Back to Journals » Clinical Ophthalmology » Volume 9
Delusional infestation: are you being bugged?
Authors Thakkar A, Ooi KG, Assaad N, Coroneo M
Received 25 October 2014
Accepted for publication 22 December 2014
Published 2 June 2015 Volume 2015:9 Pages 967—970
DOI https://doi.org/10.2147/OPTH.S76420
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Angeli Thakkar, Kenneth GJ Ooi, Nagi Assaad, Minas Coroneo
Department of Ophthalmology, Prince of Wales Hospital, Sydney, NSW, Australia
Abstract: This case report documents a 58-year-old male who presented to the clinic with a 12-month history of a burrowing sensation in his eyelids that he attributed to a parasitic infestation. After being extensively investigated and reviewed by relevant specialties, no evidence of parasitic infestation was found. He was diagnosed with and treated for blepharitis. Psychiatric referral for presumed delusional infestation (DI) was recommended. Despite this, he remained insistent in his belief of infestation, and was inevitably lost to follow-up. DI, previously known as delusional parasitosis, is a rare delusional disorder where affected individuals have a fixed, false belief that they have a parasitic infestation. Diagnosis can be challenging. Practitioners need to evaluate between primary and secondary DI carefully, as management differs depending on the etiology. Despite this, patients diagnosed with primary DI tend to be resistant to psychiatric referral. This report aims to optimize management by giving the reader a guideline for appropriate investigations and advice on patient approach. It is important to recognize hallmark features of DI to minimize self-inflicted trauma and associated psychosocial consequences. Effective treatment for DI is available, and devastating consequences, including blindness, can be avoided.
Keywords: delusions, parasitosis, infestation, ocular trauma
Case presentation
A 58-year-old man presented to an ophthalmology clinic with a 12-month history of a sensation of infestation with parasites. He felt the infestation was triggered after he had purchased a mattress from a local tip 2 years previously.
The sensation was spread across his entire body, but concentrated predominantly in his eyelids. He described a feeling of parasites jumping across and burrowing into his skin. He felt his symptoms varied according to the behavior and life cycle of the parasites, which he described in detail. He had had pustule formation and ulceration of the skin associated with this problem in the past.
In relation to his eyes, he had a chronic foreign-body sensation bilaterally, with intermittent redness, lid swelling, and discharge. In acute exacerbations, his symptoms resolved quickly with topical antibiotic medication.
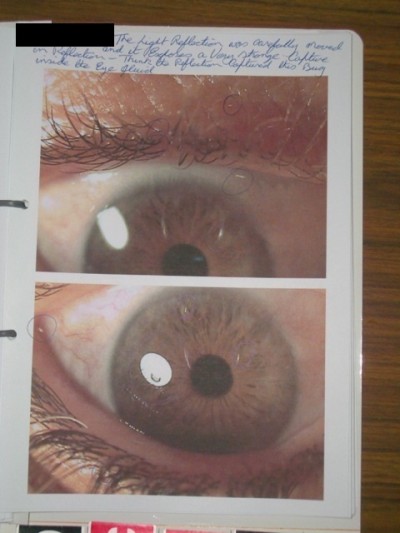
This sensation of infestation caused him great distress, and he had sought several opinions and undergone numerous investigations to confirm the presence of parasites. He attempted to document the presence of parasites on his skin through a catalog of digital photographs (Figures 1 and 2). He also mentioned that two friends in his neighborhood were having similar sensations and also having difficulties confirming the presence of parasites.
There was no significant past ophthalmic history. Medical history included hypertension, diverticulitis, and chronic back pain. His medications were irbesartan and morphine sulfate. He denied a history of illicit drug use or exposure to unusual chemicals.
Best-corrected visual acuities were 6/4 in both eyes. Intraocular pressures were normal. Slit-lamp examination revealed bilateral anterior blepharitis, with scales along the lid margin and on the lashes. There was also mild punctate fluorescein staining over his inferior bulbar conjunctiva bilaterally. The remainder of his anterior-segment examination was unremarkable, and no parasites were identified. Dilated fundus examination revealed cup:disk ratios of 0.6 with healthy neuroretinal rims bilaterally.
Eyelashes were removed for analysis, and microbiology results revealed no evidence of parasites. The patient was also reviewed by the dermatology team, who reported no evidence of primary skin disorder or infestation. Basic blood tests, including a full blood count and biochemistry, were unremarkable. Unfortunately, more extensive investigations were unable to be performed, due to the patient’s unwillingness to comply.
The patient’s symptoms of chronic ocular irritation with intermittent exacerbations were attributed to blepharitis with secondary conjunctivitis. He was instructed on lid hygiene until his symptoms settled and advised to use ocular lubricants as necessary for his irritative symptoms. A recommendation was made to review his optic disks, intraocular pressures, and visual fields annually. He refused psychiatric referral and treatment for a presumptive diagnosis of delusional infestation (DI), and was subsequently uncontactable. His general practitioner and family indicated that he spent many years after the consultation “doctor-hopping” in Australia and overseas in an attempt to find a cure for his ailment.
