Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
"De-escalation" strategy using micafungin for the treatment of systemic Candida infections: budget impact in France and Germany
Authors van Engen A, Casamayor M, Kim S, Watt M, Odeyemi I
Received 10 May 2017
Accepted for publication 22 August 2017
Published 5 December 2017 Volume 2017:9 Pages 763—774
DOI https://doi.org/10.2147/CEOR.S141548
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Anke van Engen,1 Montserrat Casamayor,2 Soyoung Kim,3 Maureen Watt,4 Isaac Odeyemi4
1Quintiles Consulting, Hoofddorp, the Netherlands; 2Quintiles Consulting, Barcelona, Spain; 3Astellas Pharma Singapore Pte., Singapore; 4Astellas Pharma Inc., Chertsey, UK
Background: The incidence of azole-resistant Candida infections is increasing. Consequently, guidelines for treating systemic Candida infection (SCI) recommend a “de-escalation” strategy: initial broad-spectrum antifungal agents (e.g., echinocandins), followed by switching to fluconazole if isolates are fluconazole sensitive, rather than “escalation” with initial fluconazole treatment and then switching to echinocandins if isolates are fluconazole resistant. However, fluconazole may continue to be used as first-line treatment in view of its low acquisition costs. The aim of this study was, therefore, to evaluate the budget impact of the de-escalation strategy using micafungin compared with the escalation strategy in France and Germany.
Methods: A budget impact model was used to compare de-escalation to escalation strategies. As well as survival, clinical success (resolution/reduction of symptoms and radiographic abnormalities associated with fungal infection), was considered, as was mycological success (eradication of Candida from the bloodstream). Health economic outcomes included cost per health state according to clinical success and mycological success, and budget impact. A 42-day time horizon was used.
Results: For all patients with SCI, the budget impact of using de-escalation rather than escalation was greater, but improved rates of survival, clinical success and mycological success were apparent with de-escalation. In patients with fluconazole-resistant isolates, clinical success rates and survival were improved by ~72% with de-escalation versus escalation, producing cost savings of €6,374 and €356 per patient in France and Germany, respectively; improvements of ~72% in mycological success rates with de-escalation versus escalation did not translate into cost savings.
Conclusion: Modeling provides evidence that when treating SCI in individuals at risk of azole-resistant infections, de-escalation from micafungin has potential cost savings associated with improved clinical success rates.
Keywords: Candida, de-escalation, fluconazole, micafungin
Background
Recent years have witnessed a worldwide increase in invasive fungal infections.1 This reflects an increase in the number of immunocompromized patients in the nosocomial setting (e.g., patients with cancer and chemotherapy-induced neutropenia) and prolonged survival of patients, which increases the risk of opportunistic fungal infections.2
The list of fungi causing serious life-threatening infections grows each year, but Candida species are the predominant cause of opportunistic fungal infections worldwide.3 Systemic Candida infections (SCIs) commonly cause morbidity and mortality, prolong hospitalization and result in substantial care-related costs.4–6 For example, the estimated mortality may be as high as 47%, and the additional cost of each episode of invasive candidiasis in hospitalized adults was estimated to be ~US$40,000.7 Historically, Candida albicans has accounted for most fungal infections, and fluconazole is an established treatment option.8,9 Consequently, there has been widespread use of fluconazole to treat and prevent SCI.10,11 Prophylaxis with fluconazole has increased over the last decade,12 and this has coincided with a shift in the epidemiology of Candida species toward non-albicans species.13 For example, population-based surveillance programs in the northern hemisphere have shown that Candida glabrata may account for up to 30% of bloodstream infections among adult and elderly patients, while Candida parapsilosis is more prevalent in pediatric patients and in southern or Pacific parts of the world.14
Non-albicans Candida species such as C. glabrata and C. krusei can be resistant to azoles,11 and infection with fluconazole-resistant isolates can be associated with increased mortality: in a prospective cohort study of 96 hospitalized patients with candidemia, the odds ratio (95% confidence interval [CI]) for death in those with fluconazole-resistant isolates was 5.3 (0.8–33.4), P=0.08.15
The echinocandins are the most recent class of antifungal agents with potent fungicidal activity against Candida species, including azole-resistant pathogens.16 Although concerns have arisen concerning management of C. parapsilosis, in view of its reduced susceptibility to echinocandins, recent data have suggested that initial use of an echinocandin-based therapeutic regimen does not appear to negatively influence outcomes17 – initial use of an echinocandin was not associated with an increased risk of clinical failure (all-cause mortality between days 3 and 30, or persistent candidemia for ≥72 hours after initiation of therapy).17 These results confirm a previous meta-analysis that found comparative efficacy between echinocandins and non-echinocandins against C. parapsilosis infection.18 Micafungin is an echinocandin with a broad spectrum of activity against Candida species, including fluconazole-resistant Candida,19 and is the subject of the current analysis.
