Back to Journals » Medical Devices: Evidence and Research » Volume 16
Design and Development of a Novel System for Remote Control of Stationary Oxygen Concentrator Flow Rate
Authors Gadiraju N , Peterson N , Shah J , Chu A, Larbie II MA, Bu A, Saterbak A
Received 20 February 2023
Accepted for publication 5 April 2023
Published 18 April 2023 Volume 2023:16 Pages 91—100
DOI https://doi.org/10.2147/MDER.S407233
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nikhil Gadiraju,* Nikhil Peterson,* Jessica Shah,* Annabelle Chu, Michael A Larbie II, Amy Bu, Ann Saterbak
Pratt School of Engineering, Duke University, Durham, NC, USA
*These authors contributed equally to this work
Correspondence: Ann Saterbak, Department of Biomedical Engineering, Duke University, 101 Science Drive, Campus Box 90281, Durham, NC, 27708-0281, USA, Tel +1 919 660 5899, Fax +1 919 684 4488, Email [email protected]
Purpose: Long-term oxygen therapy involves utilizing stationary oxygen concentrators to allow patients with respiratory illnesses to attain sufficient blood oxygenation via supplemental oxygen. Disadvantages of these devices include their lack of remote adjustability and domiciliary accessibility. To adjust oxygen flow, patients typically walk across their homes – a physically taxing activity – to manually rotate the knob of the concentrator flowmeter. The purpose of this investigation was to develop a control system device that allows patients to remotely adjust the oxygen flow rates on their stationary oxygen concentrator.
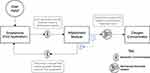
Methods: The engineering design process was used to develop the novel FLO2 device. The two-part system is composed of 1) a smartphone application and 2) an adjustable concentrator attachment unit that mechanically interfaces with the stationary oxygen concentrator flowmeter.
Results: Product testing indicates that users successfully communicated to the concentrator attachment from a maximum distance of 41m in an open field, suggesting usability from anywhere inside a standard home. The calibration algorithm adjusted oxygen flow rates with an accuracy of ± 0.019 LPM and a precision of ± 0.042 LPM.
Conclusion: Initial design testing suggests the device as a reliable and accurate method of wirelessly adjusting oxygen flow on a stationary oxygen concentrator, but further testing should be performed on different stationary oxygen concentrator models.
Keywords: oxygen therapy, respiratory disease, flow meter, wireless adjustment, medical device
Introduction
More than 1.5 million patients in the United States utilize supplemental oxygen to manage various respiratory conditions, including chronic obstructive pulmonary disease, lung cancer, cystic fibrosis, and amyotrophic lateral sclerosis.1 Patients with these respiratory diseases are unable to perform the normal physiological exchange of oxygen and carbon dioxide, resulting in oxygen deficiencies or hypoxia. Although the lung damage and resulting deficiencies associated with chronic obstructive pulmonary disease (COPD) are irreversible, home oxygen therapy can increase life expectancy and standard of living for patients who would otherwise struggle to complete daily tasks.2 More specifically, COPD patients experiencing chronic hypoxemia have demonstrated susceptibility to the health advantages and quality of life improvements afforded by long-term home oxygen therapy.3–5
Advancements in home oxygen delivery have enabled both stationary and portable oxygen concentrators to provide supplemental oxygen. Portable oxygen concentrators are utilized primarily in mobile, lightweight applications, and in the absence of a permanent power source.6 Most home oxygen therapy patients that are prescribed long-term oxygen therapy receive supplemental oxygen for over 15 hours a day through a stationary oxygen concentrator.3,7 Oxygen concentrators function using a technique called pressure swing absorption in which nitrogen from atmospheric air is adsorbed onto zeolite, a porous aluminosilicate material, producing an oxygen-rich gas. With this method, the oxygen concentration of 21% in atmospheric air can be increased to 90–95% in the gaseous product.8 Patients require different amounts of supplemental oxygen when active, resting, and sleeping. Excessive oxygen can result in hyperoxia, while insufficient oxygen can result in hypoxia. Therefore, accurate adjustment of oxygen flow rate is necessary to avoid hypoxia and hyperoxia throughout daily changing activities.2 Oxygen supplementation may need to be increased during periods of increased demand when patient activity increases, such as during exercise or when walking.9 Prior studies have shown that patients with COPD and moderate hypoxia frequently have significant periods of poor oxygenation during daily activities and at night.10