Ethics
This paper was written in accordance with the Declaration of Helsinki, 1995. Informed consent was obtained from the patient for publication of this case report and accompanying images.
Discussion
DI is a rare delusional disorder of a somatic subtype. Affected individuals have a fixed, false belief that they are infested by organisms, including parasites, insects, and worms. Classically, patients complain of scratching, biting, burrowing, and wriggling sensations under the skin (formication). Unlike paranoia, patients erroneously insist that their fear is rational.1
There are two subcategories: primary and secondary. Primary DI is a psychiatric disorder that refers to an isolated, persistent thought disorder of formication.2 In order for a diagnosis of primary DI, the following Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria must be met: a delusion of at least 1 month’s duration, no underlying psychiatric disorder, any coexisting mood disorder is of shorter duration than the delusion, and the delusion is not a result of substance abuse or an organic cause.3 Secondary DI refers to symptoms caused by other etiologies, and is further divided into functional and organic categories. Secondary functional DI is correlated with an underlying psychiatric disorder, eg, depression or schizophrenia, and differentiating from primary DI can be challenging. By contrast, secondary organic DI is triggered by an underlying medical illness or medication/drug use.3 Many DI cases have a history of alcohol, cocaine, and/or methamphetamine usage and references, have also been made to monoamine oxidase inhibitors, corticosteroids, and attention-deficit disorder medications.4–8 Predisposing organic medical conditions include vitamin B12 deficiency, endocrine disorders/tumors, renal disease, hypertension, heart failure, multiple sclerosis, hepatitis, syphilis, stroke, pneumonia, tuberculosis, lymphoma, acquired immunodeficiency syndrome, and Lyme disease.8–12
Like most psychiatric disorders, the pathogenesis is unclear. One theory suggests that the condition is secondary to an amplification of common, troubling symptoms, eg, pruritus triggered by a patient’s newfound awareness of a known disease.13 A minority of people may experience amplified symptoms (unclear cause) as they uncover more about the disease at hand. Furthermore, this can be perpetuated as patients may misinterpret preexisting sensations as new symptoms, reaffirming their delusion. This would be in keeping with many patients affected with DI reporting a previous history of skin disorders.14 It has been postulated that DI is associated with heightened dopamine levels in the striatum and limbic areas of the cerebral cortex secondary to anomalous dopamine-transporter protein function.15 This is supported by the efficacy of dopamine antagonists.
The true prevalence of DI is uncertain and difficult to assess, due to the varying terms used to describe the same condition and the patient’s reluctance to report their symptoms due to the judgmental connection between socioeconomic status and infestation.16 DI is associated with recurring presentations to numerous specialists, including dermatologists, general practitioners, infectious diseases specialists, and/or psychiatrists to resolve symptoms.16
DI has an insidious onset, and can affect any age-group; however, there is a higher prevalence in the fifth decade of life, with a 3:1 female predominance.8 Younger patients with DI are more likely to have the secondary form of the disorder.8 Many patients are functional; however, a minority are severely debilitated.13 Median duration of delusions is 12 months; however, tertiary care is only sought after 1.3 years, and the diagnosis itself may take several more years to ascertain.18 In 8%–10% of cases, patients living in close proximity display a shared psychosis, also known as “folie à deux”, as was the case with our patient.8 While there may not be evidence of a preceding psychiatric illness, a premorbid history of social isolation is common.
Patients usually offer exhaustive descriptions of the infesting organisms, including their life cycle. They typically produce nonspecific specimens in a small container as proof of infestation, referred to as the “matchbox sign”, or as in our case, image captured on compact disk.8 Examination of the specimens is necessary; however, generally they are found to be dirt, cloth fibers, or skin debris. Commonly, excoriation, traumatic ulcers, or secondary dermatitis following repeated scratching, hand washing, and cleansing are seen.16,17 There are also reports of corneal abrasions, lacerations, fungal infections, and vision loss resulting from patients attempting to eradicate the infesting organisms.19–21 In the case of our patient, there was no significant periorbital skin trauma; however, his history of pustule formation and skin ulceration, as well as the inferior bulbar conjunctival punctate erosions, could be consistent with psychosomatic self-inflicted trauma.
In order to diagnose primary DI, true parasitic infestation, as well as coexisting psychiatric or organic conditions, should be excluded. Initial assessments should include a mental state examination, full blood count and chemistry panel, thyroid-function test, urinalysis, and urine toxicology. Other investigations for consideration include: B12/folate, computed tomography brain scan, and microbiology of tissue samples. Psychiatry and dermatology should be consulted if indicated.
Despite an exhaustive examination and reassurance, the patient typically continues to hold onto their beliefs. They often accuse their doctor of incompetence due to an inability to identify the parasites. In order to overcome this, rapport between the patient and health professional must be established, and the patient’s symptoms must be acknowledged with empathy. Management of these patients may be challenging, as many resist psychiatric referral and commonly move on to find a new health professional who is in agreement with their beliefs.8 Unfortunately, as in our case, most patients bounce between practitioners/specialists, and are eventually lost to follow-up as they become increasingly frustrated and subsequently lose faith in the capability of the health system.
