Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Dermoscopic Features and Their Diagnostic Values Among Common Inflammatory and Infectious Dermatoses: A Cross-Sectional Study
Authors Pakornphadungsit K , Suchonwanit P , Thadanipon K , Visessiri Y, Rutnin S
Received 11 November 2022
Accepted for publication 14 January 2023
Published 24 January 2023 Volume 2023:16 Pages 211—220
DOI https://doi.org/10.2147/CCID.S397212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Kallapan Pakornphadungsit,1 Poonkiat Suchonwanit,1 Kunlawat Thadanipon,2 Yingluck Visessiri,3 Suthinee Rutnin1
1Division of Dermatology, Department of Internal Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Department of Clinical Epidemiology and Biostatistics, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 3Department of Pathology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Suthinee Rutnin, Division of Dermatology, Department of Internal Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchathewi, Bangkok, Thailand, 10400, Tel +66-2-2011141, Fax +66-2-201-1211 ext 4, Email [email protected]
Background: Dermoscopy is a non-invasive tool widely used to improve the diagnostic accuracy of general dermatological conditions.
Objective: To determine the dermoscopic features and their diagnostic value in distinguishing common inflammatory and infectious dermatoses.
Materials and Methods: A cross-sectional study was conducted on patients clinically diagnosed with common inflammatory or infectious skin diseases. Baseline characteristics and clinical and dermoscopic findings were recorded. Dermoscopic variables were analyzed using a correlation matrix. A skin biopsy was performed for each patient for a definitive diagnosis.
Results: Of 102 patients, 43 with dermatitis, 30 with psoriasis, 14 with lichen planus (LP), 5 with pityriasis rosea (PR), and 10 with others were included. Dull red background, patchy vessels, and scales showed significant positive correlations with dermatitis (r = 0.401, 0.488, and 0.327, respectively; p < 0.01), whereas bright red background, glomerular vessels, regular vascular distribution, and diffuse scales revealed significant positive correlations with psoriasis (r = 0.412, 0.266, 0.798, and 0.401, respectively; p < 0.01). For LP, whitish reticulate structures, purplish background, and dotted vessels mixed with linear vessels in the peripheral distribution were significantly positively correlated (r = 0.831, 0.771, 0.224, and 0.558, respectively; p < 0.05). Yellowish background and peripheral scales were predictive of PR diagnosis (r = 0.254 and 0.583, respectively; p < 0.01).
Conclusion: Dermoscopy can be used as an adjunctive tool to differentiate conditions among common inflammatory and infectious dermatoses in order to minimize unnecessary invasive diagnostic procedures.
Keywords: dermoscopy, dermoscopic finding, inflammatory skin disease, infectious skin disease, papulosquamous disease
Introduction
Inflammatory and infectious dermatoses presenting with erythematous desquamative rashes are common dermatologic problems in routine outpatient care. The diagnosis of these conditions is usually established clinically based on morphology, distribution of the lesions, and bedside laboratory investigations. However, clinical diagnosis may sometimes be challenging and require histopathological confirmation due to overlapping manifestations. Although skin biopsy is generally safe, complications can occur. Consequently, delayed diagnosis and management may negatively affect treatment outcomes.
Dermoscopy is a non-invasive tool that allows magnified in vivo observation of the characteristics of skin structures that are invisible to the naked eye. It has been widely recognized and used to improve the diagnostic accuracy of pigmented and non-pigmented skin tumors.1–3 Likewise, it has increasing applications and currently serves as a useful technique to assist in the diagnosis of skin lesions in general dermatology, including inflammatory and infectious dermatoses.4–7 However, information on individuals with Fitzpatrick skin types III–V remains limited and needs further investigation. Thus, this study aimed to determine dermoscopic features and their diagnostic value in distinguishing common inflammatory and infectious dermatoses, particularly in patients with dark skin types. We also described dermoscopic features based on expert consensus by the International Dermoscopy Society8 and provided additional details on dermoscopic parameters, including background color, vascular structures and scale distribution.
