Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 11
CYP2D6 genotype can help to predict effectiveness and safety during opioid treatment for chronic low back pain: results from a retrospective study in an Italian cohort
Authors Dagostino C, Allegri M , Napolioni V , D'Agnelli S , Bignami E, Mutti A , van Schaik RHN
Received 25 July 2018
Accepted for publication 24 August 2018
Published 24 October 2018 Volume 2018:11 Pages 179—191
DOI https://doi.org/10.2147/PGPM.S181334
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Concetta Dagostino,1,2 Massimo Allegri,2–4 Valerio Napolioni,5 Simona D’Agnelli,1 Elena Bignami,1 Antonio Mutti,1 Ron HN van Schaik6
1Department of Medicine and Surgery, University of Parma, Parma 43126, Italy; 2Study In Multidisciplinary Pain Research (SIMPAR), Milan 20100, Italy; 3Anesthesia and Intensive Care Department, IRCCS Multi Medica Hospital, Milan 20099, Italy; 4Italian Pain Institute, Milan 20100, Italy; 5Department of Neurology and Neurological Sciences, Stanford University, Stanford, CA 94305, USA; 6Department of Clinical Chemistry, Erasmus MC, 3000 Rotterdam, The Netherlands
Background: Opioids are widely used for chronic low back pain (CLBP); however, it is still unclear how to predict their effectiveness and safety. Codeine, tramadol and oxycodone are metabolized by CYP/CYP450 2D6 (CYP2D6), a highly polymorphic enzyme linked to allele-specific related differences in metabolic activity.
Purpose: CYP2D6 genetic polymorphisms could potentially help to predict the effectiveness and safety of opioid-based drugs in clinical practice, especially in the treatment of CLBP.
Patients and methods: A cohort of 224 Italian patients with CLBP treated with codeine or oxycodone was retrospectively evaluated to determine whether adverse reactions and effectiveness were related to CYP2D6 single-nucleotide polymorphisms. CYP2D6 genotyping was performed using the xTAG® CYP2D6 Kit v3 (Luminex) to determine CYP2D6 metabolizer phenotype (poor, intermediate, rapid and ultrarapid). Subjects from the cohort were categorized into two groups according to the occurrence of side effects (Case) or benefit (Control) after chronic analgesic treatment. The impact of CYP2D6 polymorphism on treatment outcome was tested at the metabolizer phenotype, diplotype and haplotype levels.
Results: CYP2D6 polymorphism was significantly associated with opioid treatment outcome (Omnibus P=0.018, for both global haplotype and diplotype distribution test). CYP2D6*6 and *9 carriers, alleles characterized by a reduced (*9) or absent (*6) enzymatic activity, were significantly (P<0.05) associated with therapeutic failure. CYP2D6 ultrarapid metabolizers (CYP2D6*2N patients) showed an increased risk of side effects, as would be predicted. Despite their low frequency, CYP2D6 *1/*11, *4/*6 and *41/* 2N diplotypes showed significant (P<0.05) associations of efficacy and side effects with chronic opioid treatment.
Conclusion: Our results showed that reduced CYP2D6 activity is correlated with lack of therapeutic effect. We found that the pharmacogenetic analysis of CYP2D6 could be helpful in foreseeing the safety and effectiveness of codeine or oxycodone treatment in CLBP.