Treatment guidelines for the management of invasive Candida infection7,20 include recommendations for initial use of broad-spectrum antifungal agents, such as an echinocandin, with a subsequent switch to fluconazole if isolates are shown to be fluconazole sensitive (minimum inhibitory concentration [MIC] ≤8 mg/L). This strategy, known as “de-escalation,” involves prescribing a treatment regimen intended to cover the most likely pathogens associated with infection, aiming to minimize resistance by narrowing the regimen when the pathogen susceptibility profile has been determined as well as by using the shortest clinically acceptable course of therapy.21 Nevertheless, fluconazole has traditionally often been regarded as the preferred first-line antifungal therapy in clinical practice because of its demonstrated efficacy against C. albicans and low acquisition costs.9 In such instances, treatment is switched to a broad-spectrum antifungal agent such as an echinocandin only if Candida isolates are subsequently identified as non-albicans species or fluconazole resistant, a strategy known as “escalation.”22 However, it has been shown that inappropriate antifungal therapy, such as treatment delay or incorrect dosing, increases mortality, hospital length of stay and hospital costs.6 For example, a retrospective analysis of candidemia in an Italian hospital showed that 30-day crude mortality was ~20% in patients receiving appropriate antifungal therapy within 48 hours, increasing to 27%–33% with administration of antifungals after 48–72 hours, 53%–56% after 72–96 hours, and 57%–60% after >96 hours; when no antifungal treatment was administered, mortality reached 71%–75%.23 Given that fluconazole-resistant isolates account for an increasing proportion of SCIs, prompt initiation of broad-spectrum antifungal treatment is likely to benefit patient outcomes. Indeed, a recent analysis comparing de-escalation from the echinocandin, micafungin, to escalation from fluconazole in the treatment of patients with SCI22 found the former approach to be cost-effective from a UK National Health Service perspective. As France and Germany are two of the most populous countries in Europe,24 we now describe an evaluation of the budget impact of the de-escalation strategy (initial micafungin treatment switching to fluconazole if isolates are fluconazole sensitive) compared with the escalation strategy (initial fluconazole with switching to micafungin if isolates are fluconazole resistant) in these two countries.
Methods
Model structure
A budget impact model, based on the cost-effectiveness model used by Masterton et al,22 was used to compare a de-escalation strategy using micafungin as initial treatment to an escalation strategy in which patients initially received fluconazole (Figure S1). Ethical approval for the model was not required.
The model used several key assumptions:22 patients received treatment as soon as samples were taken for laboratory analysis; SCI was identified in all patients and results from susceptibility testing of Candida isolates were available within 3 days of treatment initiation; patients subject to the de-escalation strategy were initially treated with micafungin 100 mg/day and those remaining alive after 3 days (when susceptibility test results were available) were switched to fluconazole 400 mg/day if the Candida isolates were fluconazole sensitive (MIC ≤8 mg/L) or continued with micafungin 100 mg/day if the isolates were fluconazole resistant (MIC ≥64 mg/L). The cut-points for fluconazole sensitivity were based on information from Pfaller et al.25 Drug dosages were in line with the Summary of Product Characteristics (SmPC) for each agent (Diflucan SmPC,26 Mycamine SmPC27). Patients subject to the escalation strategy received a loading dose of fluconazole 800 mg on day 1 and fluconazole 400 mg/day thereafter; patients remaining alive after 3 days continued to receive fluconazole 400 mg/day if the Candida isolates were fluconazole sensitive or were switched to micafungin 100 mg/day if fluconazole-resistant isolates had been identified. For both strategies, if susceptibility testing showed the Candida isolates to be fluconazole dose-dependent (MIC 16–32 mg/L), patients were treated with fluconazole 800 mg/day.
