Back to Journals » Journal of Asthma and Allergy » Volume 14
Demographics, Treatment Patterns, and Morbidity in Patients with Exercise-Induced Bronchoconstriction: An Administrative Claims Data Analysis
Authors Lanz MJ , Gilbert IA , Gandhi HN, Goshi N, Tkacz JP, Lugogo NL
Received 29 September 2021
Accepted for publication 24 November 2021
Published 11 December 2021 Volume 2021:14 Pages 1485—1495
DOI https://doi.org/10.2147/JAA.S338447
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Miguel J Lanz,1 Ileen A Gilbert,2 Hitesh N Gandhi,2 Nadia Goshi,3 Joseph P Tkacz,4 Njira L Lugogo5
1Allergy and Asthma, AAADRS Clinical Research Center, Coral Gables, FL, USA; 2BioPharmaceuticals Medical – US, AstraZeneca, Wilmington, DE, USA; 3BioPharmaceuticals Global Medicines Development – US, AstraZeneca, Wilmington, DE, USA; 4IBM Watson Health, Cambridge, MA, USA; 5Pulmonary Clinic, Michigan Medicine, University of Michigan, Ann Arbor, MI, USA
Correspondence: Ileen A Gilbert
BioPharmaceuticals Global Medicines Development – US, AstraZeneca, 1800 Concord Pike, Wilmington, DE, 19803, USA
Tel +1 262-227-0686
Email [email protected]
Purpose: Exercise-induced bronchoconstriction (EIB) is generally treated with short-acting β2-agonists (SABA) before exercising, to prevent symptoms. Real-world data on treatments and outcomes for patients with EIB alone (EIBalone), or with asthma (EIBasthma), in the USA are limited. This study compared demographics, treatment patterns, morbidity, and costs of treating EIB between these two groups of patients.
Patients and Methods: Administrative claims from US IBM® MarketScan® Research databases were analyzed retrospectively. Patients aged ≥ 4 years filling a SABA claim between 1/1/2011 and 12/31/2016 were evaluated. Patients were indexed on a random SABA claim and required to have 12 months’ continuous eligibility pre- and post-index, ≥ 1 maintenance medication and/or SABA fill post-index, and were designated EIBalone or EIBasthma according to diagnostic codes (EIB only or EIB plus asthma, respectively). Descriptive statistics were used.
Results: In total, 13,480 patients had EIBalone and 14,862 had EIBasthma. Compared with EIBasthma, the EIBalone group was older (mean[SD] 20.4[13.6] vs 17.8[13.6] years), had more females (60.7% vs 54.7%), and filled fewer SABA claims (1.9[1.4] vs 2.5[2.2]) (all p< 0.001). A smaller proportion of patients in the EIBalone than EIBasthma group had maintenance therapy claims (79.9% vs 90.6%, p< 0.001). The EIBalone group also had a lower proportion of patients with oral or injectable corticosteroid claims (29.4% vs 32.0%) and asthma and/or EIB-related emergency department (1.0% vs 13.0%) or outpatient visits (65.1% vs 72.3%; all p< 0.0001). Annual days’ supply of oral corticosteroids was similar between groups (mean[SD] EIBalone: 20.7[30.8] vs EIBasthma: 19.8[28] days).
Conclusion: Individuals with EIBalone or EIBasthma demonstrate considerable morbidity. New treatment paradigms may be needed to optimize outcomes for both patient groups.
Keywords: asthma, drug prescriptions, healthcare costs, short-acting beta2-agonist
Introduction
Exercise-induced bronchoconstriction (EIB) is the transient narrowing of the lower airways during or after exercise and commonly occurs in patients with asthma but can also occur in the absence of asthma.1–4
The decline in lung function that occurs during or after exercise was initially termed exercise-induced asthma but has more recently been subdivided and reclassified as EIB without asthma (EIBalone) or EIB with asthma (EIBasthma).2,5 EIB occurs in up to 90% of patients with a confirmed asthma diagnosis,6 and is more frequent among patients with poor asthma control or more severe asthma.3 EIBalone is particularly prevalent in children and in patients with atopy or rhinitis,5 and is associated with atopy in athletes.7 In children aged ≤16 years, the prevalence estimates of EIB with or without asthma vary between 3–35%,3 with one estimate of the global mean prevalence of EIB in children/adolescents with asthma as high as 46%.8 The wide range of these estimates is due to variability in, and lack of consensus on, the diagnostic criteria and methods of clinical confirmation, as well as variability in testing conditions (environmental conditions such as relative humidity, temperature, pollutant levels1 or exercise conditions such as type and intensity of exercise5).
