M. Synthesis, characterization, and biological properties of novel bioactive Poly(xanthoneamide‐triazole‐ethersulfone) and its multifunctional nanocomposite with polyaniline. Adv Polym Technol. 2017;36:309–319. doi:10.1002/adv.21609
15. Uzun L, Turner APF. Molecularly-imprinted polymer sensors: realising their potential. Biosens Bioelectron. 2016;76:131–144. doi:10.1016/j.bios.2015.07.013
16. Huang Y, Tian Z, Sun LP, et al. High-sensitivity DNA biosensor based on optical fiber taper interferometer coated with conjugated polymer tentacle. Opt Express. 2015;23:26962–26968. doi:10.1364/OE.23.026962
17. Markos C, Yuan W, Vlachos K, Town GE, Bang O. Label-free biosensing with high sensitivity in dual-core microstructured polymer optical fibers. Opt Express. 2011;19:7790–7798. doi:10.1364/OE.19.007790
18. Donnet JB, Ehrburger P. Carbon fibre in polymer reinforcement. Carbon. 1977;15:143–152. doi:10.1016/0008-6223(77)90047-1
19. Makvandi P, Gu JT, Zare EN, et al. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020;101:69–101. doi:10.1016/j.actbio.2019.09.025
20. Johnsson B, Löfås S, Lindquist G, Edström Å, Müller Hillgren R‐M, Hansson A. Comparison of methods for immobilization to carboxymethyl dextran sensor surfaces by analysis of the specific activity of monoclonal antibodies. J Mol Recognit. 1995;8:125–131. doi:10.1002/jmr.300080122
21. Hench LL, Joens JR. Biomaterials, Artificial Organs and Tissue Engineering. Woodhead Publish; 2005.
22. Puoci F. Advanced Polymers in Medicine. Springer; 2015.
23. Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2:32. doi:10.1186/2228-5326-2-32
24. Chen YS, Hung YC, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4:858. doi:10.1007/s11671-009-9334-6
25. Baek YW, An YI. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb 2 O 3) to escherichia coli, bacillus subtilis, and streptococcus aureus. Sci Total Environ. 2011;409:1603–1608. doi:10.1016/j.scitotenv.2011.01.014
26. Pan Y, Neuss S, Leifert A, et al. Size‐dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi:10.1002/smll.200700378
27. Hone DC, Walker P, Evans-Gowing R, et al. Generation of cytotoxic singlet oxygen via phthalocyanine-stabilized gold nanoparticles: a potential delivery vehicle for photodynamic therapy. Langmuir. 2002;18:2985–2987. doi:10.1021/la0256230
28. Li JJ, Zou L, Hartono D, Ong CN, Bay B‐H, Lanry Yung LY. Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro. Adv Mater. 2008;20:138–142. doi:10.1002/adma.200701853
29. Rekha K, Manjula N, Nair G, Anukaliani A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Physica B Condens Matter. 2010;405:3180–3185. doi:10.1016/j.physb.2010.04.042
30. Jańczyk A, Krakowska E, Stochel G, Macyk W. Singlet oxygen photogeneration at surface modified titanium dioxide. J Am Chem Soc. 2006;128:15574–15575. doi:10.1021/ja065970m
31. Wang C, Guo Z-X, Fu S, Wu W, Zhu D. Polymers containing fullerene or carbon nanotube structures. Prog Polym S. 2004;29:1079–1141. doi:10.1016/j.progpolymsci.2004.08.001
32. Berlin AA, Basin VE. The Cause of Adhesion in Polymers. Moscow; 1974.
33. Lipatov YS. Physical Chemistry of Filled Polymers. Moscow: Khimiya; 1977.
34. Shtarkman BP. Fundamentals of the Development of Thermoplastic Polymer Materials. Nizhny Novgorod: Nizhny Novgorod Humanitarian Center; 2004.
