Back to Journals » OncoTargets and Therapy » Volume 9
Deferred radiotherapy and upfront procarbazine–ACNU–vincristine administration for 1p19q codeleted oligodendroglial tumors are associated with favorable outcome without compromising patient performance, regardless of WHO grade
Authors Hata N , Yoshimoto K, Hatae R, Kuga D, Akagi Y, Suzuki SO, Iwaki T , Shono T, Mizoguchi M, Iihara K
Received 27 June 2016
Accepted for publication 23 September 2016
Published 17 November 2016 Volume 2016:9 Pages 7123—7131
DOI https://doi.org/10.2147/OTT.S115911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Li
Nobuhiro Hata,1,2 Koji Yoshimoto,1 Ryusuke Hatae,1 Daisuke Kuga,1 Yojiro Akagi,1 Satoshi O Suzuki,3 Toru Iwaki,3 Tadahisa Shono,1,4 Masahiro Mizoguchi,1,5 Koji Iihara1
1Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, 2Department of Neurosurgery, Clinical Research Institute, National Hospital Organization Kyushu Medical Center, 3Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, 4Department of Neurosurgery, Harasanshin Hospital, Fukuoka, 5Department of Neurosurgery, Kitakyushu Municipal Medical Center, Kitakyushu, Japan
Abstract: Recently updated phase III trials revealed the favorable effect of add-on procarbazine-lomustine-vincristine chemotherapy (CT) to radiotherapy (RT) in treating anaplastic oligodendrogliomas with 1p19q codeletion (codel). However, the underlying rationality of deferring RT and upfront CT administration for these tumors is yet to be elucidated. Here, we retrospectively analyzed the long-term outcome of our case series with oligodendroglial tumors treated with deferred RT and upfront procarbazine+nimustine+vincristine (PAV) in the introduction administration. We enrolled 36 patients with newly diagnosed oligodendroglial tumors (17, grade II and 19, grade III) treated during 1999–2012 and followed up for a median period of 69.0 months. Their clinical and genetic prognostic factors were analyzed, and progression-free survival, overall survival (OS), and deterioration-free survival (DFS) were evaluated. Regardless of the WHO grade, the 25 patients with 1p19q codel tumors never received RT initially, and of these 25, 23 received PAV treatment upfront. The 75% OS of patients with 1p19q codel tumor was 135.3 months (did not reach the median OS), indicating a favorable outcome. Multivariate analysis revealed that IDH mutation and 1p19q, not WHO grade, are independent prognostic factors; furthermore, IDH and 1p19q status stratified the cohort into 3 groups with significantly different OS. The DFS explained the prolonged survival without declining performance in patients with both grade II and III 1p19q codel tumors. Deferred RT and upfront PAV treatment for 1p19q codel oligodendrogliomas were associated with favorable outcomes without compromising performance status, regardless of WHO grade.
Keywords: oligodendroglioma, IDH, PAV, 1p19q codel
Introduction
Oligodendroglial tumors are relatively rare brain tumors, and the standard treatment remains controversial. However, since the efficacy of procarbazine+lomustine+vincristine (PCV) chemotherapy (CT) for these tumors was reported,1 PCV with or without radiotherapy (RT) had become the most common therapeutic approach.2 Thereafter, temozolomide (TMZ) replaced PCV overwhelmingly,3 and this substitution was accelerated by the interval reports of phase III trials, RTOG 9402 and EORTC 26951, which reported that PCV as an add-on treatment to RT showed no improvement in the overall survival (OS) of patients with anaplastic oligodendrogliomas.4,5 The benefit of TMZ in treating oligodendroglial tumors is presently being evaluated by 2 large ongoing phase III trials, CATNON and CO-DEL. Meanwhile, recently updated trials RTOG 9402 and EORTC 26951 demonstrated that the addition of PCV to RT is associated with an improved long-term OS of patients with 1p19q codeleted (1p19q codel) anaplastic oligodendroglioma, and this regimen has been reinstituted as the standard treatment for these tumors.6,7 Another recent trial proved the positive effect of PCV also for patients with 1p19q codel grade II oligodendroglioma.8 The efficacy of PCV treatment for 1p19q codel oligodendroglioma was also revealed by studies reporting the long-term follow-up of growth kinetics of the tumors, showing prolonged decrease in tumor volume during and after treatment.9,10
Another controversial issue is whether upfront CT can be a treatment strategy for oligodendroglial tumors. An international retrospective analysis showed that in recent years, patients with 1p19q codel anaplastic oligodendroglial tumors were significantly more likely to receive upfront CT.11 A previous retrospective study indicated that deferring RT did not compromise the OS of patients with 1p19q codel oligodendroglial tumors.12 Upfront CT is an attractive treatment strategy for 1p19q codel oligodendroglial tumor patients who are more likely to develop cognitive dysfunction due to early RT in their highly expected long-term survival duration.13
In our institute, in order to avoid early neurocognitive deterioration, deferring RT with upfront procarbazine+nimustine+vincristine (PAV) CT has been practiced for newly diagnosed oligodendroglial tumors since 1999. This treatment strategy has been stratified by 1p19q status since 2004, regardless of WHO grade. We herein report the results of long-term follow-up of our institutional case series. The primary goal of our study is the evaluation of the rationale behind deferring RT with upfront CT for oligodendroglial tumors by analyzing survival data. The secondary goal is the analysis of the correlation between the patient’s genetic background and outcome.