Keywords: polymorphisms, pharmacogenetics, codeine, oxycodone, analgesic drugs, personalized medicine
Introduction
Chronic low back pain (CLBP) is a common disease difficult to manage due to its complex pathogenesis.1 Several prognostic factors, such as demographic (sex, age and cultural/educational environment),2 occupational (type of work activity)3 and psychological (anxiety, pain catastrophizing and depression),4 have been claimed as important determinants of CLBP. Given the inflammatory nature of CLBP, several studies have evaluated biomarkers related to inflammatory response, rather than those related to disk degeneration.5 Indeed, the combination of age, leptin and MCP-1 predicted 61% of the risk of LBP duration, suggesting these analytes as promising biomarkers for the diagnosis of acute low back pain (LBP) and its risk to become chronic.6 During the last years, the scientific community has witnessed the mounting interest in genomics and pharmacogenetics as possible tools to predict both the risk to develop CLBP and the effectiveness and safety of pharmacological treatments.7–10 Moreover, some patients experience pain processing disorders and they may be also at risk for opioid addiction.11,12 Recently, it has been suggested that pharmacokinetic and pharmacodynamic evaluation of drugs could be helpful in improving the effectiveness13 and in preventing, at the same time, the onset of tolerance and hyperalgesia.14 Hence, personalized medicine is one of the most promising solutions to maximize the effectiveness and reduce the risk of side effect in patients’ treatment.15
Most of the drugs, especially opioids used for chronic pain therapy, are being metabolized by the hepatic enzyme CYP/CYP450 2D6 (CYP2D6, located on chromosome 22q13.2, OMIM*124030).16 Indeed, the genetic polymorphism of cytochrome 450 (P450) determines interindividual variability in the metabolism of many drugs, with relevant implications in terms of efficacy and safety, and significantly affecting the pharmacokinetics of about 50% of the drugs in clinical use.17–20
Among opioids, codeine and oxycodone are the most prescribed drugs being activated by CYP2D6.21–24 Based on the extent of CYP2D6 substrate metabolism, the population can be categorized into four different groups: extensive metabolizers (EMs), intermediate metabolizers (IMs), poor metabolizers (PMs) and ultrarapid metabolizers (UMs).25
The frequencies of CYP2D6 metabolizer phenotypes in Caucasians are: PMs, 5%–10%; IMs, 10%–15%; EMs, 65%–80% and UMs, 5%–10%.17,26–28
As ultrarapid CYP2D6 metabolizer’s patients would need higher drug doses, they are more at risk to develop not only more side effects19 but also lower drug’s dosages in the meantime lower dosage.29
Morlock and Braunstein have underlined the positive pharmacoeconomic impact of a pharmacogenetics-based treatment in patients with chronic pain.30 Nevertheless, there is no clear evidence of the role of widespread genetic analysis in patients with chronic pain.
In this retrospective study, we investigated how CYP2D6 genotyping could be helpful in predicting the effectiveness and side effects in a cohort of CLBP Italian patients clinically treated with codeine/acetaminophen or oxycodone.
Patients and methods
Subjects
The patients were enrolled in the PainOMICS study, a large multicenter study funded by the European Community in the Seventh Framework Program (FP7) – THEME (HEALTH.2013.2.2.1-5 – understanding and controlling pain) to evaluate the genomics, glycomics and activomics of biomarkers related to pain. The protocol7 has been approved by the Ethical Committee at the University of Parma and all the participating clinical centers. It has been registered on clinicaltrials.gov (NCT02037789). All patients enrolled have signed a written informed consent.
Inclusion and exclusion criteria of PainOMICS project have been previously published.7 In the present study, we considered all CLBP patients whose physicians had decided to prescribe codeine/acetaminophen or oxycodone for pain treatment, and categorized the patients showing side effects and no pain relief from the opioid treatment as “Case” and those patients who reported pain relief with opioid treatment without side effects as “Control”. We excluded all patients with possible drug–drug interaction (DDI) with ongoing treatments.13,31
As the primary endpoint, we evaluated the correlation between pharmacokinetic CYP2D6 pattern and the effectiveness/safety of codeine/acetaminophen or oxycodone in the visit after the first prescription. We considered the treatment effective if it had led to a reduction of at least 30% of pain, evaluated by the Brief Pain Inventory. As a safety measure, we registered the onset of severe side effects (nausea, vomiting, dizziness) that would determine the patients’ decision to withdraw from the treatment.
Patients were stratified into two groups according to the genetic factors that may contribute to variability in drug response and that can help maximize the likelihood of efficacious treatment by minimizing the adverse drug reactions (ADRs):32,33 patients showing effectiveness of the drug and no severe side effects and patients showing no effectiveness of the drug and/or severe side effects that prejudice the treatment continuation.
Patients enrolled were also stratified into four groups according to their corresponding CYP2D6 metabolizing phenotype.17,26–28
Pharmacogenetic analysis
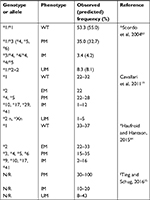
Blood sample collection and analysis were performed according to the previously determined personalized standard operating procedures.34 CYP2D6 genotyping was performed using the xTAG® CYP2D6 Kit v3 (Luminex). Briefly, genomic DNA, extracted from whole blood and collected in vacutainer with EDTA, was purified with a DNA purification Kit (cod. AS1010; Promega) using an automatic system Maxwell16 (Promega). Genomic DNAs were quantified using a NanoDrop ND-1000 spectrophotometer, adjusting the concentration of each sample in the range of 24–1,800 ng. The xTAG CYP2D6 Kit v3 detects a comprehensive panel of functional CYP2D6 nucleotide variants, including gene rearrangements associated with the deletion (*5) and duplication genotypes. The assay incorporates multiplex PCR and multiplex Allele-Specific Primer Extension with Luminex’s Universal Tag sorting system on the Luminex® 100/200™ xMAP® platform based on the principles of flow cytometry which integrates key xMAP detection components such as lasers, optics, fluidics and high-speed digital signal processors. The test automatically determines the diplotype of each sample (Table 1).