There is broad agreement that the aims of pharmacologic treatment of EIBalone and EIBasthma should be prevention and/or amelioration of exercise-related bronchoconstriction and symptoms.1,4,9 For patients with EIBalone, use of inhaled short-acting β2-agonists (SABA) taken 5–20 minutes before exercise and/or if symptoms develop post-exercise is generally recommended.1,10 In contrast to the usual management of EIBalone, which targets symptom prevention and/or relief with SABA only,1,4 patients diagnosed with EIBasthma should be treated with daily maintenance therapy for optimal asthma control,1,9,10 according to the severity of their asthma, and following clinical practice guidelines. Patients with EIBalone may also be prescribed daily inhaled corticosteroid (ICS) or leukotriene modifier (LM) treatment if they use SABA on a frequent or even daily basis.1,4 Chronic/daily use of SABA or long-acting β2-agonist (LABA) alone for patients with EIBalone should be avoided, because of the risk of developing tachyphylaxis to the bronchoprotective and bronchodilatory effects of β2-agonists and subsequent risk of serious adverse effects including exacerbations requiring hospitalization.1,4,11–13
Other treatment options include low-dose ICS-formoterol taken as needed and before exercise in patients with EIBasthma,9,14 inhaled short-acting anticholinergics,4,15 and mast cell stabilizers (inhaled cromolyn sodium or nedocromil sodium) given before exercise. Although mast cell stabilizers are recommended for patients with EIBalone or EIBasthma who continue to have EIB despite SABA use or who require an inhaled SABA daily or more frequently,4 they attenuate EIB only by ~50%, have a limited duration of effect and no bronchodilator activity,1 and are only available in the USA in a formulation requiring nebulization.
Real-world treatment patterns and clinical outcomes of EIB in patients with or without asthma in the USA have not been studied in detail to date. The aim of this study, therefore, was to compare demographic characteristics, treatment patterns, morbidities, healthcare resource use, and costs between patients with EIBalone versus those with EIBasthma in a real-world setting.
Materials and Methods
Data Source and Patients
This was a retrospective analysis of administrative claims data from the IBM® MarketScan® Research Databases in the USA, which includes the Commercial, Medicare Supplemental, and Medicaid databases. These databases contain healthcare data for patients insured commercially or via the Medicare or Medicaid programs. They include detailed cost, use and outcomes data for healthcare services, including prescription drug claims, provided in inpatient or outpatient settings. The online analytic platform Treatment Pathways 4.0 was used to access the MarketScan databases.
Unique identifiers are used to link person-level enrollment data to medical and outpatient prescription drug claims. All database studies using US data that are conducted by AstraZeneca comply with the United States Health Insurance Portability and Accountability Act of 1996 Privacy Rule, which allows for use of health information that neither identifies nor provides a reasonable basis to identify an individual. This study used fully de-identified data and as such was not classified as research involving human participants as defined by 45 CFR 46.104(d)(4). For the use of fully de-identified US data, AstraZeneca complies with the procedures set forth in Sections 164.514 (a)–(b)1ii of the Health Insurance Portability and Accountability Act of 1996 Privacy Rule, which allows for use of health information that neither identifies nor provides a reasonable basis to identify an individual. Therefore, approval from an institutional review board was not sought.
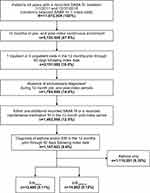
Patients aged ≥4 years with a SABA fill between January 1, 2011 and December 31, 2016 were assessed for inclusion. A random SABA claim within the analysis period was chosen as the index date. Randomization was conducted through the random number generator (RANUNIT function) using SAS 9.4. Eligible patients were required to have continuous enrolment in the MarketScan databases for 1 year prior to (pre-index period) and 1 year following this index date (post-index period), 1 inpatient or 2 outpatient non-diagnostic claims (ie visits) and a separate diagnostic code for EIB (International Classification of Diseases (ICD)-9-CM: 493.81; ICD-10-CM: J45990), asthma (ICD-9-CM: 493.xx, except 493.81; ICD-10-CM: J45xx, except J45990), or both, at any time in the pre-index through 60 days in the post-index period, and ≥1 additional SABA fills, or for patients with a single SABA fill (index fill only), ≥1 maintenance medication fills in the post-index period. Thus, patients in the EIBalone group had a diagnostic code for EIB, but not asthma, and patients in the EIBasthma group had diagnostic codes for both EIB and asthma. Patients with diagnostic codes on an inpatient claim or non-diagnostic outpatient claim any time during the pre- or post-index periods associated with chronic lower respiratory diseases other than asthma, primary eosinophilic disorders, cancer, or autoimmune conditions were excluded from the analysis.