35. Zimon AD. Adhesion of Films and Coatings. Moscow: Khimiya; 1977.
36. Samiei E, Luka GS, Najjaran H, Hoorfar M. Integration of biosensors into digital microfluidics: impact of hydrophilic surface of biosensors on droplet manipulation. Biosens Bioelectron. 2016;81:480–486. doi:10.1016/j.bios.2016.03.035
37. Luka G, Samiei E, Dehghani S, Johnson T, Najjaran H, Hoorfar M. Label-free capacitive biosensor for detection of cryptosporidium. Sensors. 2019;19:258. doi:10.3390/s19020258
38. Tang F, Meng X, Chen D, Ran J, Zheng C. Glucose biosensor enhanced by nanoparticles. Sci Chin Ser B Chem. 2000;43:268–274. doi:10.1007/BF02969521
39. Yu Q, Wu Z, Chen H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015;16:1–13. doi:10.1016/j.actbio.2015.01.018
40. Burunkova J, Denisiuk I, Vorzoba N, et al. Fabrication and characterization of gold/acrylic polymer nanocomposites. Eur Polym J. 2013;49:3072–3077. doi:10.1016/j.eurpolymj.2013.05.024
41. Burunkova J, Kokenyesi S, Csarnovics I, Bonyár A, Veres M, Csík A. Influence of gold nanoparticles on the photo-polymerization processes and structure in acrylate nanocomposites. Eur Polym J. 2015;64:189–195. doi:10.1016/j.eurpolymj.2015.01.011
42. Zhuk DI, Burunkova JA, Denisyuk IY, et al. Peculiarities of photonic crystal recording in functional polymer nanocomposites by multibeam interference holography. Polymer. 2017;112:136–143. doi:10.1016/j.polymer.2017.02.004
43. Cheng G, Zhang Z, Chen S, Bryers JD, Jiang S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials. 2007;28:4192–4199. doi:10.1016/j.biomaterials.2007.05.041
44. Mankoci S, Kaiser RL, Sahai N, Barton HA, Joy A. Bactericidal peptidomimetic polyurethanes with remarkable selectivity against escherichia coli. ACS Biomater Sci Eng. 2017;3:2588–2597. doi:10.1021/acsbiomaterials.7b00309
45. Salton MRJ, Kim KS. Structure. In: Baron S, editor. Medical Microbiology.
46. Kim SJ, Chang J, Singh M. Peptidoglycan architecture of Gram-positive bacteria by solid-state NMR. Biochim Biophys Acta. 2014;1848:350–412. doi:10.1016/j.bbamem.2014.05.031
47. Cochet F, Peri F. The role of carbohydrates in the lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) signalling. Int J Mol Sci. 2017;18:2318. doi:10.3390/ijms18112318
48. Maldonado RF, Sá-Correia I, Valvano MA. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev. 2016;40:480–573. doi:10.1093/femsre/fuw007
49. Chaffin WL, López-Ribot JL, Casanova M, Gozalbo D, Martínez JP. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–180. doi:10.1128/MMBR.62.1.130-180.1998
50. Reis RL, Neves NM, Mano JF, Gomes ME, Marques AP, Azevedo HS. Natural-Based Polymers for Biomedical Applications. Woodhead Publishing; 2008.
51. Malanovic N, Lohner K. Gram-positive bacterial cell envelopes: the impact on the activity of antimicrobial. Biochim Biophys Acta. 2016;1858:936–946. doi:10.1016/j.bbamem.2015.11.004
52. Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs. Fron Cell Infect Microbiol. 2014;4:112.
53. Denisiuk I, Burunkova J, Zhuk D, et al. Fabrication and properties of luminescence polymer composites with erbium/ytterbium oxides and gold nanoparticles. Beilstein J Nanotechnol. 2016;7:630–636. doi:10.3762/bjnano.7.55
54. Barbieri A, Accorsi G, Armaroli N. Luminescent complexes beyond the platinum group. Chem Com. 2008;19:2185–2278. doi:10.1039/b716650h
55. Tao B, Cheng F, Jiang X, Xia H. Synthesis, crystal structures and luminescent properties of nickel(II) and copper(II) hexaazamacrocyclic compounds with 1,3,5- benzenetricarboxylate ligands. J Mol Struct. 2012;1028:176–180. doi:10.1016/j.molstruc.2012.06.043
56. Bonyár A, Csarnovics I, Veres M, et al. Investigation of the performance of thermally generated gold nanoislands for LSPR and SERS applications. Sensors Actuators B Chem. 2018;255:433–439. doi:10.1016/j.snb.2017.08.063
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