Documented cases of ocular DI are few, though they describe a similar progression. The case reports by Sherman et al describe four patients, none of which continued with formal psychiatric evaluation.21 In contrast, Meraj et al illustrated a case of DI resulting in self-inflicted corneal abrasions, where the patient agreed to inpatient psychiatric evaluation and subsequently received group therapy and insight-oriented counseling. He later agreed to be treated with olanzapine.19 This stresses the importance of developing a therapeutic connection, and even in some cases conceding that the symptoms and even the infestations are real in order to facilitate treatment.
Antipsychotics are the mainstay of treatment, and should be commenced after the diagnosis has been established. Previously, the first line was pimozide; however, it has been replaced due to its harmful side effects.22 Subsequently, atypical antipsychotics, such as olanzapine, have been deemed the medication of choice. One study demonstrated that primary DI required 10 weeks of treatment for maximum effect, compared to 3 weeks in secondary DI. Overall, full remission was achieved in approximately 70% of patients on olanzapine.23 More recently, aripiprazole has been reported for the treatment of DI with ocular and dermatologic presentations.24 Management should be tailored to each individual patient, and in some situations concurrent medications, such as anxiolytics, antidepressants, and corticosteroid creams, may be beneficial.
This report aims to create awareness of DI with ophthalmic manifestations among health professionals. DI continues to be a diagnostic and management challenge for physicians. Early recognition and psychiatric referral and treatment may improve the likelihood of an advantageous outcome, including that of vision preservation.
Author contributions
AT carried out the literature review and drafted the manuscript. KO was involved in drafting the manuscript and designing the case report. NA and MC were responsible for the clinical management of the patient, participated in the design of the case report, and revised the manuscript. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Bishop ER Jr. Monosymptomatic hypochondriacal syndromes in dermatology. J Am Acad Dermatol. 1983;9(1):152–158. | ||
Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47–53. | ||
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington (VA): APA; 2013. | ||
Catalano MC, Glass JM, Catalano G, Burrows SL, Lynn WA, Weitzner BS. Gamma butyrolactone (GBL) withdrawal syndromes. Psychosomatics. 2001;42(1):83–88. | ||
Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988; 42(4):273–274. | ||
Marschall MA, Dolezal RF, Cohen M, Marschall SF. Chronic wounds and delusions of parasitosis in the drug abuser. Plast Reconstr Surg. 1991;88(2):328–330. | ||
Riding J, Munro A. Pimozide in the treatment of mono-symptomatic hypochondriacal psychosis. Acta Psychiatr Scand. 1975;52(1):23–30. | ||
Lyell A. The Michelson Lecture. Delusions of parasitosis. Br J Dermatol. 1983;108(4):485–499. | ||
Wilson JW, Miller HE. Delusions of parasitosis (acarophobia). Arch Dermatol Syphilol. 1946;54:39–56. | ||
Berrios GE. Delusional parasitosis and physical disease. Comp Psychiatry. 1985;26(5):395–403. | ||
Holmes VF. Treatment of monosymptomatic hypochondriacal psychosis with pimozide in an AIDS patient. Am J Psychiatry. 1989; 146(4):554–555. | ||
Saverly VR, Leitao MM, Stricker RB. The mystery of Morgellons disease: infection or delusion? Am J Clin Dermatology. 2006;7(1):1–5. | ||
Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med. 1999;130(11):910–921. | ||
Lyell A. Delusions of parasitosis. J Am Acad Dermatol. 1983;8(6):895–897. | ||
Huber M, Kirchler E, Karner M, Pycha R. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351–1358. | ||
Bak R, Tumu P, Hui C, Kay D, Burnett J, Peng D. A review of delusions of parasitosis, part 1: presentation and diagnosis. Cutis. 2008; 82(2):123–130. | ||
Driscoll MS, Rothe MJ, Grant-Kels JM, Hale MS. Delusional parasitosis: a dermatologic, psychiatric and pharmacologic approach. J Am Acad Dermatol. 1993;29(6):1023–1023. | ||
Trabert W. 100 Years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238–246. | ||
Meraj A, Din AU, Larsen L, Liskow BI. Self inflicted corneal abrasions due to delusional parasitosis. BMJ Case Rep. 2011:1–4. | ||
Trager MJ, Hwang TN, McCulley TJ. Delusions of parasitosis of the eyelids. Ophthal Plast Reconstr Surg. 2008;24(4):317–319. | ||
Sherman MD, Holland GN, Holsclaw DS, Weisz JM, Omar OH, Sherman RA. Delusions of ocular parasitosis. Am J Ophthalmol. 1998;125(6):852–856. | ||
Meehan WJ, Badreshia S, Mackley CL. Successful treatment of delusions of parasitosis with olanzapine. Arch Dermatol. 2006;142(3):352–355. | ||
Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500–508. | ||
Huang WL, Chang LR. Aripiprazole in the treatment of delusional parasitosis with ocular and dermatologic presentations. J Clin Psychopharmacol. 2013;33(2):272–273. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.