Methods
Patients
In our institute, all patients with gliomas are registered in the brain tumor database of our department. From this database, we extracted the information of newly diagnosed oligodendroglial tumor patients treated between 1999 and 2012. Among them, 36 patients whose 1p19q status was determined using snap-frozen tumor tissue samples obtained by surgery were enrolled. Based on the WHO Classification of Tumours of the Central Nervous System, 4th Edition, published in 2007, the tumors were diagnosed histologically by qualified neuropathologists (SOS and TI) as follows: oligodendrogliomas, 15; anaplastic oligodendrogliomas, 15; oligoastrocytomas, 2; and anaplastic oligoastrocytomas, 4.
PAV treatment
The PAV treatment regimen was as follows: nimustine (ACNU), 75 mg/body; procarbazine, 100 mg/(body·day) on days 8–21; and vincristine, 2.0 mg/body on days 8 and 29 in cycles of 6 weeks. A maximum of 6 cycles were performed, although the intervals were modified by the physician according to the side effects.
Samples and DNA preparation
A part of the surgical specimens was saved for histopathological examination; the rest was snap-frozen in liquid nitrogen and stored at −80°C. Tumor and corresponding wild-type DNA extraction was carried out using the QIAamp DNA mini Kit (Qiagen Science, Germantown, MD, USA) following the manufacturer’s protocol. The present investigation was approved by the Ethics Committee of Kyushu University and complied with the current laws of the country in which it was performed. Written informed consent was obtained from all participants.
Genetic analysis
For IDH1 and IDH2, polymerase chain reaction (PCR) and sequencing were performed as described previously.14 The allelic status of chromosomes 1p, 10, and 19q was evaluated using a PCR-based loss of heterozygosity (LOH) assay as described previously.14 To evaluate 1p and 19q codel, 10 microsatellite markers, 3 on the distal side of 1p (1p36), 3 on the 1p centromere side, and 4 on 19q, were used in the LOH analysis. In the present study, 1p19q codel was defined as the complete loss of both chromosome arms and was determined when all the informative markers of 1p and 19q showed LOH.