Patients received 14 days of appropriate treatment (i.e., 17 days overall for those in whom the initial therapy was not appropriate), after which the success or failure of the treatment was assessed. The treatment duration was based on Chalmers et al28 and the Mycamine SmPC.27 Treatment success was based on clinical and mycological outcomes, with survival also being considered (percent of patients alive at 42 days). Clinical success was defined as resolution (complete response) or improvement (partial response) of all attributable signs, symptoms and abnormal radiographic findings associated with fungal infection since baseline at the end of treatment (14 or 17 days). In the event of treatment failure (i.e., the absence of clinical success), patients who had received fluconazole were switched to micafungin 100 mg/day and those who had been treated with micafungin had the dose increased from 100 to 200 mg/day. Mycological success was defined as eradication of Candida from the bloodstream (confirmed by negative results on microscopy and culture) at 14 or 17 days.
Health economic outcomes considered were the cost per health state according to clinical success and mycological success, and budget impact. A 42-day horizon was used for these costs, in line with the time point at which mortality was assessed in Phase III micafungin studies.29–31
Table 1 shows the clinical and treatment-related model input parameters and their sources.
  | Table 1 Clinical and treatment-related model input parameters (based on Masterton et al22) Notes: *Values for fluconazole-sensitive Candida infections were also applied to dose-dependent Candida infections, unless otherwise specified; **values were applied to DE and ES strategies, unless otherwise specified. Data from: aSlavin et al32; bAnaissie et al34; cFluconazole: Anaissie et al,34 Phillips et al,35 Rex et al,36 Sipsas et al37; dMicafungin: Marfo and Guo,38 Kubiak et al,33 Pappas et al,29 Sidhu et al,51 Dupont et al,41 Kuse et al30; eKujath et al,39 Nolla-Salas et al,40 Marfo and Guo,38 Pappas et al,29 Dupont et al41; fChalmers and Bal28 and Mycamine Summary of Product Characteristics.27 Abbreviations: DE, de-escalation; ES, escalation; SA, sensitivity analyses. |
Mortality data
Early (3-day) mortality data used in the model were based on information provided by Slavin et al,32 who describe mortality at day 5 for patients receiving appropriate and inappropriate treatment. Early mortality was 14.49% for patients with fluconazole-resistant isolates in the escalation strategy, and 9.84% for all other patients. Late (42-day) mortality for patients with fluconazole-sensitive or dose-dependent isolates, also based on information provided by Slavin et al,32 was set at 21.26%, after subtracting the early mortality rate. Late mortality for patients with fluconazole-resistant isolates varied according to treatment strategy: 50.85% for the escalation strategy and 19.85% for the de-escalation approach (the weighted average mortality of patients treated in the previously reported micafungin studies mentioned earlier).29,30,33
Clinical success rates
For patients with fluconazole-sensitive or dose-dependent isolates, regardless of the regimen on which they started, the clinical success rate used in the model was 65.57%; this is the weighted average from four previously reported fluconazole studies.34–37 For patients with fluconazole-resistant isolates, a clinical success rate of 76.91% was used; this was a weighted average value in line with four previously reported micafungin studies.29,30,33,38 For patients in the escalation arm, the 3 days of inappropriate therapy were assumed not to influence overall efficacy. This means costs in the escalation arm would be conservative estimates. As the only patients in the escalation arm who receive inappropriate therapy are those who subsequently receive micafungin, a patient who receives micafungin on day 0 (de-escalation) would be expected to have better clinical outcomes than one who receives micafungin on day 3 (escalation).