Materials and Methods
This cross-sectional study was conducted at Ramathibodi Hospital, Bangkok, Thailand, between September 2016 and December 2020 and was approved by the Mahidol University Institutional Review Board for Ethics in Human Research (MURA2020/275). Written informed consent was obtained from all participants before enrollment.
Patients over 18 years who presented with scaly erythematous/papulosquamous rash and were provisionally diagnosed with inflammatory or infectious skin diseases affecting the face, trunk, and upper or lower extremities were recruited from the Dermatology Outpatient Clinic. Exclusion criteria were lesions on the scalp, palm, and sole; incomplete dermoscopic or histopathologic data; pregnancy; lactation; bleeding diathesis; immunocompromised status; and treatment with topical agents within 2 weeks or systemic treatments (ie, corticosteroids, cyclosporine, methotrexate, retinoids, biologics and antimicrobial agents) within 4 weeks before enrollment.
Patient demographics and clinical data were recorded. The most recently developed lesion was selected for dermoscopic and histopathological examination in individuals with multiple lesions. Digital photographs and dermoscopic images were taken before histologic evaluation using a digital camera (EOS 80D, Canon, Japan), handheld dermoscope DermLite® (DL3N, 3 Gen Inc., California, USA), and digital microscope (Dino-Lite®, AnMo Electronics Corp., Hsinchu, Taiwan) at 10x and 50x magnification, respectively. Finally, a routine histopathological examination was performed in each case to provide a definitive diagnosis.
Dermoscopic images were evaluated by a blinded dermatologist based on expert consensus by the International Dermoscopy Society as a basic guide used in general dermatology.8 Dermoscopic parameters were reported as follows: I) background color, (II) vessels (morphology and distribution), (III) scales (color and distribution), and (IV) other structures (structures other than vessels/scales or specific clues) (Figures 1–4).
 |
Figure 1 Dermoscopic images of background color: (A) bright red, (B) dull red, (C) light red, (D) yellowish, and (E) purplish (x10 original magnification). |
 |
Figure 3 Dermoscopic images of scale morphology: (A) diffuse, (B) central, (C) peripheral (D) patchy, and (E) circular (x10 original magnification). |
 |
Figure 4 Dermoscopic images of some crucial features described: (A) Wickham striae, (B) morse code-like hair, (C) translucent hair, and (D) grey dots (x50 original magnification). |
Statistical Analysis
Data were statistically analyzed using STATA 16.1 (StataCorp LLC, College Station, TX, USA). Variables were calculated as frequencies and percentages and further analyzed via a correlation matrix created using Spearman’s rho. Statistical significance was set at p < 0.05.
Results
Of 126 patients, 24 were excluded due to diagnosis of malignant skin lesions (n=13; Bowen disease, n=6; actinic keratosis, n=3; mycosis fungoides, n=3; and squamous cell carcinoma, n=1), receiving topical/systemic therapy within 2 weeks (n=6), incomplete dermoscopic data (n=4), and biopsy from the scalp region (n=1). One hundred and two patients were included in the study for evaluation. The mean age was 51.8±17.3 years. Fifty-seven (55.9%) patients were females, and 45 (44.1%) were males. Fitzpatrick skin types III, IV, and V were found in 55 (53.9%), 46 (45.1), and one (1%) patients, respectively. Histopathological diagnosis revealed 43 (42.3%) patients with dermatitis, 30 (29.5%) with psoriasis, 14 (13.7%) with lichen planus (LP), five (4.9%) with pityriasis rosea (PR), three (2.9%) with dermatophytosis, two (1.9%) with secondary syphilis (SY), two (1.9%) with lichen striatus (LS), two (1.9%) with dermatomyositis (DM), and one (1%) with pityriasis rubra pilaris (PRP).