Statistical analyses
The objective of this study was to assess OS, progression-free survival (PFS), and deterioration-free survival (DFS), measured in months, with censoring at the date of last follow-up for survivors. In the present study, “progression” was defined according to the RANO criteria15 and “deterioration” was defined as the decline of performance status, determined by at least a 10-point decrease in the Karnofsky performance score,16 relating to disease progression or treatment-related events including neurocognitive dysfunction due to RT, radiation necrosis, or vincristine-associated severe polyneuropathy. The Kaplan–Meier analysis was used to evaluate OS, PFS, and DFS, and the log-rank test was used for comparing the survival distributions. Cox’s proportional hazards regression models were used to estimate the hazard ratios with a 95% confidence interval for the putative prognostic factors. All P-values were two sided, and a level of 0.05 was considered statistically significant. JMP Pro v. 11.0.0 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patient characteristics
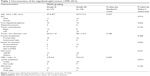
All 36 patients were followed up for a median period of 69.0 months. Their putative prognostic factors are summarized in Table 1. Except the prevalence rate of 1p19q codel (P=0.031), no significant bias was noted between the clinical factors of grade II and III tumors. Furthermore, the difference in IDH status (P=0.106) was less significant than that in 1p19q status; however, this was probably a biased factor because all IDH wild-type tumors are grade III. Notably, 1p19q codel exclusively occurred in IDH1/2 mutant tumors, ie, according to the genetic stratification of grade II–III gliomas in our previous study, 1p19q and IDH status segregated our cohort into the following 3 genetic subtypes:17 Type I, 1p19q codel and IDH mutation (n=25); Type II, 1p19q noncodel and IDH mutation (n=7); and Type III, 1p19q noncodel and IDH wild-type (n=4). Putative prognostic factors according to tumor type are summarized in Table 2. LOH on chromosome 10 was detected exclusively in 3 patients categorized with Type III tumors. In addition, Type III tumors were prone to show unusual characteristics, such as astrocytic components, nonfrontal localization, and high MIB-1 index, as well as being more common in older age patients.
  | Table 2 Characteristics of tumor Types I–III |
Treatments
In our institute, all patients with oligodendroglial tumors had been treated with PAV CT upfront during 1999–2004. Thereafter, patients with 1p19q codel oligodendroglial tumors (Type I) have been treated with PAV CT until present, and those with noncodel tumors (Types II and III) were treated with concurrent radio-CT (ACNU-IFN/RT; 2004–2006 and TMZ/RT, 2006–present), if consent was obtained. Thus, treatments for Type II and III tumors varied over the years. Of the 25 patients with Type I tumors, none received initial RT. Twenty-three patients were treated with PAV upfront instead of initial RT (Table 3), while 2 patients had a different treatment course as per their personal preference (not their physician’s recommendation). Both these patients had 1p19q codel oligodendroglioma (grade II). One requested prolonged CT after 5 cycles of PAV, underwent ACNU therapy for 2 years, developed a recurrent tumor 4 years later, which was completely removed, and underwent concurrent TMZ-RT followed by TMZ maintenance. Currently, 6 years after tumor onset, the patient is alive. The other patient refused adjuvant treatment, underwent recraniotomy for the progression of the tumor 4 years later, and although no progression was noted, he died due to myocardial infarction 6 years after tumor onset (his OS has been censored).
  | Table 3 First-line treatment of primary oligodendroglial tumors |
Upfront PAV CT
Among the 23 patients treated with PAV upfront, 10 discontinued before 6 cycles because of tumor progression (n=2), toxicity (n=5; myelosuppression, 3; liver dysfunction, 1; and stomach pain, 1) or 3 cycles of treatment as decided by the physician (n=3). The remaining 13 patients underwent 6 cycles of treatment; however, 1 patient discontinued procarbazine due to dermatitis and 2 were administered ACNU monotherapy at midtreatment due to severe polyneuropathy and patients’ own preference.
Treatment after progression
Among the 23 patients with Type I tumors treated with PAV upfront, 13 developed progressive lesions, 7 remained progression-free, and 3 remained progression-free but died due to reasons unrelated to the disease. After progression, 2 of the 13 were initially treated with PAV for the second time, followed by recraniotomy and RT after tumor regrowth; 2 others received concurrent TMZ-RT; and the remaining 9 underwent recraniotomy. Of the 9 patients who underwent recraniotomy, 7 were treated with concurrent TMZ-RT and maintenance TMZ, 1 was treated with TMZ monotherapy for 1 year, and the remaining 1 refused adjuvant treatment. In all, 11 of the 23 Type I tumor patients treated with PAV upfront consequently received RT due to progression. The Kaplan–Meier analysis showed that the median RT-free survival in the 23 Type I tumor patients was 104.8 months. All the patients with noncodeleted tumors treated with upfront PAV (n=4: Type II =2, Type III =2) developed progressive lesions within several years (range, 3.5–40.0 months) and underwent RT. Among them, 3 patients died shortly after treatment, and 1 patient remains recurrence-free 2 and a half years later.