Results have been reported with the xTAG Data Analysis Software (TDAS CYP2D6, applies algorithms to the mean fluorescence intensity values produced57 by each sample to generate automated genotyping calls for each mutation easy). Data acquisition protocols for Luminex IS and xPONENT3.1 software were designed for protocol-based data acquisition with robust data regression analysis. Each run included at least one DNase/RNase-free water controls (TDAS automatically assigns the last sample the primary negative) and commercially available CYP2D6 controls as positive controls. The assignment of CYP2D6 genotypes to each metabolizer phenotype was carried out according to Table S1, as reported in the Luminex xTAG CYP2D6 Kit.
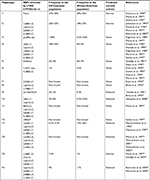
CYP2D6 haplotype frequencies in the population analyzed were compared to the ones reported in the literature (Table 2).
Statistical analysis
Demographic variables were reported as mean±SD. Associations between the three assigned phenotype groups (poor, intermediate to extensive and ultrarapid) and outcomes (poor pain control, adverse reaction or either) were assessed using chi-squared tests for categorical variables.
CYP2D6 diplotype and haplotype frequencies were tested using UNPHASEDv. 3.1.765 under an additive model and adjusting for sex and age at sampling. We set the statistical significance for the haplotype-based analyses at a global P-value of <0.05. Analyses excluded haplotypes with a frequency <1%.
Results
One thousand patients were enrolled in the University Parma’s Pain Center for PainOMICS study between June 2014 and February 2016.
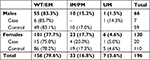
Two hundred and twenty-four Italian patients (22.4% of PainOMICS Project participants), 78 males and 146 females, with a mean age 66.8 years (±14.3), were selected since they had been treated with one of the two drugs considered (109 patients treated with codeine/acetaminophen and 115 with oxycodone). Thirteen patients were excluded for possible DDI,66 while 15 patients were excluded because of genotyping failure. Hence, a total of 196 patients (66 males and 130 females) were analyzed.
Ninety-seven and 99 patients had been treated with codeine/acetaminophen and oxycodone or oxycodone/naloxone, respectively.
Twenty-seven (13.8%) patients showed side effects/or no pain relief from opioid treatment (Case) and 169 patients (86.2%) reported pain relief with opioid treatment (Control), as shown in Figure 1.
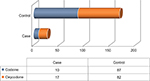  | Figure 1 Patients chronically treated with codeine or oxycodone for the low back pain in each group selected to investigate the onset of the side effects and lack of benefit. |
Approximately 15% of the Italian population considered in the two groups receive one or more prescriptions in addition to the opioid.
Demographic and clinical characteristics of the volunteers enrolled are summarized in Table 3.
The EM phenotype was the most frequent in both Case and Control groups, representing 78% and 80%, respectively, of the group studied; the IM/PM (reduced metabolic activity) phenotype frequencies were 15% and 17%, and the UM (increased metabolic activity) accounted for 7% and 3% of the studied groups (Figure 2).
Distribution of CYP2D6 metabolizer phenotypes in the two groups studied according to sex showed no significant differences (Case, Fisher’s exact test P=0.304; Control, Fisher’s exact test P=0.302), as shown in Table 4.
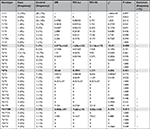
CYP2D6 haplotype and diplotype distributions displayed significant differences in the “Case” and “Control” groups (Omnibus P=0.018, for both global haplotype and diplotype distribution test) (S1). CYP2D6*6 (PM) and *9 (IM) haplotypes were significantly overrepresented in the Case group (Table 5). At the diplotype level, CYP2D6*1/*11 (EM), *4/*6 (PM), and *41/*2N (UM) were individually associated with an increased risk of treatment failure and toxic effect (Table 6).