Descriptive comparative and analytical results of the dermoscopic features in our study are shown in Table 1. For dermatitis (Figure 5), a dull red background color was the most commonly observed color in 31 (72.1%) patients, followed by dull red and yellowish (n=5, 11.6%) and light red (n=4, 9.3%). A dull red background was a significant positive predictor of dermatitis (r = 0.401; p < 0.01). Dotted vessels were the most commonly found vascular type in 22 (51.2%) patients and were predominantly distributed in a patchy pattern in 37 (86%) patients. The patchy distribution of vessels and scales showed a significant positive correlation with dermatitis (r = 0.488 and 0.327, respectively; p < 0.01). White scaling was the most frequently observed color in 31 (72.1%) patients; however, no significant correlation was found.
 |
Table 1 Dermoscopic Findings with Prevalence and Correlation Analysis |
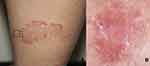 |
Figure 5 (A) Clinical image of dermatitis, square indicates (B) dermoscopic image showing dull red background, patchy distribution of vessels and scales (x10 original magnification). |
Concerning the dermoscopic features of psoriasis (Figure 6), the most common background color observed in 14 (46.7%) patients was bright red, indicating a significant positive correlation with psoriasis. (r = 0.412, p<0.01). Dotted vessels were most commonly observed in 19 (63.3%) patients. Although glomerular vessels were found in only eight (26.7%) patients, they were positively and significantly correlated with psoriasis (r = 0.266; p < 0.01). The regular pattern of the vessels was highly suggestive of psoriasis (r = 0.798; p < 0.01) and was found in 27 (90%) patients. The diffuse scale distribution was also a positive indicator of psoriasis (r = 0.401; p < 0.01) in 21 (70%) patients. White scaling was most frequently observed in 26 (86.7%) patients without any statistically significant correlation.
Regarding the dermoscopic findings of LP (Figure 7), background colors were mostly purplish in 13 (92.9%) patients. In comparison, dotted vessels mixed with linear vessels were commonly observed in the vessel characteristics of 10 (71.4%) patients. The purplish background color and dotted vessels mixed with linear vessels were diagnostic of LP (r = 0.771 and 0.224, respectively; p < 0.01 and < 0.05, respectively). The distribution of peripheral vessels was frequently found in seven (50%) patients, with a significant positive correlation (r = 0.558; p < 0.01). The presence of whitish reticulate structures or “Wickham striae” (WS) was a constant finding in the LP observed in all cases (r = 0.831; p < 0.01).
The dermoscopic features of PR in our study (Figure 8) were characterized by a peripheral scale distribution in all patients (r = 0.583; p < 0.01). Additionally, two (40%) lesions displayed a yellowish background color, which was positively correlated with PR (r = 0.254; p < 0.01). Finally, dotted vessels mixed with linear vessels in a patchy distribution were found in three (60%) patients without any correlation with PR diagnosis.
 |
Figure 8 (A) Clinical image of pityriasis rosea, square indicates (B) dermoscopic image showing yellowish background with peripheral scales (x10 original magnification). |
For the rest of the dermoscopy of included dermatological conditions, dermatophytosis demonstrated a dull red mixed with yellowish background and scales in a patchy distribution in two (66.7%) lesions. Dotted and linear vessels in a patchy pattern were also observed in all three (100%) patients. Morse code-like and translucent hairs (Figure 4) were found in three (100%) and two (66.67%) patients, respectively. Two patients with SY were characterized by a dull red mixed with a yellowish background, dotted vessels in a patchy distribution, and white scales in a circular distribution. LS patients presented with dull red and dull red mixed yellowish background in each case. Additionally, two (100%) LS cases displayed dotted and linear vessels with regular and clustered distributions in each patient. On the other hand, DM lesions showed a dull red background and patchy white scales in two (100%) cases. Gray dots and vessels distributed in a linear pattern and branches (Figure 4) were observed in all the patients with DM. One PRP case demonstrated a dull red mixed with yellowish background, dotted and linear vessels in a patchy distribution, and white scales in a diffuse arrangement. The characteristics of the included skin conditions providing diagnostic value are summarized in Table 2.
 |
Table 2 Characteristic Dermoscopic Findings in Each Disease |
Discussion
The current study revealed dermoscopic features of patients with common inflammatory and infectious dermatoses, including dermatitis, psoriasis, LP, PR, dermatophytosis, SY, LS, DM, and PRP. We demonstrated their diagnostic performance that aids in differentiating among these conditions. Furthermore, a combination of each indicative dermoscopic finding could enhance diagnostic accuracy.