Survival analyses
The median follow-up time of the enrolled 36 patients was 69.0 months (range, 6.5–169). The median PFS of patients with Type I tumor was 59.1 months (Figure 1A). The 75% OS of patients with Type I tumors was 135.3 months (did not reach the median OS; Figure 1B). Although IDH was a significant prognostic factor according to PFS (Table 4), no significant difference in PFS was seen between patients with 1p19q codel and nondel tumors. On the other hand, screening of the putative prognostic factors by univariate analyses suggested that WHO grade, tumor laterality, and 1p19q and IDH status were significantly associated with OS (Table 4). Further multivariate analysis of OS confirmed that 1p19q and IDH status remained as the independent prognostic factors (Table 4). These findings were validated using the Kaplan–Meier analyses of genetic stratification. Patients with Type I and II tumors showed prolonged PFS compared to those with Type III tumor (Type I vs II, P=0.71; Type II vs III, P=0.004). The 75% PFSs of Type I and II tumor patients were 33.0 and 32.8 months, respectively, whereas that of Type III was 6.9 months (Figure 1A). OS differed significantly among all the patients (Type I vs II, P=0.0031; Type II vs III, P=0.004); 75% OS of patients with Type I, II, and III tumors were 135.3, 60.6, and 8.9 months, respectively (Figure 1B). Additionally, the independent effect of 1p19q, not WHO grade, was implied by the OS of patients with 1p19q codel grade III, which significantly approximated to codel grade II, not nondel grade III (codel grade II vs III, P=0.93; grade III codel vs nondel, P=0.002; Figure 2). Notably, the effect of 1p19q showed the discrepancy between PFS and OS (Figure 2A and B). We hypothesized that this was at least partly due to the fact that the progressions after upfront PAV treatment for 1p19q codel tumors generally appear as less aggressive lesions that barely affect patient performance and can be well controlled by second-line treatments, as seen in our case series (Figure 3). The evaluation of DFS verified this speculation, as shown in Figure 2C, presenting a favorable survival with maintained performance in patients with 1p19q codel grade III as well as grade II tumors, and this was significantly different in those with 1p19q nondel tumors (1p19q codel grade II vs III, P=0.78; grade III codel vs nondel, P=0.01). The 75% DFSs of patients with 1p19q codel grade II and III and nondel grade III were 133.5, 93.1, and 8.9 months, respectively. These results indicate that deferring RT with upfront CT treatment was associated with the long-term survival of patients with 1p19q codel tumors without early deterioration of performance, regardless of WHO grade.
Discussion
Our retrospective study demonstrated that the genetic markers 1p19q and IDH showed higher correlation with the outcome of patients with oligodendroglial tumors than did other putative prognostic factors; nonetheless, intensive treatments were administered initially to the patients in the poorer prognostic group. WHO grade was also seen to be a significant prognostic factor according to univariate analysis; however, the confounding effect of 1p19q codel was strongly suspected according to the multivariate analysis and the bias of the prevalence rate of 1p19q codel between WHO grades II and III. Furthermore, the outcome of oligodendroglial tumors was clearly segregated by subtyping based on IDH and 1p19q status. Taken together, the present study proved that for oligodendroglial tumors, the prognostic effect of genetic stratification supersedes that of WHO grading. The concept that 1p19q and IDH status can stratify anaplastic oligodendrogliomas into 3 groups showing distinct prognoses has been proposed previously.18–20 The survival curve of 1p19q codel grade III tumors was noted to be similar to that of 1p/19q codel grade II, but not 1p19q noncodel grade III; nonetheless, we treated patients with PAV upfront deferring RT, regardless of WHO grade. These results indicated that deferring RT and administering PAV upfront for oligodendrogliomas should be performed based on the stratification of genetic markers, not WHO grade.