As currently designed, patients or caregivers need to manually adjust the knob on the front of the device to increase or decrease oxygen flow rates. As a result, patients must walk across their homes – and sometimes up and down stairs – to turn the knob of the stationary oxygen concentrator flow meter to maintain adequate oxygenation. However, manual adjustment of oxygen flow is time-consuming and burdens patients, which often reduces therapy compliance.11 Patients undergoing long-term oxygen therapy have previously expressed a need for remote control capabilities for stationary oxygen concentrators.12 Furthermore, the American Thoracic Society (ATS) has highlighted the development of remote-control flowrate adjustment technology as a key funding priority for overcoming barriers to supplemental oxygen delivery via stationary oxygen concentrators.1
The limitations of current oxygen delivery methods led to the advances of new intelligent portable oxygen concentrators. For example, Sanchez-Morillo et al developed a portable oxygen concentrator that uses physiological sensors and predictors of oxygen desaturation to automatically adjust oxygen flow rates.13 However, there are few developments in stationary concentrator technology that address the need for improved oxygen flowrate control and overall device accessibility.1
To adapt oxygen therapy to the dynamic needs of patients and minimize oxygen desaturation episodes, we developed FLO2, a novel oxygen concentrator control system that is an external add-on for existing stationary concentrators. FLO2 allows patients to wirelessly control the flow rate of their oxygen concentrator using a mobile application. This innovation removes any physical activity associated with oxygen flow adjustment for both patients and family members, which will lower the risks of hypoxia and hyperoxia associated with home oxygen therapy.
Materials and Methods
Engineering Design Process
To design and build the FLO2 oxygen concentrator control system, the team used the engineering design process.14 The engineering design process is an iterative workflow utilized to create novel products. As a part of this process, a problem was identified and researched, potential solutions were brainstormed and evaluated using a set of design criteria, a prototype solution was designed and produced, and this initial prototype was iteratively improved upon following testing. In narrowing down the initial set of potential solutions, the design criteria that were utilized during solution evaluation were: range of 30 meters, mobile application reliability of 97.5%, flow rate accuracy of 0.25%, and flow rate precision of 0.25% (Table 1). In summary, the team utilized the iterative engineering design process to produce FLO2, a device that met all established design criteria.
 |
Table 1 Design Criteria with Definitions |
Initially, a preliminary proof of concept was designed for both the communication and attachment modules. The initial communication module was composed of a simple Arduino-based Bluetooth transceiver that allowed users to utilize push buttons to elicit a verifiable response on another Arduino board; this proved user interface and wireless communication capabilities. The attachment proof of concept was a simple elastic clamping mechanism that modeled the module mounting contraption; all attachment proof of concepts were initially designed around the in-house oxygen concentrator, the Invacare Mobilaire V. Given these proof of concepts, iterative prototyping, involving rapid 3D-printing and design review cycles, was utilized to create an initial product prototype. This allowed for prototype iterations to be rapidly tested and refined until a final, viable prototype was created. This iterative prototyping process was repeated multiple times, with in-depth design reviews being conducted between processes. This led the product to transition from an attachment module designed purely for our in-house oxygen concentrator and a physical remote, to an adaptable attachment module and a mobile application.