Data Collected
The following patient data were collected: demographics (age, sex), diagnoses of interest, and medication claims in the 1-year post-index period (SABA, maintenance medication, and systemic corticosteroids [SCS; oral or injectable] claims per National Drug Code).
Data on healthcare resource use and costs in the 1-year post-index period were gathered: both all-cause and disease (EIB and/or asthma)-specific healthcare service utilization by place of service, including medical services (inpatient admission, outpatient medical services) and outpatient prescriptions. Disease-specific medical costs included EIB claims in the EIBalone group and both EIB and asthma claims in the EIBasthma group.
Healthcare costs were calculated based on amounts paid for adjudicated claims; this included insurer and health plan payments, as well as patient cost-sharing in the form of copayments, deductibles or coinsurance. All costs were inflation-adjusted using the medical care component of the Consumer Price Index (CPI), and were standardized to annualized 2017 US dollars (US$).
Statistical Analysis
Descriptive statistics were used to summarize patient demographics, annual medication claims, and all-cause and disease-specific healthcare costs. Comparisons between patients with EIBalone and those with EIBasthma for treatment patterns and healthcare utilization were made using the healthcare Student’s t-test and chi-squared (χ2) tests, and for costs using unpaired Student’s t-tests, at a pre-determined alpha significance level of 0.05.
Results
A total of 13,480 patients with EIBalone and 14,862 with EIBasthma were identified and included in this analysis (Figure 1). Of note, the population of patients having diagnostic codes for both asthma and EIB made up 1.3% of the population with a diagnostic code for asthma (N=1,134,143). Patients in the EIBalone group were older (mean [standard deviation (SD)] 20.4 [13.6] vs 17.8 [13.6] years) and had a greater proportion of females (60.7% vs 54.7%) than the EIBasthma group (both χ2 p<0.001; Table 1). EIBalone was more common than EIBasthma in all the age groups except in the 4–11 years’ group, where there were fewer patients with EIBalone than with EIBasthma (20.9% vs 34.2%; χ2 p<0.001) (Table 1).
 |
Table 1 Demographic Characteristics of Patients Aged ≥4 Years with a Diagnosis of EIBalone or EIBasthma |
Treatment Patterns
The annual mean [SD] SABA claims were lower in the EIBalone than the EIBasthma group (1.9 [1.4] vs 2.5 [2.2]; p<0.001), but the median number of SABA claims was 2.0 in both groups.
Significant between-group differences (p<0.0001) were observed according to annual quantity of SABA claims: in the EIBalone group, more patients had filled one or two SABA claims compared with the EIBasthma group (44.4% vs 34.8% and 31.3% vs 26.9%, respectively; Figure 2). In contrast, significantly fewer patients in the EIBalone group than EIBasthma group had 3 or 4 or more SABA fills in the post-index period (12.8% vs 15.9% and 11.6% vs 22.4%, respectively).
Maintenance medication fills were less common in the EIBalone than the EIBasthma group (Figure 3). The most common maintenance therapy regimen in both groups was a LM alone or in combination with other maintenance treatments, recorded for 40.2% of patients in the EIBalone group and 45.0% of patients in the EIBasthma group (between-group difference p<0.0001). Between-group differences were significant (p<0.0001) for all categories of maintenance medication prescription (Figure 3). Maintenance medication prescriptions were higher in the EIBasthma than EIBalone group for ICS in combination with other maintenance medication, ICS only or ICS plus LM only (montelukast, zafirlukast, zileuton), ICS-LABA only, and ICS-LABA in combination with any other maintenance medication, while prescriptions for LM only were higher with EIBalone versus EIBasthma (all p<0.0001) (Figure 3).