The major frequency of haplotypes *6 (6%) and *9 (6%) in the group of the cases with side effects and without benefit (Case) in respect to the group with benefit (Control) is actually responsible for the no efficacy. Furthermore, the highest percentage of *2N in the same group (7% compared to 3%; Figure 2) increased the risk of side effects. Moreover, the risk of treatment failure and toxic effect is noted in the genotypes *1/*11, *4/*6, and *41/*2N, though with less significance, and the results are reported in bold in Tables 5 and 6.
Conclusion
Nowadays medicine is going toward a “patient-centered” and “personalized” approach in which omics represent an important pillar to characterize each patient both for diagnostic and for therapeutic purposes.67,68 The principle of “one size fits all”69 is not applicable in clinical practice; drugs’ metabolism and interaction with other drugs have to be always considered. Hence, genomics could be helpful in stratifying subgroups of patients in whom treatments are potentially more effective, improving the therapeutic index of drugs in pain therapy.70 Nowadays precision medicine is a great opportunity with a revolutionary impact on the “health system”, especially in terms of effectiveness, safety, ethics and cost savings.30 Genomics is also promising in improving safety by reducing ADRs. ADRs and treatment failures represent a significant albeit avoidable expenditure even today, in terms of both cost and quality of life, as they are one of the leading causes of morbidity and mortality in health care. An estimated 2.2 million serious ADRs occur yearly in the US, resulting in >100,000 deaths.71 Pharmacogenetics approach may help to choose the most appropriate drug and dosage for a specific patient, maximizing the therapeutic efficacy and improving the drug safety.72,73
Many of the pain killers currently used, such as opioids and antidepressant drugs, are metabolized by CYP2D6 enzyme. Polymorphisms of the CYP2D6 gene may potentially induce clinically important effects across a wide range of therapeutic areas.74,75 Identification of patient CYP2D6 genotypes could help clinicians to tailor drug treatment by selection of appropriate therapies minimizing ADRs. Studies of genetic polymorphisms linked to pain syndromes and medication metabolism herald a fresh therapeutic approach based on genotype with targeted analgesia and fewer side effects.76,77 Furthermore, pharmacoeconomics analysis showed how pharmacogenetic analysis prior to starting a specific pain treatment can reduce the direct and indirect therapeutic costs.30
In our study, we evaluated if and how CYP2D6 pharmacogenetic analysis could be helpful in predicting the effectiveness and safety of codeine or oxycodone treatment in CLBP Italian cohort.
In our cohort, we observed that >10% of patients, who were initially treated with codeine or oxycodone, stopped the treatment due to the occurrence of side effects and/or because of no improvement in pain relief. Codeine and oxycodone are frequently prescribed as a drug for treatment of chronic severe pain that cannot be treated with other pharmacological or invasive treatment. Nevertheless, a scarce analgesic effect may be expected in 5%–10% of Caucasian people who have PM phenotype.61 In fact, prodrugs such as codeine, which must be metabolized by CYP2D6 to morphine to provide analgesia, are often not effective in these patients.62
On the other hand, UMs have higher quantities of expressed enzymes because of gene duplication (allele *2N) with production of higher plasma concentration of active drug. In our cohort, we confirmed that UM patients complain more frequently of side effects compared to normal metabolizer patients (7% vs 2.96%). It has to be underlined that these results are concordant both in codeine and in oxycodone treatments, even though they have two different metabolic ways: codeine is an inactive prodrug with active metabolites (morphine), while oxycodone is an active drug with active metabolites. Our results showed, concordantly with previous studies, that in both treatments, an increased metabolism (*2N) exposes patients to more toxic effects. On the other hand, we found a result discordant with previous studies,78,79 which was that IMs and PMs reported higher effectiveness. This apparently paradoxical ambiguity in our results could be attributed to the significant allele frequency (6%) in*6 (PM) and *9 (IM) compared to the Control (0% and 1%, respectively) in which although a greater number of patients presented with a slow metabolism, the beneficial effect was still present. This result is an evidence that the alleles *6 and *9 seem to be responsible for the lack of therapeutic effect in the Case group, which was not observed in the Control group despite having the most number of representing PMs.
To our knowledge, our study represents the first study about the pharmacogenetic usefulness of screening CYP2D6 in a well-phenotyped, large cohort of Italian patients with CLBP (Figure 3), where patients with already existing possible DDIs have been excluded. It is mandatory to acknowledge the limitations and biases of the present work.
The sample size was extremely small, since only 27 out of 224 patients ended up in the “Case” group. Notwithstanding, the association testing of CYP2D6 haplotype and diplotypes showed global significant differences according to “the no benefit/benefit status”. Replication studies using larger cohorts are clearly warranted to extend and confirm the present results.