For more informed refinements of the initial prototype, informal feedback was sought from community members with chronic lung diseases using home oxygen therapy (see Appendix for survey). From a total of 12 survey responses, 91% of responders were prescribed multiple oxygen flow rates each day depending on their level of exertion. Within this group, two members stated that they were prescribed certain flow rates for different levels of exertion but never changed their flow rate due to the difficulty in adjusting it. The survey received mixed feedback on the preference for a slider or button form of flow rate adjustment, so both methods were included in the development of the mobile application. Responders pointed to the ability to adjust to half increments using a slider and the ease of use of pressing a button when explaining their preferences. Finally, the community members expressed a high degree of interest in the continued development of this product.
The final device solution, named FLO2, consists of two parts: 1) an adjustable oxygen concentrator attachment that clamps onto the flow meter of various oxygen concentrator models and 2) a mobile application that allows patients to wirelessly adjust their flow rate (Figure 1). The oxygen concentrator attachment is an external add-on that modifies the flow rate without interfering with the internal workings of the existing oxygen concentrator. The innovation allows patients to use an app on their smart device to change their flow rate from anywhere in their homes. After the FLO2 smartphone application receives user input, it wirelessly communicates with the oxygen concentrator attachment module via Bluetooth. This input prompts the attachment module to utilize a motor and gear system to mechanically rotate the knob on the flow meter of the oxygen concentrator accordingly. Subsequently, the attachment electronically updates the app with the new flow rate value via Bluetooth, which is then displayed to the user.
Attachment Module Housing
The attachment module was designed using Computer Aided Design (CAD) software and 3D-printed using polylactic acid (PLA). The attachment is set atop the oxygen concentrator flowmeter and works by using an adjustable horizontal clamping mechanism that laterally surrounds the flow meter (Figure 2). The right horizontal clamp was designed such that the user can read the oxygen flow rate ball position through the horizontal slot cutout in the case of product failure. Additionally, the vertical slider (Figure 2) can be appropriately adjusted to interface with the flow meter gear to allow for flow rate adjustment. The flow meter gear (Figure 3) attaches to the knob of the flowmeter using four adjustable set screws. This horizontal and vertical clamping mechanism was intentionally designed to allow for oxygen concentrator adaptability. Rather than utilizing a static flowmeter slot for mounting, this designed clamp can adjust to various flowmeter and concentrator geometries, which allows this device to be compatible with many oxygen concentrator models.
 |
Figure 3 (a) Concentrator attachment module and gear system clamped to flow meter. (b) Concentrator attachment side view with an insert designed for users to read the oxygen flow rate ball position. |
Motor and Potentiometer
The stepper motor was placed to rotate the motor driver gear, which was connected to a gear attached to the flow meter knob and a gear attached to the potentiometer (Figure 3). Electronically, a circuit was designed using a Bluno Beetle BLE microcontroller (DFR0339; DFRobot), a 10kΩ 2W wire-wound linear multi-turn potentiometer (3590S-1-103L, Bourns Inc.), and a 12V 4 phase geared stepper motor (28BYJ-48; CenryKay). The microcontroller was powered using a 9V 2A AC/DC power supply (B087ZWS7CG; GuanTing), which was compatible with standard 110 V wall outlets; its power line runs alongside the oxygen concentrator’s power line (Figure 4).
 |
Figure 4 Full concentrator attachment view with electronics plugged into the 110V wall outlet. |
The microcontroller was programmed using Arduino to control the rotational direction and position of the stepper motor based on the potentiometer input. The potentiometer was used to track the position of the flow meter, even when the concentrator attachment or the oxygen concentrator is not on. This was implemented as a safety mechanism for the FLO2 control system to track when the user manually turns the flow meter without the app or if the concentrator attachment accidentally powers off.
When the motor drive gear rotates, it adjusts the flow rate by rotating the flow meter knob via the flow meter gear and sends the current flow rate to the app by rotating the potentiometer via the potentiometer gear. During the calibration process, the potentiometer values that are associated with 0, 1, 2, 3, 4, and 5 LPM are recorded by the microcontroller and sent to the app. The potentiometer values associated with each 0.5 LPM increment are then interpolated. When a target flow rate is selected on the app, the app sends the previously stored potentiometer value to the microcontroller in the concentrator attachment. The microcontroller then signals the 12V DC stepper motor to rotate the flowmeter knob until the potentiometer value matches the target value.