Mycological success rates
For patients with fluconazole-sensitive or dose-dependent isolates, regardless of the regimen on which they started, the mycological success rate used in the model was 61.62%, a weighted average from information published by Kujath et al39 and Nolla-Salas et al.40 For patients with fluconazole-resistant isolates, a mycological success rate of 83.77% was used, being similarly derived from other published data29,38,41 (Table 1).
Candida epidemiology data
Candida strain profiles for France and Germany were based on information provided by Leroy et al42 and Schmalreck et al,43 respectively. In both countries, the most commonly isolated Candida species were C. albicans and C. glabrata (Figure 1). Fluconazole susceptibility data42,43 are also shown in Figure 1. For Germany, these values were based on data provided by Schmalreck et al.43 For France, these values were based on data from Leroy et al.42 Consequently, fluconazole resistance was apparent in 2.6% and 8.0% of C. albicans isolates in France and Germany, respectively. The highest resistance rates were apparent in C. parapsilosis (42%, France) and C. krusei (91%, Germany).
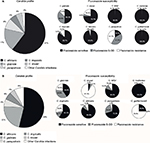  | Figure 1 Candida epidemiology and fluconazole susceptibility. (A) France (calculated from Leroy et al,42 as described in the text); (B) Germany (calculated from Schmalreck et al43). Notes: Fluconazole S-DD, fluconazole sensitivity is dose-dependent. Percentages may not total 100 due to rounding. Abbreviation: C, Candida. |
Health resource utilization
Health resource consumption by patients with SCI was used to calculate the cost per health state (clinical/mycological success). The model covered the cost of first- and second-line antifungal treatment (fluconazole, micafungin) and “excess” hospitalization (rather than overall length of stay) arising from inappropriate treatment. Excess hospitalization was set at 7.7 days on a general ward, with no additional intensive care stay; this was based on data from Zilberberg et al.6
Model input parameters and their sources are shown in Table 2; the costs considered in the model were in euros (€) for 2014 (or inflated to 2014 prices).
  | Table 2 Costs and health resource utilization used in the model (based on Masterton et al22) Notes: *Values apply to all Candida fluconazole-susceptibility isolates, ES and DE strategies and both countries, unless otherwise stated; **additional hospitalization resulting from inappropriate treatment, i.e., initial fluconazole treatment of fluconazole-resistant infections. From: aRépertoire des Spécialités Pharmaceutiques, France, 2014; bWHO-CHOICE initiative 2008, inflated to 2014 prices; cZilberberg et al6; dAgence Regionale de Sante 2012 and Institut National de la Statistique et des Études Économiques 2014, inflated to 2014 prices. Abbreviations: CI, confidence interval; DE, de-escalation; ES, escalation. |
Sensitivity analyses
The robustness of the budget impact model to changes in inputs was tested by performing one-way sensitivity analyses in which each input parameter was varied between the lower and upper limits of that parameter’s 95% CI.
Results
In France and Germany, the incidence of SCI was estimated at 4.10 and 10.05/100,000 population/year, respectively (linear extrapolation of incidence over time from the study by Bitar et al44 [France] and based on data in the study by Arendrup et al12 [Germany]).
Considering all patients with SCI over a 42-day period, the relative improvements in clinical success and survival with the de-escalation and escalation strategies in France and Germany, as well as the associated costs are shown in Table 3. Clinical success rates for the escalation and de-escalation strategies were 45.5% and 47.2%, respectively, in France, and 44.3% and 48.0%, respectively, in Germany. The corresponding total costs were €2,806 and €3,363, respectively, for France (representing a cost excess of €557 for de-escalation), and €3,754 and €5,906, respectively, for Germany (cost excess of €2,152 for de-escalation). The corresponding budget impact per hospital per year of using the de-escalation strategy rather than the escalation strategy to manage SCI was €1,115 and €5,367 in France and Germany, respectively, reflecting the small numbers of patients requiring treatment.
  | Table 3 (A) Clinical outcomes and costs and (B) mycological success and costs of the escalation and de-escalation strategies in all patients with systemic Candida infection (42-day horizon) |
Mycological success rates and the corresponding costs for the overall population are summarized in Table 3. Mycological success rates for the escalation and de-escalation strategies were 43.1% and 45.0%, respectively, in France, and 42.4% and 46.4%, respectively, in Germany. The corresponding total costs were €2,935 and €4,002, respectively, for France (representing a cost excess of €1,067 for de-escalation), and €3,882 and €6,670, respectively, for Germany (cost excess of €2,788 for de-escalation). The corresponding budget impact per hospital per year of using the de-escalation strategy rather than the escalation strategy was €2,134 in France and €11,153 in Germany. The reason for the difference in the total cost results between the two clinical outcome measures is due to lower mycological success rates compared to clinical success rates.