The background colors reported in this study can be used as clues for diagnosing dermatitis. The dull red background color was the most common dermoscopic feature of the disease and showed a significant positive correlation. Corresponding to Lallas’s study, red was the most common background color, although not statistically significant, compared to other papulosquamous disorders.9 Moreover, the patchy distribution of blood vessels and scales revealed significant positive predictors for eczema, corresponding to pre-existing reports.4–11 Yellowish scales were previously demonstrated as the most helpful sign for distinguishing eczema from psoriasis.9,10 However, white scales were more commonly found in our study than yellowish ones. These findings were compatible with a previous study,9 and could be explained by the varying stages of the disease. In the acute exudative stage of eczema, yellow scales/crusts are typically seen, while white scales are found in chronic and lichenoid lesions.4–6
Our study revealed that bright red was the most common background color in psoriasis. In contrast to pre-existing data, light red was the most common.9,10 The difference could be explained by the dissimilarity in the Fitzpatrick skin type of the study population between our study and previous studies.9,10 In terms of vessels, dotted vessels were most frequently found in psoriasis without a statistically significant difference. As reported by Errichetti et al, this type of vessel represented the most frequently seen morphology,8 and its regular distribution demonstrated the dermoscopic keystone in psoriasis.4–15 Consistent with the current study, we found a strong positive predictor of uniform vessel distribution for the diagnosis of psoriasis. Diffuse scales showed a positive correlation with psoriasis in our research, which was in line with previous studies.4–10,14,15 Importantly, a previous study reported the combination of uniform dotted vessels over a red background and diffuse white scales as highly predictive indicators of psoriasis with a sensitivity of 84.9% and a specificity of 88.0%.9
Several recent studies have demonstrated varied dermoscopic LP patterns according to the disease stage.4–6,16 Notably, WS was reported to be the most significant dermoscopic feature exclusively seen in all LP patients in previous studies, including ours.9,11,13,17 WS is histologically correlated with compact orthokeratosis above the zones of wedge-shaped hypergranulosis. In terms of background color, a purplish background was the most common and yielded a significant solid indicator of LP. This finding was in line with a study by Gungor et al.18 Likewise, dotted mixed with linear vessels in a peripheral arrangement was reported in several studies.4–6,9,11,17 These values were the most common findings and revealed a positive correlation with LP in the current study.
Our study’s significant dermoscopic indicators of PR were peripheral scaling and yellow background color. Correspondingly, previous studies reported that peripheral scaling was the hallmark dermoscopic pattern of PR.4,5,9,11,19–21 Remarkably, the finding could be observed in the herald patch and secondary lesions.4
Due to the limited sample size in the dermatophytosis, SY, LS, DM, and PRP groups, our study could not establish dermoscopic findings with distinguishability among these conditions. The present study showed that dotted and linear vessels distributed in a patchy arrangement with patchy white scaling over a dull red and yellowish background were the predominant dermoscopic features of dermatophytosis. Nevertheless, few reports have described the dermoscopic features of dermatophyte skin infections.22,23 Bhat et al demonstrated diffuse erythema, follicular micropustules, and brown spots surrounded by a white‑yellowish halo, broken hairs, wavy hairs, and morse code-like hairs were dermoscopic findings in tinea corporis.22 Comma, corkscrew, and translucent hairs were additionally described in tinea capitis.24–27 Morse code-like and translucent hairs were commonly found in the dermatophyte infection group in our study. These features could be explained by intense fungal invasion of the hair shaft.24–27