Mobile Application
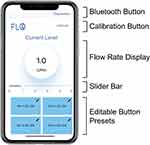
Mobile applications were developed for iOS and Android devices using Swift and Java, respectively, to allow users to control the oxygen flow using their smartphone (Figure 5). The app communicates with the Bluno Beetle microcontroller using Bluetooth 4.0 (BLE).
The app first requires the user to connect to the oxygen concentrator attachment via Bluetooth 4.0 (BLE) (Figure 6, Step 1 and 2). To initially calibrate their device, the user manually turns the flow meter knob to each one integer increment (eg 0, 1, 2, 3, 4, and 5 LPM) and presses a button on the app to confirm the flow rate (Figure 6, Step 3 and 4). The potentiometer values at each of these flow rates are stored in the app. The potentiometer values associated with each 0.5 LPM increment are then interpolated. After the one-time calibration is finished, users can use the slider bar or one of the four editable button presets (Figure 6, Step 5 and 6) to choose their desired oxygen flow rate (Figure 5). The editable button presets were included to allow for easy adjustment to commonly used flow rates such as those prescribed by a physician for the varying activity levels of sleep, rest, and high activity. Each time the desired flow rate is selected, the phone updates the user with the current flow rate and sends the corresponding potentiometer value to the Bluno Beetle BLE microcontroller in the concentrator attachment. The microcontroller then signals the 12V DC stepper motor to rotate the flowmeter knob until the multi-turn potentiometer value matches the value corresponding to the desired flow rate.
 |
Figure 6 iOS application wireframe displaying Bluetooth connectivity (Steps 1 and 2), calibration (Steps 3 and 4), and editing capability of the four favorite buttons (Steps 5 and 6). |
Results
To evaluate the design, the team tested the prototype according to the following design criteria: Bluetooth range, reliability, flow rate accuracy, and precision (Table 2). Testing was performed on the Invacare Mobilaire V stationary oxygen concentrator.
 |
Table 2 Summary of Prototype Testing results |
The Bluetooth range was defined as the maximum distance the app remained connected to the concentrator attachment. A range of at least 30 meters was desirable to ensure that the device would stay connected within the confines of even a relatively large home. The range was tested by identifying how far the app could be from the concentrator attachment before the Bluetooth disconnected in an open field. Through testing, it was found that the distance before disconnection was 41 meters in open space, which met the desired goal of ≥ 30 meters.
Reliability assessed two different facets of the system: 1) the app’s ability to connect via Bluetooth to the concentrator attachment in under 10 seconds and 2) the communication of actions performed on the app to the concentrator attachment. For these reliability standards to be met, at least 195 out of 200 (97.5%) trials performed had to be successful. In testing for reliability, there was successful Bluetooth pairing between the app and the attachment in under 10 seconds for 200 out of 200 (100%) trials. To test for the app adjustment reliability, a button press was administered on the app, and if successful there would be an associated flow rate change. We found that 200 out of 200 (100%) trials were successful, which met the desired standard for app adjustment reliability.
To determine the accuracy and precision of the system, the performance of 122 flow rate adjustments, which included all possible start and end flow rate combinations, was analyzed. Accuracy was accessed by determining the absolute difference between the observed flow rate and the expected flow rate inputted by the user. The overall accuracy was ±0.019 LPM, and the average flow difference satisfied the target of 0.25 LPM for all target flow rates (Figure 7a). Precision was accessed by calculating the standard deviation of the observed flow rate for each expected flow rate inputted by the user. The overall precision was ±0.042 LPM, and the average standard deviation satisfied the target of 0.25 LPM across all target flow rates (Figure 7b). For both accuracy and precision, a maximum absolute difference or standard deviation of 0.25 LPM was acceptable because supplemental oxygen is prescribed in increments of 0.5 LPM. Therefore, any difference from the desired value of less than 0.25 LPM is highly unlikely to negatively affect the patient. Overall, the data demonstrated that the system can adjust the flow rate of the oxygen concentrator with a high level of accuracy and precision.