According to our model, over a 42-day period, in patients with fluconazole-resistant isolates, in both France and Germany, the clinical success rate was improved by 72% with the de-escalation strategy (72.3% minus 42.0% divided by 42.0%); this was accompanied by an ~72% improvement in survival (55.6% minus 32.3% divided by 32.3%) (Figure 2). The improved clinical success produced cost savings with de-escalation compared to the escalation strategy for hospitals in both France and Germany – incremental costs of €6,374 and €356 per patient, respectively (Figure 3). The cost savings resulted from the decreased hospitalization costs associated with de-escalation. In patients with fluconazole-resistant isolates, in both France and Germany, mycological success rates were also improved by ~72% with de-escalation compared with escalation (60.5% minus 35.2% divided by 35.2%), although this did not translate into cost savings with the former strategy (Figure 3).
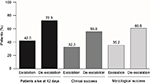  | Figure 2 Outcomes of the escalation and de-escalation strategies in patients with fluconazole-resistant systemic Candida infection (42-day horizon). |
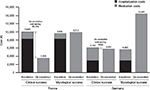  | Figure 3 Costs per patient of the escalation and de-escalation strategies in patients with fluconazole-resistant systemic Candida infection (42-day horizon). |
Figure 4 shows the results of one-way sensitivity analyses for the top 10 input parameters that were found to be the most influential. In both Germany and France, for clinical success as well as for mycological success, the parameter that most greatly influenced the results of the budget impact model was the duration of additional hospitalization.
Discussion
Prompt and appropriate treatment of invasive Candida infection is critical, as a delay can prolong hospitalization6,45 and is associated with a significant increase in mortality per day of inappropriate treatment.23 However, selection of initial treatment is complicated by a lack of differentiating clinical features between C. albicans and non-albicans infections46 (which therefore requires laboratory confirmation) and the resistance of some non-albicans species to fluconazole.8 Treatment guidelines for the management of invasive Candida infection7,47 include recommendations for initial use of broad-spectrum antifungal agents, such as echinocandins, with a subsequent switch to fluconazole if isolates are shown to be sensitive. The aim of this “de-escalation” strategy is to improve clinical outcomes and overall survival, particularly among patients with fluconazole-resistant Candida strains.
The treatment outcomes evident in the current budget impact model support the recommendation to use de-escalation rather than escalation in those at high-risk of azole-resistant Candida infection. The results suggest that, with a de-escalation strategy, better outcomes and cost savings can be expected in fluconazole-resistant patients. In these patients, de-escalation was associated with ~72% improvements for clinical and mycological success rates and survival rates compared to escalation. These outcomes are associated with cost savings in both Germany and France and were greater in France mainly due to the difference in cost for the stay in a general hospital ward (€1,252 and €455 per day, respectively). Furthermore, France had greater cost savings due to lower fluconazole resistance (7.68%) compared to Germany (15.68%). However, apparent benefits to mycological outcomes with de-escalation did not translate into cost savings. The absence of cost savings based on mycological success compared with a cost-saving gain based on clinical success may reflect the considerably higher medication costs associated with de-escalation. Patients with treatment failure at the end of the treatment period (day 14 or 17) were either switched to micafungin 100 mg/day (escalation group) or their dose of micafungin increased to 200 mg/day (de-escalation group). Since the medication cost of micafungin is much higher than fluconazole, small differences (in this case around 2%) between clinical and mycological success rates can produce large differences in incremental medication costs and consequently total costs. In addition, as the model only captures hospitalization costs due to inappropriate treatment, then with lower mycological success rates, hospitalization costs would also increase.