Dermoscopic data in secondary SY are scarce and limited to case reports and small case series.28–30 In the present study, dotted vessels in a patchy distribution and white scales in a circular distribution over a dull red mixed with yellowish background were prevalent in secondary SY. Tognetti et al emphasized circular, thin scaling (termed Biett’s sign) surrounded by an erythematous halo as a diagnostic dermoscopic indicator in SY, albeit clinically non-scaling lesions.28 The circular scale in SY can be distinguished from other annular scaling lesions, especially in PR, by the continuous homogenous rings of scales progressing in an outward direction in SY. Multiple fine scaling edges with undefined peeling directions were found in the peripheral ring of the PR.28
Dermoscopic features of LS have rarely been reported in the literature, revealing non-specific background color, vessels, and scales, corresponding to our study.31,32 Conversely, other previously reported findings,31,32 including erythematous blotches, gray granular pigmentation, a white scar-like line, cerebriform pattern, and milia-like cysts, were not observed. Namiki et al33 described dotted vessels on homogeneous pink background, scattered scales, and slightly brown structureless areas as DM dermoscopic features. Slawinska et al34 described diverse vascular structures (irregularly branched vessels, red irregular globules of different sizes, and tortuous vessels) on a homogenous pink background and slight pigmentations in a parallel ridge pattern with a lump of several large surface scales in DM lesions. In our study, all patients with DM showed a dull red background, white scales with grey dots, and linear with branch vessels. These features correlate with pigmentary incontinence and telangiectasia on histopathological examination. Our study supported previously demonstrated dermoscopic features for PRP, which presented a yellowish-red area surrounded by non-specifically distributed mixed morphology vessels.35–39 Although whitish keratotic plugs reported by Lopez-Gomez et al exclusively indicated PRP, this finding was not observed in our study.37
The present study had some limitations. First, the relatively small number of patients in each subgroup limited the statistical power to detect undersized differences. Second, this study did not provide an association between dermoscopic findings and disease progression and/or severity. Third, it is difficult to compare our findings with those of previous studies because of the heterogeneity of dermoscopic terminology. Finally, dermoscopic features from this study population, which included Fitzpatrick skin types III–V, might not apply to those with different Fitzpatrick skin types. Further prospective studies in a large number of patients with appropriate controls are required to obtain more precise results.
Conclusion
In summary, dermoscopy may provide an advantage in aiding the differential diagnosis of common inflammatory and infectious skin conditions. Although some dermoscopic features highly indicate particular diseases, combined with other non-specific findings, they could also be valued, assisting in narrowing down the differential diagnosis. Our study highlights the utility of dermoscopic examination as an adjunctive tool to distinguish conditions among common inflammatory and infectious dermatoses presenting with erythematous plaque(s) with or without scales in order to minimize unnecessary invasive investigative procedures.
Data Sharing Statement
The data sets used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the principles of the Declaration of Helsinki. The protocol was approved by the Mahidol University Institutional Review Board for Ethics in Human Research (MURA2020/275). Informed consent was obtained from all participants before enrollment, and data anonymization was performed before analysis.
Acknowledgments
We would like to thank the Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand for the preparation of biopsy specimens.
Disclosure
The authors declare that there is no conflict of interest.
References
1. Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159(3):669–676. doi:10.1111/j.1365-2133.2008.08713.x
2. Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricalà C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part I. melanocytic skin tumors. J Am Acad Dermatol. 2010;63(3):361–374. doi:10.1016/j.jaad.2009.11.698
3. Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricalà C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part II. nonmelanocytic skin tumors. J Am Acad Dermatol. 2010;63(3):377–386. doi:10.1016/j.jaad.2009.11.697
4. Errichetti E, Stinco G. Dermoscopy in general dermatology: a practical overview. Dermatol Ther (Heidelb). 2016;6(4):471–507. doi:10.1007/s13555-016-0141-6
5. Lallas A, Giacomel J, Argenziano G, et al. Dermoscopy in general dermatology: practical tips for the clinician. Br J Dermatol. 2014;170(3):514–526. doi:10.1111/bjd.12685
6. Errichetti E. Dermoscopy in monitoring and predicting therapeutic response in general dermatology (non-tumoral dermatoses): an Up-To-Date Overview. Dermatol Ther (Heidelb). 2020;10(6):1199–1214. doi:10.1007/s13555-020-00455-y
7. Guida S, Longhitano S, Ardigò M, et al. Dermoscopy, confocal microscopy and optical coherence tomography features of main inflammatory and autoimmune skin diseases: a systematic review. Australas J Dermatol. 2022;63(1):15–26. doi:10.1111/ajd.13695
8. Errichetti E, Zalaudek I, Kittler H, et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol. 2020;182(2):454–467. doi:10.1111/bjd.18125
9. Lallas A, Kyrgidis A, Tzellos TG, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166(6):1198–1205. doi:10.1111/j.1365-2133.2012.10868.x
10. Tubanur C, Aylin TE, Peyker T. Dermoscopic clues of palmoplantar hyperkeratotic eczema and palmoplantar psoriasis: a prospective, comparative study of 90 patients. J Dermatol. 2020;47(10):1157–1165. doi:10.1111/1346-8138.15487
11. Sultan AB, Demet C, Betul D. Dermoscopy in differential diagnosis of inflammatory dermatoses and mycosis fungoides. Int J Dermatol. 2020;59(7):843–850. doi:10.1111/ijd.14925
12. Vazquez Lopez F, Kreusch J, Marghoob AA. Dermoscopic semiology: further insights into vascular features by screening a large spectrum of nontumoral skin lesions. Br J Dermatol. 2004;150(2):226–231. doi:10.1111/j.1365-2133.2004.05753.x
13. Vázquez-López F, Manjón-Haces JA, Maldonado-Seral C, Raya-Aguado C, Pérez-Oliva N, Marghoob AA. Dermoscopic features of plaque psoriasis and lichen planus: new observations. Dermatology. 2003;207(2):151–156. doi:10.1159/000071785
14. Golinska J, Sar-Pomian M, Rudnicka L. Dermoscopic features of psoriasis of the skin, scalp and nails - a systematic review. J Eur Acad Dermatol Venereol. 2019;33(4):648–660. doi:10.1111/jdv.15344
15. Lallas A, Apalla Z, Argenziano G, et al. Dermoscopic pattern of psoriatic lesions on specific body sites. Dermatology. 2014;228(3):250–254. doi:10.1159/000357914
16. Makhecha M, Singh T, Malladi N, Rambhia K. Dermoscopic features of various stages of lichen planus. Indian. J Dermatol Venereol Leprol. 2020;86(2):191–194. doi:10.4103/ijdvl.IJDVL_931_18
17. Dash S, Behera B, Palit A, Sethy M, Nayak AK, Ayyanar P. Dermoscopy of lichen planus under polarized versus nonpolarized mode: a retrospective analysis of 14 patients. Clin Exp Dermatol. 2021;46(4):752–756. doi:10.1111/ced.14565
18. Gungor S, Topal IO, Goncu EK. Dermoscopic patterns in active and regressive lichen planus and lichen planus variants: a morphological study. Dermatol Pract Concept. 2015;5(2):45–53. doi:10.5826/dpc.0502a06
19. Rashmi J, Payal C, Sheenam S. Dermoscopic characterization of guttate psoriasis, pityriasis rosea, and pityriasis lichenoides chronica in dark skin phototypes: an observational study. Dermatol Ther. 2021;34(1):e14631. doi:10.1111/dth.14631
20. Chuh AA. Collarette scaling in pityriasis rosea demonstrated by digital epiluminescence dermatoscopy. Australas J Dermatol. 2001;42(4):288–290. doi:10.1046/j.1440-0960.2001.00538.x
21. Chuh AA. The use of digital epiluminescence dermatoscopy to identify peripheral scaling in pityriasis rosea. Comput Med Imaging Graph. 2002;26(2):129–134. doi:10.1016/S0895-6111(01)00036-2
22. Bhat YJ, Keen A, Hassan I, Latif I, Bashir S. Can dermoscopy serve as a diagnostic tool in dermatophytosis a pilot study. Indian Dermatol Online J. 2019;10(5):530–535. doi:10.4103/idoj.IDOJ_423_18
23. Knöpfel N, Del Pozo LJ, Escudero Mdel M, Martín-Santiago A. A Dermoscopic visualization of vellus hair involvement in tinea corporis: a criterion for systemic antifungal therapy. Pediatr Dermatol. 2015;325(5):548.