Our major bias could be related to the “not appropriate” drug selection by the physician, but we are confident that this possible bias cannot be considered as all the patients have been enrolled from the same center and have been seen by a few physicians who have same therapeutic approach. Another bias is related to the retrospective design of the study. Even though we have excluded possible DDI, we could not follow-up the patients prospectively looking at possible drug adjustments or better definition of no effect/side effect. A prospective study that could overcome this bias through a better definition of people who are candidates to these treatments is needed, as already suggested by Lloyd et al.80
We confirmed that pharmacogenetics has the potential to improve pain management by predicting the individual response to a specific analgesic before initiation of therapy and, therefore, to streamline the way physicians prescribe medications to the individual.15 CYP2D6 genetic polymorphism analysis might represent a helpful support for the clinicians in the optimization of pharmacological therapies, as also suggested by Linares et al in a small study.81
However, while the pharmacokinetic consequences of polymorphic metabolism are relatively well characterized for many drugs, the documentation of its importance with respect to therapeutic response and dosing remains scanty.15 Added to this is the fact that our study is the first attempt to apply the pharmacokinetic knowledge to opioids for the chronic treatment of LBP. Further studies are required to assess the clinical significance of these differences in the treatment outcome and optimal dosage of drugs metabolized by these polymorphic enzymes. It is of potential clinical importance to be able to identify individuals who have altered pharmacokinetics for CYP2D6 substrates as appropriate dosage strategies for these drugs can be adopted and ADRs can be avoided.
The principle that a good therapy is based on a good diagnostic determines, therefore, a wide field of action for the precision of personalized medicine, not only in relation to diagnostic innovations introduced by “omics”, but also in the rediscovery of the traditional quality and accuracy of analytical and biological variability of the value of the study.
The success of treatment is also closely linked to the precocity of therapeutic intervention because the health expenditure increases exponentially with the progress of the disease. Prevention is, therefore, a considerable resource for health systems. This way you can minimize the “cost-toxic” treatment by improving the quality of life of patients and optimize the management of available financial resources.
Data sharing statement
The authors intend to share, only for research purposes, individual deidentified genetic and clinical data after 1 year of the publication of the paper. The data will be accessible for 3 years and it will be possible to have access only through an official request sent to the corresponding author of the paper.
Acknowledgments
We would like to thank all participants enrolled in this study through Anesthesia, Critical Care and Pain Medicine Unit, Division of Surgical Sciences, Department of Medicine and Surgery, University of Parma, Parma, Italy. Thanks to Dr Lorenzo Boggiani and Dr Mariateresa Dagostino for their contribution to the data collection and processing, respectively. This trial is a funded academic research; it is supported by funding from the European Commission in the context of the Seventh Framework Program (Grant agreement no.: 602736) of the European Community for Research, Technological Development and Demonstration Activities – (FP7) – THEME (HEALTH.2013.2.2.1-5 – understanding and controlling pain). The funds received from the European grant provided support in the form of salaries for the author CD, but did not have any additional role in the study design, data collection and analysis, decision to publish, or in the preparation of the manuscript. The LX200 for readings of the pharmacokinetic tests was given by Luminex and Lagitre s.r.l. on loan.