In the overall population of patients with SCI, an increase in the cost of managing this condition was observed with the de-escalation strategy compared to escalation. However, this increase is limited (€557 in France; €2,152 in Germany) and in part offset by the improvement in survival rates achieved using the de-escalation strategy (42-day survival rates were 68.8% for escalation and 71.1% for de-escalation in France, and 66.4% and 71.2%, respectively, in Germany).
A potential for cost saving with de-escalation when treating patients with fluconazole-resistant SCI is in line with the results of the UK cost-effectiveness model published by Masterton et al.22 Cost savings for de-escalation versus escalation were £1,621 per treated patient with fluconazole-resistant isolates.22 Furthermore, when the results were extrapolated over a lifetime horizon, the de-escalation strategy was found to be cost-effective: the incremental cost per quality-adjusted life year (QALY) was £25,673, which falls within the cost-effectiveness acceptability threshold set by the National Institute for Health and Care Excellence (£20,000–£30,000).48
The extent of any cost savings that may be achieved using the de-escalation strategy depends, of course, on a number of factors including the incidence of SCI and the degree of azole resistance. As this can vary from country to country and region to region, physicians should consider the risk of azole resistance in their own centers when deciding whether to use a de-escalation strategy for the treatment of SCI. Indeed, Ruhnke49 has recently proposed that consideration of local fungal epidemiology and information of antifungal resistance rates should be included as aspects of antifungal stewardship programs in SCI – programs in which de-escalation can play a key role. Herein lies one of the main advantages of the budgetary impact model used in the current study, as it can be tailored using data from hospitals in specific localities.
There are some limitations associated with the current analysis. As with all economic analyses, a number of assumptions were inherent to the model. This includes a simplified representation of clinical practice which assumed that SCI developed and was identified in all patients initially treated with micafungin or fluconazole; however, in actual clinical practice in an intensive care unit setting, the prevalence of candidemia has been reported at ~15% of bloodstream infections.5,50 Nevertheless, in an analysis comparing empiric therapy with micafungin versus fluconazole for suspected candidemia based on a prevalence of 14%, micafungin was cost-effective, with an incremental cost-effectiveness of US$34,734 per QALY.5 Finally, the cost of treating a patient with SCI was based on the direct cost of medication and the cost of prolonged hospitalization due to inappropriate initial treatment; it did not include the other potential costs of inappropriate initial management. In the escalation arm, these costs would likely be conservative estimates since the inclusion of other costs related to inappropriate initial management would likely increase the cost differential between de-escalation and escalation strategies in favor of de-escalation. The model also assumed that in the escalation arm, the 3 days of fluconazole treatment does not impact the overall efficacy of micafungin for the fluconazole-resistant patients. Clinical data on the efficacy of micafungin in this setting are not available; however, it would be expected that fluconazole-resistant patients who had inappropriate treatment for 3 days would not have the same clinical response as those who started micafungin 3 days earlier.
Conclusion
In summary, the management of SCI should be both prompt and appropriate in order to reduce morbidity and mortality. Advances in new diagnostic techniques for invasive fungal infection will likely facilitate this process in the future. In the meantime, the current model provides evidence that when treating SCI in individuals at risk of azole-resistant infections, such as those who have previously received azole prophylaxis or treatment, de-escalation from micafungin has potential cost savings associated with improved clinical success rates.
Availability of data and material
Data are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by Astellas Pharma Inc. Writing and editorial assistance in the preparation of this manuscript, which was provided by Bioscript Medical, was funded by Astellas Pharma Inc. The work of Quintiles Consulting was also funded by Astellas Pharma Inc.
Author contributions
AvE, MC and MW conceived and designed the analysis; AvE and MC performed the analysis; MW analyzed the data; SK and IO contributed analysis tools; all authors contributed to the writing of the manuscript. All authors agree to be accountable for all aspects of the work.
Disclosure
The economic model was developed by Nicola Trevor (NCT Enterprises), an employee of Astellas Pharma Inc. at the time of its development. The authors report no other conflicts of interest in this work.