The proportion of patients with ≥1 annual claim for SCS was lower in the EIBalone group than the EIBasthma group (29.4% vs 32.0%; p<0.0001) (Table 2). However, when analyzed by type of SCS (oral versus injectable), with the between-group difference being significantly higher only for oral corticosteroids (22.5% [EIBalone] vs 26.6% [EIBasthma]; p<0.0001). Approximately 3 weeks’ worth of oral corticosteroid prescriptions were filled in the post-index year in both groups: a mean of 20.7 days (SD 30.8) in the EIBalone group and 19.8 days (28.8) in the EIBasthma group (p=0.188). Chronic SCS use was rare and was observed in a similar proportion of patients in both groups (both 0.2%; p=0.448).
 |
Table 2 Systemic Corticosteroid Prescriptions for Patients Aged ≥4 Years with a Diagnosis of EIBalone or EIBasthma |
Healthcare Resource Utilization
More than two-thirds of patients in both groups had disease-specific outpatient healthcare professional (HCP) office visits in the post-index period, fewer in the EIBalone than the EIBasthma group (65.1% vs 72.3%; p<0.0001) (Table 3). While uncommon overall, fewer patients with EIBalone than patients with EIBasthma required urgent care visits in the post-index period year (0.3% vs 2.0%; p<0.0001). Emergency department (ED) visits were much less common among patients with EIBalone than patients with EIBasthma (1.0% vs 13.0%; p<0.0001). Fewer patients with EIBalone had a disease-related hospitalization (0.4%) than patients in the EIBasthma group (2.0%; p<0.0001).
 |
Table 3 Disease-Specific Healthcare Resource Use for Patients Aged ≥4 Years with a Diagnosis of EIBalone or EIBasthma |
All-Cause and Disease-Related Costs
All-cause total healthcare costs were higher in the EIBasthma than the EIBalone group (US$6511 vs US$5432; P<0.0001). This was likely attributable to significantly higher outpatient costs, such as HCP office visits and pharmacy prescriptions costs in the EIBasthma versus EIBalone group (Table 4). However, all-cause inpatient costs were higher in the EIBalone than the EIBasthma group (mean costs per patient per year: US$25,542 vs US$21,013; P<0.0001).
 |
Table 4 All-Cause and Disease-Specific Healthcare Costs (US$) for Patients Aged ≥4 Years with a Diagnosis of EIBalone or EIBasthma |
Disease-specific healthcare costs for EIBalone represented 11.6% of all-cause healthcare costs in this group, while for the EIBasthma group it was 26.2%. Although disease-specific inpatient and ED costs were higher in the EIBalone than EIBasthma group (mean costs per patient per year: US$18,110 vs US$15,463; P<0.0001, and US$822 vs US$764; P=0.002, respectively), the overall total healthcare costs were higher in the EIBasthma group (US$1708 vs US$633 in the EIBalone group; P<0.0001). This was driven by significantly higher medication costs (SABA and maintenance medication) and outpatient visit costs in the EIBasthma versus EIBalone group (Table 4).
Discussion
Prescription claims and healthcare resource utilization data from 28,342 US patients with EIB, of whom 14,862 also had a diagnosis of asthma, were analyzed in this study. To the best of our knowledge, this is the first investigation of its kind in which real world evidence data is analyzed to better understand the burden of disease in patients with EIB. Systemic corticosteroid claims, healthcare resource utilization, and cost of care were high in both cohorts, suggesting considerable unmet need in patients with EIB.
Almost one-third of patients with EIBalone had ≥1 SCS claim (29.4% of patients), with 3-weeks’ worth of oral corticosteroids being filled in the post-index year. Healthcare resource use data confirmed the extent of unrecognized morbidity in EIBalone; 65.1% of patients in this group had outpatient HCP office visits associated with EIB. In the EIBasthma group, 32.0% of patients had ≥1 SCS claim in the post-index period, and similar to the EIBalone population, this group also filled close to 3 weeks’ worth of oral corticosteroids. Additionally, the majority of patients with EIBasthma (72.3%) had ≥1 outpatient office visit and 13.0% attended an ED for an asthma exacerbation.