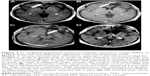
Our study showed the effect of 1p19q status only by OS not by PFS. Our study is not sufficient for assessing PFS results, because of the difference in treatment for each type of tumor that fundamentally displays different prognoses. However, these results were at least partly due to the fact that we deferred RT initially for patients with 1p19q codel tumors and this could prolong tumor progression. Instead, we found a favorable DFS of patients with 1p19q codel tumors, suggesting that upfront PAV administration could provide favorable survival for patients with 1p19q codel tumors without compromising their performance even though disease progression began early in their clinical course. Our case series included examples of patients who developed tumor progressions recurring as less aggressive lesions around the residual tumor or resection cavity, as shown in Figure 3, and these progressions were associated with no clinical deterioration. It is well known that long-term survivors of low-grade glioma who received RT showed a progressive decline in cognitive function, including the patients who received fractional doses that are regarded as safe.21 A subanalysis of the EORTC 26951 trial identified that a substantial proportion of long-term progression-free survivors who had undergone RT for anaplastic oligodendroglioma suffer from cognitive impairment.22 Taken together, our retrospective study shows that in patients with 1p19q codel tumors, deferring RT and administering CT upfront maintained performance without compromising survival.
Our case series showed that majority of the patients with 1p19q codel oligodendrogliomas, including those with grade II and III tumors, lived for over 10 years and their 75% OS was 135.3 months (did not reach the median OS). This outcome is almost equivalent to that of the 1p19q codel grade II oligodendrogliomas in a recent large clinical trial8 and is favorable in comparison with those of patents with 1p19q codel anaplastic oligodendroglioma treated with PCV and RT in RTOG 9402 and EORTC 26951 trials.6,7 Grade II tumors included in our case series were not associated with the superior outcome because, as shown in Figure 2B, our grade II and III tumors with 1p19q codel showed similar outcomes. As a previous report13 regarding PAV treatment showed a favorable outcome comparable to that of our study, the difference between PAV and PCV might be noteworthy. Nonetheless, the most important difference between the 2 large clinical trials and our study was the timing of RT – initial and deferred. RT-related toxicities such as brain atrophy or radiation necrosis might have led to a negative effect in previous clinical trials. In our study, we could defer RT administration to 1p19q codel tumors for a mean interval of 104.8 months without compromising the performance of patients as shown by DFS analysis, indicating the efficacy of deferred RT and upfront PAV administration. We evaluated the DFS in our study by using the Karnofsky performance score, which may not be sufficiently sensitive to detect several aspects of quality of life and neurocognitive function that are generally important end points in contemporary neuro-oncology studies.
Our study has several limitations, such as the large sample size differences among tumor types, insufficient clinical data related to baseline characteristics, incomplete exclusion of potential selection biases due to nonrandomized study design, the small number of patients treated at a single institution, and the follow-up time; also, a median follow-up time of 69 months is not long enough for oligodendroglial tumors. In contrast to our study, recent clinical trials, such as RTOG 9802, EORTC-intergroup study, and CODEL, suggested that upfront CT alone may compromise survival, and deferring RT may be detrimental for OS.8 Future clinical trials elucidating the effect of deferred RT for oligodendrogliomas are warranted.
Another unsolved issue is whether PAV, as well as PCV, can be replaced by TMZ. No randomized controlled study has compared the efficacy of PCV and TMZ one-on-one in the treatment of oligodendroglial tumors. Meanwhile, the comparable efficacy of these 2 regimens has been implied by several studies.23,24 On the contrary, there are reports proposing the superiority of PCV to TMZ. A previous retrospective study demonstrated that patients treated with PCV can achieve prolonged and persistent responses, despite a shorter duration of treatment, than those treated with TMZ.9 In addition to the replacement by TMZ, modified PCV regimens, such as PC alone, has been attempted in previous studies.25 As shown in our results as well as previous reports,26 a considerable number of patients discontinued PCV treatment due to its toxicities. Therefore, replacing PCV with regimens with a favorable adverse effect profile could be beneficial.
In this study, we estimated the 1p19q status by LOH analysis using multiple microsatellite markers of 1p and 19q. A recent next-generation sequencing study, having unveiled more 1p19q codel than fluorescence in situ hybridization (FISH), validated the overall accuracy of LOH analysis in estimating 1p19q status by using multiple microsatellite markers.27 FISH is the most frequently used technique in routine diagnostic laboratories. However, it cannot be used to clearly distinguish between partial and complete loss of a chromosomal arm. 1p19q codel in oligodendrogliomas is due to the translocation of unbalanced t(1;19).28 Partial losses of 1p in oligodendrogliomas were proved to be associated with poorer survival than that with complete losses.29 Thus, it is vital that stringent criteria of 1p19q codel, like those used in the current study, be employed in future clinical trials evaluating the effect of genetic stratification in the treatment of oligodendroglial tumors.