Discussion
With the increasing prevalence of chronic lung diseases and the COVID-19 pandemic, it is essential that home oxygen therapy patients have immediate control over their supplemental oxygen flow. To address this, the FLO2 oxygen concentrator control system was developed to reduce the risks of hypoxia and hyperoxia associated with movement for manual oxygen flow adjustment. For example, many patients turn the flow meter to 4–6 liter/min and leave it there all day due to the difficulty in changing it, which may risk toxic effects when the dose of oxygen is higher than the body’s demand. With our system, complications of hyperoxia can be avoided with the ability to turn flow rates down once the patient returns to a resting position. When paired with a pulse oximeter, our system also ensures with prescribed target SpO2 range (ex. a prescription of 90% to 92% SpO2). Increased accessibility may improve therapy compliance, increase patients’ quality of life, and lower the risks of adverse blood oxygen levels associated with home oxygen therapy. This two-part control system includes a mobile user application as well as a stationary attachment module to control for a 0–5 liter/min oxygen output flow rate.
Since home oxygen therapy is a medical treatment, the process of home oxygen therapy and any adjustments to the established practice must be accurate, precise, and closely monitored for reliability. Extensive testing of the FLO2 system concluded that the device successfully met the design criteria for precision, accuracy, and reliable performance. An accuracy of ±0.019 LPM proved that the device adjusts the oxygen concentrator to the desired flow rate, which is essential for patients who have exact calculated oxygen prescriptions from their physicians. Similarly, a standard deviation of ±0.042 LPM provides evidence of a high level of precision in receiving consistent flow rate values from the oxygen concentrator. Finally, there is reliable pairing of the smartphone app with the attachment module and consistent registration of any inputs by the user. Due to the combination of these experimental successes, patients may confidently rely on the device to provide the desired flow rate without a risk of a failed connection between the app and attachment module. Even in the case of an unforeseen malfunction, the device preserves the natural function of the oxygen concentrator, allowing patients to manually adjust the flow rate whenever necessary.
While the final prototype met all pre-determined design criteria, certain limitations in design and testing allow for future improvements. The maximum distance of Bluetooth connectivity was measured in open space instead of inside a residence, the likely setting for the device. Future testing should take into account various building materials, wall thickness, or size of the residence to accurately determine the Bluetooth range. Additionally, flow rate measurements were read from a floating ball flow meter attached to the concentrator, which is less precise than a digital readout found on more modern oxygen concentrators. To improve the accuracy of flow rate measurements, future trials should measure the actual output from the nozzle of the machine.
While our user needs assessment demonstrated a concern for the lack of remote adjustability and domiciliary accessibility, we had a small survey sample size of 12. To strengthen our user needs assessment, future work will conduct a more extensive survey with a larger, diverse sample of stationary oxygen concentrator users. Further testing of the device will involve evaluating its accuracy and reliability through feedback from oxygen therapy patients, with iterative improvements to be made based on their user experience. Future studies should investigate the compatibility of the FLO2 system with other stationary oxygen concentrator models and evaluate its clinical impact on patients with various respiratory conditions through randomized trials. The device can also be expanded to work with other oxygen therapy devices such as portable oxygen concentrators or compressed gas tanks. Moreover, we can integrate pulse oximetry with this oxygen control system to automate flow rate adjustment based on a patient’s blood-oxygen concentration. This system could also be adapted to control hospital oxygen, improving the accessibility of oxygen control for both patients and doctors in the future.