Analysis of treatment patterns may help explain the observed morbidity associated with EIB in both sets of patients. The EIBasthma group relied heavily on SABA use, with 22.4% filling 4 or more SABA claims in the year. This would equate to at least 400 doses annually (each canister contains 200 puffs, ie 100 doses), constituting an average use of SABA at least once daily. Regular use of SABA may not only lead to an increase in bronchial hyperresponsiveness, but also a tolerance to the bronchodilator effect and an increase in bronchoconstriction in response to the stimulus of exercise.4,11,13 Patients with EIBasthma should be treated for optimum asthma control, ie with maintenance therapy, to reduce the frequency and severity of EIB and the use of SABA.1,4,9,10 Although over 90% of patients with EIBasthma were receiving maintenance therapy, the reliance on SABA suggests their asthma was uncontrolled. Indeed, the level of SCS use in this group adds further support to this explanation.
Although there was no difference in the median number of SABA fills per year between the EIBasthma and EIBalone populations (2.0), the majority of patients in the EIBalone group had one or two refills (~76%). This is what would be expected if patients were using their SABA intermittently prior to exercise, as recommended,1,4 since one canister provides 100 doses and two canisters over 52 weeks would equate to usage of 3.8 times a week, which seems reasonable for prophylactic use during regular exercise. However, 80% of patients with EIBalone in our analysis were also prescribed daily maintenance medication. Taken together with the number of SCS claims in the EIBalone group, our analysis raises several important issues that should be brought to the attention of US healthcare providers: patients classified as EIBalone may be misdiagnosed, have unrecognized asthma, or be placed on inadequate maintenance medication. EIB needs to be identified objectively as per current guidelines,2,4 and such rigor in making this diagnosis most likely occurred in a very small proportion of the groups identified in this study. For many assumed to have EIB, symptomatic assessment alone can be inadequate for assessing both the presence and severity of EIB. The effective treatment of EIB may require daily ICS with or without additional ICS prior to exercise, where mast cell degranulation due to airway drying results in inflammatory mediator release.1 In fact, it has been proposed that many EIBasthma patients have active EIB due to poor adherence to regular ICS;4 thus, as-needed ICS in EIB may be a problem in those whose asthma is of a severity requiring daily maintenance ICS to achieve asthma control. Furthermore, the increased disease severity observed in this study for patients with EIBalone may point to this condition having a more severe phenotype than is currently recognized in US clinical practice; these patients may be more similar to EIBasthma patients or those transitioning into active asthma.
We did observe differences in healthcare resource utilization between patients with EIBalone and those with EIBasthma. Patients with EIBalone had fewer disease-related hospitalizations and outpatient, urgent care, and ED visits. The higher outpatient (medical and pharmacy) costs in the EIBasthma group could be attributable to greater vigilance in clinical assessment and determining treatment plans for patients with an established asthma diagnosis, thus helping to minimize the cost of any disease-specific inpatient admissions. Since patients with EIBalone did not have an asthma diagnosis, inpatient costs may have been higher due to a more thorough clinical evaluation needed for differential diagnosis, especially as between-group differences in disease-specific inpatient costs were not attributable to differences in mean length of stay (data not shown).
Equally important for both patient populations, more attention may need to be paid to EIB symptoms so that insufficient and/or ineffective treatments can be modified to decrease morbidity. Only a very small proportion of all patients in this real world medical and pharmacy claims database identified as having an asthma diagnosis and a diagnosis of EIB (1.3% of the 1,134,143 patients with asthma), even though it is well established that EIB and asthma frequently co-occur in up to 90% of patients with asthma.16 This finding suggests EIB may be ignored or downplayed if asthma is already diagnosed. Much the same as providers under-coding for allergic asthma in patients with a pre-existing asthma diagnosis,8 failure to enter a diagnostic code for EIB when treating a patient with asthma for exercise-related symptoms may occur. Moreover, this low prevalence of EIB in the asthma population may have also resulted from patients not exercising, and therefore not experiencing EIB, or from fear of exercising and inducing EIB.
Overall, our morbidity results indicate that the currently recommended EIB treatment paradigm of SABA prior to exercise or in response to exercise-related symptoms with or without daily maintenance therapy is not optimal.1,4 For patients with EIBasthma, the level of SABA use related to exercise is excluded from National Asthma Education and Prevention Program (NAEPP) recommendations for determining asthma control17 and is not specifically mentioned in terms of determining asthma control in the Global Initiative for Asthma (GINA) 2021 guidelines.9 This suggests that the consequent potential risk of morbidity associated with SABA as prevention and/or treatment of EIB may be overlooked by clinicians and patients, as illustrated by our results. Under-explored alternative approaches include the use of as-needed ICS-formoterol or an ICS whenever SABA is taken, for patients with any severity of asthma. In a 6-week randomized study in 59 patients with mild EIBasthma, as-needed budesonide-formoterol was superior to as-needed SABA alone in reducing EIB, and non-inferior to daily budesonide maintenance therapy plus as-needed SABA.14 While as-needed budesonide-formoterol is not recommended for the management of EIB, and the NAEPP 2020 focused update does not include any revisions on their recommendations for the management of EIBasthma,17 the 2021 GINA strategy document acknowledges that when as-needed budesonide-formoterol is used to manage mild asthma, EIB can be managed by using the same inhaler as-needed prior to exercise,9 thus avoiding the need for a separate inhaler prior to exercise, and mitigating the risks associated with SABA overuse.