References
de la Torre P, Reboli AC. Micafungin: an evidence-based review of its place in therapy. Core Evid. 2014;9:27–39. | ||
Richardson M, Lass-Florl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect. 2008;14(Suppl 4):5–24. | ||
Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–163. | ||
Karabinis A, Hill C, Leclercq B, Tancrede C, Baume D, Andremont A. Risk factors for candidemia in cancer patients: a case-control study. J Clin Microbiol. 1988;26(3):429–432. | ||
Zilberberg MD, Kothari S, Shorr AF. Cost-effectiveness of micafungin as an alternative to fluconazole empiric treatment of suspected ICU-acquired candidemia among patients with sepsis: a model simulation. Crit Care. 2009;13(3):R94. | ||
Zilberberg MD, Kollef MH, Arnold H, et al. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis. 2010; 10:150. | ||
Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. | ||
Martin MV. The use of fluconazole and itraconazole in the treatment of Candida albicans infections: a review. J Antimicrob Chemother. 1999;44(4):429–437. | ||
Charlier C, Hart E, Lefort A, et al. Fluconazole for the management of invasive candidiasis: where do we stand after 15 years? J Antimicrob Chemother. 2006;57(3):384–410. | ||
Snydman DR. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest. 2003;123(5 Suppl):500S–503S. | ||
Kabir MA, Ahmad Z. Candida infections and their prevention. ISRN Preventive Med. 2013;2013:763628. | ||
Arendrup MC, Dzajic E, Jensen RH, et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect. 2013;19(8):E343–353. | ||
Mikulska M, Del Bono V, Ratto S, Viscoli C. Occurrence, presentation and treatment of candidemia. Expert Rev Clin Immunol. 2012;8(8):755–765. | ||
Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect. 2014;20(Suppl 6):42–48. | ||
Baddley JW, Patel M, Bhavnani SM, Moser SA, Andes DR. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob Agents Chemother. 2008;52(9):3022–3028. | ||
Emri T, Majoros L, Toth V, Pocsi I. Echinocandins: production and applications. Appl Microbiol Biotechnol. 2013;97(8):3267–3284. | ||
Fernandez-Ruiz M, Aguado JM, Almirante B, et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis. 2014;58(10):1413–1421. | ||
Kale-Pradhan PB, Morgan G, Wilhelm SM, Johnson LB. Comparative efficacy of echinocandins and nonechinocandins for the treatment of Candida parapsilosis infections: a meta-analysis. Pharmacotherapy. 2010;30(12):1207–1213. | ||
Messer SA, Diekema DJ, Boyken L, Tendolkar S, Hollis RJ, Pfaller MA. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J Clin Microbiol. 2006;44(2):324–326. | ||
Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18(Suppl 7):53–67. | ||
Kollef M. Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time. Drugs. 2003;63(20):2157–2168. | ||
Masterton RG, Casamayor M, Musingarimi P, et al. De-escalation from micafungin is a cost-effective alternative to traditional escalation from fluconazole in the treatment of patients with systemic Candida infections. J Med Econ. 2013;16(11):1344–1356. | ||
Bassetti M, Molinari MP, Mussap M, Viscoli C, Righi E. Candidaemia in internal medicine departments: the burden of a rising problem. Clin MicrobiolInfect. 2013;19(6):E281–E284. | ||
European Commission. Eurostat. Total population database. Data for 1 January 2014; 2014. Available from: http://epp.eurostat.ec.europa.eu/tgm/table.do?tab=table&language=en&pcode=tps00001&tableSelection=1&footnotes=yes&labeling=labels&plugin=1. Accessed September 12, 2014. | ||
Pfaller MA, Diekema DJ, Sheehan DJ. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin Microbiol Rev. 2006;19(2):435–447. | ||
Diflucan Summary of Product Characteristics. 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Diflucan_30/WC500121908.pdf. Accessed September 10, 2014. | ||
Mycamine Summary of Product Characteristics. 2014. Available f rom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000734/WC500031075.pdf. Accessed September 10, 2014. | ||