Acknowledgment
This work was supported by JSPS KAKENHI, grant numbers 26462185, 25293311, 15K15529 and 16K10779.
Disclosure
The authors report no conflicts of interest in this work.
References
Cairncross JG, Macdonald DR. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann Neurol. 1988;23(4):360–364. | ||
Engelhard HH, Stelea A, Mundt A. Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment, and prognosis. Surg Neurol. 2003;60(5):443–456. | ||
Abrey LE, Louis DN, Paleologos N, et al. Survey of treatment recommendations for anaplastic oligodendroglioma. Neuro Oncol. 2007;9(3):314–318. | ||
Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707–2714. | ||
van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715–2722. | ||
Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. | ||
van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. | ||
Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. | ||
Peyre M, Cartalat-Carel S, Meyronet D, et al. Prolonged response without prolonged chemotherapy: a lesson from PCV chemotherapy in low-grade gliomas. Neuro Oncol. 2010;12(10):1078–1082. | ||
Taal W, van der Rijt CC, Dinjens WN, et al. Treatment of large low-grade oligodendroglial tumors with upfront procarbazine, lomustine, and vincristine chemotherapy with long follow-up: a retrospective cohort study with growth kinetics. J Neurooncol. 2015;121(2):365–372. | ||
Panageas KS, Iwamoto FM, Cloughesy TF, et al. Initial treatment patterns over time for anaplastic oligodendroglial tumors. Neuro Oncol. 2012;14(6):761–767. | ||
Lassman AB, Iwamoto FM, Cloughesy TF, et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13(6):649–659. | ||
Iwadate Y, Matsutani T, Shinozaki N, Saeki N. Anaplastic oligodendroglial tumors harboring 1p/19q deletion can be successfully treated without radiotherapy. Anticancer Res. 2011;31(12):4475–4479. | ||
Ma X, Yoshimoto K, Guan Y, et al. Associations between microRNA expression and mesenchymal marker gene expression in glioblastoma. Neuro Oncol. 2012;14(9):1153–1162. | ||
Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. | ||
Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–2007. | ||
Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–468. | ||
Labussiere M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010;74(23):1886–1890. | ||
Frenel JS, Leux C, Loussouarn D, et al. Combining two biomarkers, IDH1/2 mutations and 1p/19q codeletion, to stratify anaplastic oligodendroglioma in three groups: a single-center experience. J Neurooncol. 2013;114(1):85–91. | ||
Jiang H, Ren X, Cui X, et al. 1p/19q codeletion and IDH1/2 mutation identified a subtype of anaplastic oligoastrocytomas with prognosis as favorable as anaplastic oligodendrogliomas. Neuro Oncol. 2013;15(6):775–782. | ||
Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. | ||
Habets EJ, Taphoorn MJ, Nederend S, et al. Health-related quality of life and cognitive functioning in long-term anaplastic oligodendroglioma and oligoastrocytoma survivors. J Neurooncol. 2014;116(1):161–168. | ||
Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. | ||
Speirs CK, Simpson JR, Robinson CG, et al. Impact of 1p/19q codeletion and histology on outcomes of anaplastic gliomas treated with radiation therapy and temozolomide. Int J Radiat Oncol Biol Phys. 2015;91(2):268–276. | ||
Webre C, Shonka N, Smith L, Liu D, De Groot J. PC or PCV, that is the question: primary anaplastic oligodendroglial tumors treated with procarbazine and CCNU with and without vincristine. Anticancer Res. 2015;35(10):5467–5472. | ||
Tabouret E, Reyes-Botero G, Dehais C, et al. Relationships between dose intensity, toxicity, and outcome in patients with oligodendroglial tumors treated with the PCV regimen. Anticancer Res. 2015;35(5):2901–2908. | ||
Dubbink HJ, Atmodimedjo PN, Kros JM, et al. Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro Oncol. 2016;18(3):388–400. | ||
Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66(20):9852–9861. | ||
Idbaih A, Marie Y, Pierron G, et al. Two types of chromosome 1p losses with opposite significance in gliomas. Ann Neurol. 2005;58(3):483–487. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.