24. Lacarrubba F, Verzi AE, Micali G. Newly described features resulting from high-magnification dermoscopy of tinea capitis. JAMA Dermatol. 2015;151(3):308–310. doi:10.1001/jamadermatol.2014.3313
25. Gomez-Moyano E, Crespo Erchiga V, Martínez P, et al. Using dermoscopy to detect tinea of vellus hair. Br J Dermatol. 2016;174(3):636–638. doi:10.1111/bjd.14085
26. Dhaille F, Dillies AS, Dessirier F, et al. A single typical trichoscopic feature is predictive of tinea capitis a prospective multicenter study. Br J Dermatol. 2019;181(5):1046–1051. doi:10.1111/bjd.17866
27. Waśkiel-Burnat A, Rakowska A, Sikora M, Ciechanowicz P, Olszewska M, Rudnicka L. Trichoscopy of Tinea Capitis: a systematic review. Dermatol Ther (Heidelb). 2020;10(1):43–52. doi:10.1007/s13555-019-00350-1
28. Tognetti L, Sbano P, Fimiani M, et al. Dermoscopy of Biett’s sign and differential diagnosis with annular maculo-papular rashes with scaling. Indian J Dermatol Venereol Leprol. 2017;83:270–273. doi:10.4103/0378-6323.196318
29. Errichetti E, Stinco G. Dermoscopy in differentiating palmar syphiloderm from palmar papular psoriasis. Int J STD AIDS. 2017;28(14):1461–1463. doi:10.1177/0956462417714178
30. Mathur M, Acharya P, Karki A, Shah J, Kc N. Dermoscopic clues in the skin lesions of secondary syphilis. Clin Case Rep. 2019;7(3):431–434. doi:10.1002/ccr3.1999
31. Coto-Segura P, Costa-Romero M, Gonzalvo P, Mallo-García S, Curto-Iglesias JR, Santos-Juanes J. Lichen striatus in an adult following trauma with central nail plate involvement and its dermoscopy features. Int J Dermatol. 2008;47(12):1324–1325. doi:10.1111/j.1365-4632.2008.03730.x
32. Kim DW, Kwak HB, Yun SK, Kim HU, Park J. Dermoscopy of linear dermatosis along Blaschko’s line in childhood: lichen striatus versus inflammatory linear verrucous epidermal nevus. J Dermatol. 2017;44(12):e355–e356. doi:10.1111/1346-8138.14035
33. Namiki T, Hashimoto T, Hanafusa T, Miura K, Yokozeki H. Case of dermatomyositis with Gottron papules and mechanic’s hand: dermoscopic features. J Dermatol. 2018;45(1):e19–e20. doi:10.1111/1346-8138.14072
34. Sławińska M, Sokołowska-Wojdyło M, Sobjanek M, Golińska J, Nowicki RJ, Rudnicka L. The significance of dermoscopy and trichoscopy in differentiation of erythroderma due to various dermatological disorders. J Eur Acad Dermatol Venereol. 2021;35(1):230–240. doi:10.1111/jdv.16998
35. Abdel-Azim NE, Ismail SA, Fathy E. Differentiation of pityriasis rubra pilaris from plaque psoriasis by dermoscopy. Arch Dermatol Res. 2017;309(4):311–314. doi:10.1007/s00403-017-1727-2
36. Nair PA, Sheth N. Dermoscopy of Juvenile Circumscribed Pityriasis Rubra Pilaris. Indian Dermatol Online J. 2018;9(6):474–476. doi:10.4103/idoj.IDOJ_334_17
37. Lopez-Gomez A, Vera-Casano A, Gomez-Moyano E, et al. Dermoscopy of circumscribed juvenile pityriasis rubra pilaris. J Am Acad Dermatol. 2015;72(Suppl 1):S58–S59. doi:10.1016/j.jaad.2014.07.053
38. Errichetti E, Piccirillo A, Stinco G. Dermoscopy as an auxiliary tool in the differentiation of the main types of erythroderma due to dermatological disorders. Int J Dermatol. 2016;55(12):e616–e618. doi:10.1111/ijd.13322
39. Kumar S, Vinay K, Radotra BD. Dermoscopy of Erythrodermic Pityriasis Rubra Pilaris. Indian Dermatol Online J. 2019;10(4):500–501. doi:10.4103/idoj.IDOJ_156_18
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