Limitations of this study include the reliance on administrative claims databases, which could introduce the potential for coding and data entry errors, misclassification or misdiagnosis of asthma status and healthcare resource utilization, and incomplete assessment of cost of care. Furthermore, EIB should be diagnosed on the basis of changes in lung function provoked by exercise, not on the basis of symptoms.4 The patient selection criterion of requiring those with only the index SABA fill to also have had ≥1 maintenance medication claim could have biased our observations, although this was to ensure that patients with a one-off prescription of SABA were not included. Additionally, our findings may not be generalizable to patients that have healthcare insurance types not in the database or patients that have no insurance coverage. It is therefore possible that the two groups we assessed did not represent the full spectrum of patients with EIBalone or EIBasthma. Because the data available for analysis were inherently limited by the information available in the database, we were not able to adjust for age, comorbidities, socioeconomic status, or other variables likely to influence clinical morbidity and asthma- and all-cause healthcare costs. The cost analysis of inpatient admissions was based on a small sample size without the ability to adjust for outliers, and should be interpreted cautiously. Additionally, it was not possible to determine the role of maintenance treatment adherence on the lack of asthma control observed in these patient populations. Studies with a more detailed evaluation of demographic, clinical and treatment data would be helpful in understanding the full extent and causes of morbidity in patients with EIB. Most of these limitations may be expected from a claims-based study, but because this analysis was performed on the MarketScan database, it represents actual prescribing practices and healthcare resource use in a real-world setting.
Conclusions
To our knowledge, this is the first real-world study demonstrating the considerable burden of disease associated with EIB with or without a concomitant asthma diagnosis. Our findings underscore that there may be a lack of recognition, as well as inadequate management, of EIB. Prospective research in clinical practice settings that analyze methods of diagnosis and the relationships between EIB and asthma control are needed to determine if new therapeutic paradigms for patients with EIB can impact the associated morbidity.
Abbreviations
CPI, Consumer Price Index; ED, emergency department; EIB, exercise-induced bronchoconstriction; EIBalone, exercise-induced bronchoconstriction without asthma; EIBasthma exercise-induced bronchoconstriction with asthma; GINA, Global Initiative for Asthma; HCP, healthcare professional; ICD, International Classification of Diseases; ICS, inhaled corticosteroid; LABA, long-acting β2-agonists; LM, leukotriene modifiers; NAEPP, National Asthma Education and Prevention Program; OCS, oral corticosteroid; SABA, short-acting β2-agonists; SCS, systemic corticosteroid; SD, standard deviation.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics Approval and Informed Consent
All database studies using US data that are conducted by AstraZeneca comply with the United States Health Insurance Portability and Accountability Act of 1996 Privacy Rule, which allows for use of health information that neither identifies nor provides a reasonable basis to identify an individual. This study used fully de-identified data and as such was not classified as research involving human participants as defined by 45 CFR 46.104(d)(4). For the use of fully de-identified US data, AstraZeneca complies with the procedures set forth in Sections 164.514 (a)–(b)1ii of the Health Insurance Portability and Accountability Act of 1996 Privacy Rule, which allows for use of health information that neither identifies nor provides a reasonable basis to identify an individual. Therefore, approval from an institutional review board was not sought.
Acknowledgments
We would like to thank Tracy Harrison of inScience Communications, Springer Healthcare Ltd, for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The authors also wish to thank Laura C. Moore-Schiltz, who provided assistance with initial data analyses during the time she was employed by IBM Watson Health (Cambridge, MA, United States). Joseph Tkacz’s current affiliation is Avalere Health, 1201 New York Avenue NW, Suite 1000, Washington, DC 20005.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
AstraZeneca funded the study and had a role in study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data and had final responsibility to submit for publication.