Author contributions
CD conceived this current study, conducted the pharmacogenetic analysis and wrote the manuscript. During the patients’ enrollment and sample analysis, MA was the Principal Investigator of the granted European project “PainOMICS” and Clinician Director of the Anesthesia, Critical Care and Pain Medicine Unit in the Pain Therapy Center of University Hospital of Parma. VN elaborated the statistical results. SDA checked the clinical information on patients’ treatment by matching them with pharmacogenetic results. MA and RVS improved the experimental design and the manuscript. EB and AM are the supervisors of the project activity in the University of Parma. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Allegri M, Montella S, Salici F, et al. Mechanisms of low back pain: a guide for diagnosis and therapy. F1000Res. 2016;5:1530. | ||
Wong AY, Karppinen J, Samartzis D. Low back pain in older adults: risk factors, management options and future directions. Scoliosis Spinal Disord. 2017;12:14. | ||
Petit A, Roche-Leboucher G, Bontoux L, et al. Effectiveness of three treatment strategies on occupational limitations and quality of life for patients with non-specific chronic low back pain: is a multidisciplinary approach the key feature to success: study protocol for a randomized controlled trial. BMC Musculoskelet Disord. 2014;15(15):131. | ||
Campbell P, Foster NE, Thomas E, Dunn KM. Prognostic indicators of low back pain in primary care: five-year prospective study. J Pain. 2013;14(8):873–883. | ||
Freidin MB, Keser T, Gudelj I, et al. The association between low back pain and composition of IgG glycome. Sci Rep. 2016;6:26815. | ||
Lippi G, Dagostino C, Buonocore R, et al. The serum concentrations of leptin and MCP-1 independently predict low back pain duration. Clin Chem Lab Med. 2017;55(9):1368–1374. | ||
Allegri M, de Gregori M, Minella CE, et al. ‘Omics’ biomarkers associated with chronic low back pain: protocol of a retrospective longitudinal study. BMJ Open. 2016;6(10):e012070. | ||
Suri P, Boyko EJ, Smith NL, et al. Modifiable risk factors for chronic back pain: insights using the co-twin control design. Spine J. 2017;17(1):4–14. | ||
Malkin I, Williams FM, Lachance G, Spector T, Macgregor AJ, Livshits G. Low back and common widespread pain share common genetic determinants. Ann Hum Genet. 2014;78(5):357–366. | ||
Williams FM, Bansal AT, van Meurs JB, et al. Novel genetic variants associated with lumbar disc degeneration in northern Europeans: a meta-analysis of 4600 subjects. Ann Rheum Dis. 2013;72(7):1141–1148. | ||
Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20(2S):S111–S133. | ||
Reimer J, Wright N, Somaini L, et al. The impact of misuse and diversion of opioid substitution treatment medicines: evidence review and expert consensus. Eur Addict Res. 2016;22(2):99–106. | ||
Davis MP. Pharmacokinetic and pharmacodynamic evaluation of oxycodone and naltrexone for the treatment of chronic lower back pain. Expert Opin Drug Metab Toxicol. 2016;12(7):823–831. | ||
Muscoli C, Dagostino C, Ilari S, et al. Posttranslational nitration of tyrosine residues modulates glutamate transmission and contributes to N-methyl-D-aspartate-mediated thermal hyperalgesia. Mediators Inflamm. 2013;2013:1–12. | ||
Ting S, Schug S. The pharmacogenomics of pain management: prospects for personalized medicine. J Pain Res. 2016;9(9):49–56. | ||
Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5–6):274–285. | ||
de Gregori M, Allegri M, de Gregori S, et al. How and why to screen for CYP2D6 interindividual variability in patients under pharmacological treatments. Curr Drug Metab. 2010;11(3):276–282. | ||
Choi SW, Lam DMH, Wong SSC, Shiu HHC, Wang AXM, Cheung CW. Effects of single nucleotide polymorphisms on surgical and postsurgical opioid requirements: a systematic review and meta-analysis. Clin J Pain. 2017;33(12):1–1130. | ||
Cavallari LH, Jeong H, Bress A. Role of cytochrome P450 genotype in the steps toward personalized drug therapy. Pharmgenomics Pers Med. 2011;4:123–136. | ||
Madadi P, Ross CJ, Hayden MR, et al. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case–control study. Clin Pharmacol Ther. 2009;85(1):31–35. | ||
Chidambaran V, Sadhasivam S, Mahmoud M. Codeine and opioid metabolism: implications and alternatives for pediatric pain management. Curr Opin Anaesthesiol. 2017;30(3):349–356. | ||
Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte – an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662–673. | ||