Conclusion
We developed and tested a novel oxygen control system to wirelessly control the flow rate of supplemental oxygen during home oxygen therapy. As an external add-on to existing oxygen concentrators, this system was demonstrated to be an accurate, precise, and reliable method for oxygen therapy patients to wirelessly adjust their oxygen flow rate within 41 meters. By eliminating the risks of hyperoxia and hypoxia associated with physical adjustment of oxygen concentrator flow meters, our system offers a safer and more accessible method for patients to control oxygen flow. Future work should focus on validating the accuracy and reliability of our device in a clinical study, exploring compatibility with other oxygen therapy devices, and integrating pulse oximetry to automate flow rate adjustment based on a patient’s blood-oxygen concentration. Further research and development of this device can lead to its integration with current oxygen therapy practices, ultimately improving oxygen flow control accessibility for both patients and healthcare providers.
Acknowledgments
We would like to thank Dr. Elizabeth Bucholz for her early guidance, Murad Maksumov for supplying tools and equipment, and Dr. Alejandro Piño for his clinical insights. This work was funded by the Duke University Pratt School of Engineering and the Duke Baquerizo Innovation Grant.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jacobs SS, Lederer DJ, Garvey CM, et al. Optimizing home oxygen therapy. An Official American Thoracic Society Workshop report. Ann Am Thorac Soc. 2018;15(12):13691381. doi:10.1513/AnnalsATS.201809-627WS
2. Sanchez-Morillo D, Olaby O, Fernandez-Granero MA, Leon-Jimenez A. Automatic recognition of daily physical activities for an intelligent-portable oxygen concentrator (ipoc). Adv Comput Intell. 2017;10305:212–221. doi:10.1007/978-3-319-59153-7_19
3. Tanni SE, Vale SA, Lopes PS, Guiotoko MM, Godoy I, Godoy I. Influence of the oxygen delivery system on the quality of life of patients with chronic hypoxemia. J Bras Pneumol. 2007;33(2):161–167. doi:10.1590/s1806-37132007000200010
4. Górecka D, Gorzelak K, Sliwiński P, Tobiasz M, Zieliński J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52(8):674–679. doi:10.1136/thx.52.8.674
5. Stuart H. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681–686.
6. Hardavella G, Karampinis I, Frille A, Sreter K, Rousalova I. Oxygen devices and delivery systems. Breathe. 2019;15(3):e108–e116. doi:10.1183/20734735.0204-2019
7. McDonald CF. Oxygen therapy for COPD. J Thorac Dis. 2014;6(11):1632–1639. doi:10.3978/j.issn.2072-1439.2014.10.23
8. Chai SW, Kothare MV, Sircar S. Rapid pressure swing adsorption for reduction of bed size factor of a medical oxygen concentrator. Ind Eng Chem Res. 2011;50(14):8703–8710. doi:10.1021/ie2005093
9. Claure N, Bancalari E. Automated closed loop control of inspired oxygen concentration. Respir Care. 2013;58(1):151–161. doi:10.4187/respcare.01955
10. Casanova C, Hernández MC, Sánchez A, et al. Twenty-four-hour ambulatory oximetry monitoring in COPD patients with moderate hypoxemia. Respir Care. 2006;51(12):1416–1423.
11. Sanchez-Morillo D, Olaby O, Fernandez-Granero MA, Leon-Jimenez A. Physiological closed-loop control in intelligent oxygen therapy: a review. Comput Methods Programs Biomed. 2017;146:101–108. doi:10.1016/j.cmpb.2017.05.013
12. Shinoda M, Shinkai M, Nagashima A, Nagai K, Takagi K, Kaneko T. Modification of oxygen concentrators based on questionnaire survey. Clin Respir J. 2018;12(5):1937–1941. doi:10.1111/crj.12761
13. Sanchez-Morillo D, Muñoz-Zara P, Lara-Doña A, Leon-Jimenez A. Automated home oxygen delivery for patients with COPD and respiratory failure: a new approach. Sensors. 2020;20(4):1178. doi:10.3390/s20041178
14. Dym CL, Little P, Dym CL, Dym CL. Engineering Design: A Project-Based Introduction. New York: Wiley; 2000.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.