Disclosure
MJL is a speaker/on advisory board for ALK, Amgen, AstraZeneca, Novartis, Regeneron and Sanofi, and has received research support from AstraZeneca, Optinose, Regeneron and Sanofi. IAG is an AstraZeneca employee and shareholder and reports personal fees from Abbot, outside the submitted work. HNG is an AstraZeneca employee and shareholder. NG is an AstraZeneca employee and shareholder. JPT is a paid employee of IBM Watson Health, and was contracted by AstraZeneca to conduct this study. NLL has received research funding from Sanofi, GSK, Genentech, TEVA, Regeneron, Novartis, and AstraZeneca and consulting fees from AstraZeneca, GSK and Teva; served on Advisory Boards for Sanofi, AstraZeneca, Genentech, TEVA, Amgen and GSK; participated in clinical trials for Avillion, Janssen, and Gossamer; and was a Spanish speaker at a national conference on allergy that was sponsored by AstraZeneca. The authors report no other conflicts of interest in this work.
References
1. Weiler JM, Brannan JD, Randolph CC, et al. Exercise-induced broncho-constriction update-2016. J Allergy Clin Immunol. 2016;138(5):1292–1295 e1236. doi:10.1016/j.jaci.2016.05.029
2. Koya T, Ueno H, Hasegawa T, Arakawa M, Kikuchi T. Management of exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol Pract. 2020;8(7):2183–2192. doi:10.1016/j.jaip.2020.03.011
3. Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ Prim Care Respir Med. 2018;28(1):31. doi:10.1038/s41533-018-0098-2
4. Parsons JP, Hallstrand TS, Mastronarde JG, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2013;187(9):1016–1027. doi:10.1164/rccm.201303-0437ST
5. Bonini M, Palange P. Exercise-induced bronchoconstriction: new evidence in pathogenesis, diagnosis and treatment. Asthma Res Pract. 2015;1:2. doi:10.1186/s40733-015-0004-4
6. Gerow M, Bruner PJ. Exercise induced asthma. In: StatPearls. Treasure Island (FL); 2021.
7. Rodriguez Bauza DE, Silveyra P. Sex differences in exercise-induced bronchoconstriction in athletes: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17(19):7270. doi:10.3390/ijerph17197270
8. de Aguiar KB, Anzolin M, Zhang L. Global prevalence of exercise-induced bronchoconstriction in childhood: a meta-analysis. Pediatr Pulmonol. 2018;53(4):412–425. doi:10.1002/ppul.23951
9. Global Initiative for Asthma. 2021 GINA report, global strategy for asthma management and prevention; 2021. Available from: www.ginasthma.org/reports.
10. National Heart Lung and Blood Institute and the National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma (EPR-3); 2007. Available from: www.nhlbi.nih.gov/guidelines/asthma.
11. Hancox RJ. Concluding remarks: can we explain the association of beta-agonists with asthma mortality? A hypothesis. Clin Rev Allergy Immunol. 2006;31(2–3):279–288. doi:10.1385/CRIAI:31:2:279
12. Lommatzsch M, Lindner Y, Edner A, Bratke K, Kuepper M, Virchow JC. Adverse effects of salmeterol in asthma: a neuronal perspective. Thorax. 2009;64:763–769. doi:10.1136/thx.2008.110916
13. O’Connor BJ, Aikman SL, Barnes PJ. Tolerance to the nonbronchodilator effects of inhaled beta 2-agonists in asthma. N Engl J Med. 1992;327(17):1204–1208. doi:10.1056/NEJM199210223271704
14. Lazarinis N, Jorgensen L, Ekstrom T, et al. Combination of budesonide/formoterol on demand improves asthma control by reducing exercise-induced bronchoconstriction. Thorax. 2014;69(2):130–136. doi:10.1136/thoraxjnl-2013-203557
15. Bonini M, Cilluffo G, La Grutta S, et al. Anti-muscarinic drugs as preventive treatment of exercise-induced bronchoconstriction (EIB) in children and adults. Respir Med. 2020;172:106128. doi:10.1016/j.rmed.2020.106128
16. Weiler JM, Anderson SD, Randolph C, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010;105(6 Suppl):S1–47. doi:10.1016/j.anai.2010.09.021
17. National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. 2020 focused updates to the asthma management guidelines; 2020. Available from: https://www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.