Depriest AZ, Puet BL, Holt AC, Roberts A, Cone EJ. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 20152015;27(2):115–145. | ||
Flores CM, Mogil JS. The pharmacogenetics of analgesia: toward a genetically-based approach to pain management. Pharmacogenomics. 2001;2(3):177–194. | ||
Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116(3):496–526. | ||
Lammers LA, Achterbergh R, van Schaik RHN, Romijn JA, Mathôt RAA. Effect of short-term fasting on systemic cytochrome P450-mediated drug metabolism in healthy subjects: a randomized, controlled, crossover study using a cocktail approach. Clin Pharmacokinet. 2017;56(10):1231–1244. | ||
Fijal BA, Guo Y, Li SG, et al. CYP2D6 predicted metabolizer status and safety in adult patients with attention-deficit hyperactivity disorder participating in a large placebo-controlled atomoxetine maintenance of response clinical trial. J Clin Pharmacol. 2015;55(10):1167–1174. | ||
Rebsamen MC, Desmeules J, Daali Y, et al. The AmpliChip CYP450 test: cytochrome P450 2D6 genotype assessment and phenotype prediction. Pharmacogenomics J. 2009;9(1):34–41. | ||
St Sauver JL, Olson JE, Roger VL, et al. CYP2D6 phenotypes are associated with adverse outcomes related to opioid medications. Pharmgenomics Pers Med. 2017;10:217–227. | ||
Morlock R, Braunstein GD. Pharmacoeconomics of genotyping-based treatment decisions in patients with chronic pain. Pain Rep. 2017;2(5):e615. | ||
Ng GQ, Sklar GE, Chng HT. An evaluation of the completeness of drug–drug interaction-related information in package inserts. Eur J Clin Pharmacol. 2017;73(2):165–174. | ||
Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5(1):6–13. | ||
Bahar MA, Setiawan D, Hak E, Wilffert B. Pharmacogenetics of drug–drug interaction and drug–drug–gene interaction: a systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics. 2017;18(7):701–739. | ||
Dagostino C, de Gregori M, Gieger C, et al. Validation of standard operating procedures in a multicenter retrospective study to identify -omics biomarkers for chronic low back pain. PLoS One. 2017;12(5):e0176372. | ||
Kimura S, Umeno M, Skoda RC, Meyer UA, Gonzalez FJ. The human debrisoquine 4-hydroxylase (CYP2D) locus: sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. Am J Hum Genet. 1989;45(6):889–904. | ||
Marez D, Legrand M, Sabbagh N, et al. Polymorphism of the cytochrome P450 CYP2D6 gene in a European population: characterization of 48 mutations and 53 alleles, their frequencies and evolution. Pharmacogenetics. 1997;7(3):193–202. | ||
Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60(2):284–295. | ||
Johansson I, Lundqvist E, Bertilsson L, Dahl ML, Sjöqvist F, Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci U S A. 1993;90(24):11825–11829. | ||
Panserat S, Mura C, Gérard N, et al. DNA haplotype-dependent differences in the amino acid sequence of debrisoquine 4-hydroxylase (CYP2D6): evidence for two major allozymes in extensive metabolisers. Hum Genet. 1994;94(4):401–406. | ||
Raimundo S, Fischer J, Eichelbaum M, Griese EU, Schwab M, Zanger UM. Elucidation of the genetic basis of the common ‘intermediate metabolizer’ phenotype for drug oxidation by CYP2D6. Pharmacogenetics. 2000;10(7):577–581. | ||
Kagimoto M, Heim M, Kagimoto K, Zeugin T, Meyer UA. Multiple mutations of the human cytochrome P450IID6 gene (CYP2D6) in poor metabolizers of debrisoquine. Study of the functional significance of individual mutations by expression of chimeric genes. J Biol Chem. 1990;265(28):17209–17214. | ||
Gough AC, Miles JS, Spurr NK, et al. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature. 1990;347(6295):773–776. | ||
Hanioka N, Kimura S, Meyer UA, Gonzalez FJ. The human CYP2D locus associated with a common genetic defect in drug oxidation: a G1934-A base change in intron 3 of a mutant CYP2D6 allele results in an aberrant 3’ splice recognition site. Am J Hum Genet. 1990;47(6):995–1001. | ||
Gaedigk A, Blum M, Gaedigk R, Eichelbaum M, Meyer UA. Deletion of the entire cytochrome P450 CYP2D6 gene as a cause of impaired drug metabolism in poor metabolizers of the debrisoquine/sparteine polymorphism. Am J Hum Genet. 1991;48(5):943–950. | ||
Steen VM, Molven A, Aarskog NK, Gulbrandsen AK. Homologous unequal cross-over involving a 2.8 kb direct repeat as a mechanism for the generation of allelic variants of human cytochrome P450 CYP2D6 gene. Hum Mol Genet. 1995;4(12):2251–2257. | ||