Chalmers CM, Bal AM. Management of fungal infections in the intensive care unit: a survey of UK practice. Br J Anaesth. 2011;106(6):827–831. | ||
Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45(7):883–893. | ||
Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369(9572):1519–1527. | ||
Horn DL, Ostrosky-Zeichner L, Morris MI, et al. Factors related to survival and treatment success in invasive candidiasis or candidemia: a pooled analysis of two arge, prospective, micafungin trials. Eur J Clin MicrobiolInfect Dis. 2010;29(2):223–229. | ||
Slavin MA, Sorrell TC, Marriott D, et al. Candidaemia in adult cancer patients: risks for fluconazole-resistant isolates and death. J Antimicrob Chemother. 2010;65(5):1042–1051. | ||
Kubiak DW, Bryar JM, McDonnell AM, et al. Evaluation of caspofungin or micafungin as empiric antifungal therapy in adult patients with persistent febrile neutropenia: a retrospective, observational, sequential cohort analysis. Clin Ther. 2010;32(4):637–648. | ||
Anaissie EJ, Darouiche RO, Abi-Said D, et al. Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clin Infect Dis. 1996;23(5):964–972. | ||
Phillips P, Shafran S, Garber G, et al. Multicenter randomized trial of fluconazole versus amphotericin B for treatment of candidemia in non-neutropenic patients. Canadian Candidemia Study Group. Eur J Clin Microbiol Infect Dis. 1997;16(5):337–345. | ||
Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med. 1994;331(20):1325–1330. | ||
Sipsas NV, Lewis RE, Tarrand J, et al. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer. 2009;115(20):4745–4752. | ||
Marfo K, Guo Y. Pharmacoeconomic analysis of micafungin (Mycamine) 100 mg and 150 mg daily In the treatment of candidemia. PT. 2009;34(4):196–199. | ||
Kujath P, Lerch K, Kochendorfer P, Boos C. Comparative study of the efficacy of fluconazole versus amphotericin B/flucytosine in surgical patients with systemic mycoses. Infection. 1993;21(6):376–382. | ||
Nolla-Salas J, Sitges-Serra A, Leon-Gil C, et al. Candidemia in non-neutropenic critically ill patients: analysis of prognostic factors and assessment of systemic antifungal therapy. Study Group of Fungal Infection in the ICU. Intensive Care Med. 1997;23(1):23–30. | ||
Dupont BF, Lortholary O, Ostrosky-Zeichner L, Stucker F, Yeldandi V. Treatment of candidemia and invasive candidiasis in the intensive care unit: post hoc analysis of a randomized, controlled trial comparing micafungin and liposomal amphotericin B. Crit Care. 2009;13(5):R159. | ||
Leroy O, Gangneux JP, Montravers P, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006). Crit Care Med. 2009;37(5):1612–1618. | ||
Schmalreck AF, Willinger B, Haase G, et al. Species and susceptibility distribution of 1062 clinical yeast isolates to azoles, echinocandins, flucytosine and amphotericin B from a multi-centre study. Mycoses. 2012;55(3):e124–e137. | ||
Bitar D, Lortholary O, Le Strat Y, et al. Population-based analysis of invasive fungal infections, France, 2001-2010. Emerg Infect Dis. 2014;20(7):1149–1155. | ||
Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31. | ||
Sganga G. Clinical aspects of invasive candidiasis in the surgical patient. Drugs. 2009;69(Suppl 1):29–32. | ||
Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18(Suppl 7):53–67. | ||
National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal 2013. 2013. Available from: http://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf. Accessed November 24, 2014. | ||
Ruhnke M. Antifungal stewardship in invasive Candida infections. Clin Microbiol Infect. 2014;20(Suppl 6):11–18. | ||
Prowle JR, Echeverri JE, Ligabo EV, et al. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care. 2011;15(2):R100. | ||
Sidhu MK, van Engen AK, Kleintjens J, Schoeman O, Palazzo M. Cost-effectiveness analysis of micafungin versus caspofungin for treatment of systemic Candida infections in the UK. Curr Med Res Opin. 2009;25(8):2049–2059. |
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.