Evert B, Griese EU, Eichelbaum M. Cloning and sequencing of a new non-functional CYP2D6 allele: deletion of T1795 in exon 3 generates a premature stop codon. Pharmacogenetics. 1994;4(5):271–274. | ||
Saxena R, Shaw GL, Relling MV, et al. Identification of a new variant CYP2D6 allele with a single base deletion in exon 3 and its association with the poor metabolizer phenotype. Hum Mol Genet. 1994;3(6):923–926. | ||
Daly AK, Leathart JB, London SJ, Idle JR. An inactive cytochrome P450 CYP2D6 allele containing a deletion and a base substitution. Hum Genet. 1995;95(3):337–341. | ||
Evert B, Griese EU, Eichelbaum M. A missense mutation in exon 6 of the CYP2D6 gene leading to a histidine 324 to proline exchange is associated with the poor metabolizer phenotype of sparteine. Naunyn Schmiedebergs Arch Pharmacol. 1994;350(4):434–439. | ||
Broly F, Marez D, Lo Guidice JM, et al. A nonsense mutation in the cytochrome P450 CYP2D6 gene identified in a Caucasian with an enzyme deficiency. Hum Genet. 1995;96(5):601–603. | ||
Tyndale R, Aoyama T, Broly F, et al. Identification of a new variant CYP2D6 allele lacking the codon encoding Lys-281: possible association with the poor metabolizer phenotype. Pharmacogenetics. 1991;1(1):26–32. | ||
Broly F, Meyer UA. Debrisoquine oxidation polymorphism: phenotypic consequences of a 3-base-pair deletion in exon 5 of the CYP2D6 gene. Pharmacogenetics. 1993;3(3):123–130. | ||
Sakuyama K, Sasaki T, Ujiie S, et al. Functional characterization of 17 CYP2D6 allelic variants (CYP2D6.2, 10, 14A-B, 18, 27, 36, 39, 47-51, 53-55, and 57). Drug Metab Dispos. 2008;36(12):2460–2467. | ||
Marez D, Sabbagh N, Legrand M, Lo-Guidice JM, Boone P, Broly F. A novel CYP2D6 allele with an abolished splice recognition site associated with the poor metabolizer phenotype. Pharmacogenetics. 1995;5(5):305–311. | ||
Masimirembwa C, Persson I, Bertilsson L, Hasler J, Ingelman-Sundberg M. A novel mutant variant of the CYP2D6 gene (CYP2D6*17) common in a black African population: association with diminished debrisoquine hydroxylase activity. Br J Clin Pharmacol. 1996;42(6):713–719. | ||
Oscarson M, Hidestrand M, Johansson I, Ingelman-Sundberg M. A combination of mutations in the CYP2D6*17 (CYP2D6Z) allele causes alterations in enzyme function. Mol Pharmacol. 1997;52(6):10–1040. | ||
Wennerholm A, Johansson I, Hidestrand M, Bertilsson L, Gustafsson LL, Ingelman-Sundberg M. Characterization of the CYP2D6*29 allele commonly present in a black Tanzanian population causing reduced catalytic activity. Pharmacogenetics. 2001;11(5):417–427. | ||
Wennerholm A, Dandara C, Sayi J, et al. The African-specific CYP2D617 allele encodes an enzyme with changed substrate specificity. Clin Pharmacol Ther. 2002;71(1):77–88. | ||
Gaedigk A, Ryder DL, Bradford LD, Leeder JS. CYP2D6 poor metabolizer status can be ruled out by a single genotyping assay for the -1584G promoter polymorphism. Clin Chem. 2003;49(6 Pt 1):1008–1011. | ||
Raimundo S, Toscano C, Klein K, et al. A novel intronic mutation, 2988G>A, with high predictivity for impaired function of cytochrome P450 2D6 in white subjects. Clin Pharmacol Ther. 2004;76(2):128–138. | ||
Meyer UA. Pharmacogenetics and adverse drug reactions. Lancet. 2000;356(9242):1667–1671. | ||
Rogers JF, Nafziger AN, Bertino JS. Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450-metabolized drugs. Am J Med. 2002;113(9):746–750. | ||
Scordo MG, Caputi AP, D’Arrigo C, Fava G, Spina E. Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharmacol Res. 2004;50(2):195–200. | ||
Haufroid V, Hantson P. CYP2D6 genetic polymorphisms and their relevance for poisoning due to amfetamines, opioid analgesics and antidepressants. Clin Toxicol. 2015;53(6):501–510. | ||
Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25(2):115–121. | ||
Feng XQ, Zhu LL, Zhou Q. Opioid analgesics-related pharmacokinetic drug interactions: from the perspectives of evidence based on randomized controlled trials and clinical risk management. J Pain Res. 2017;10:1225–1239. | ||
Foroutan B. Personalized medicine: a review with regard to biomarkers. J Bioequiv Availab. 2015;07(06):244–256. | ||
Zhang XD. Precision medicine, personalized medicine, omics and big data: concepts and relationships. J Pharmacogenomics Pharmacoproteomics. 2015;06(2):2. | ||
Schleyer T, King Z, Miled ZB. A novel conceptual architecture for person-centered health records. AMIA Annu Symp Proc. 2016;2016:1090–1099. | ||
Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. | ||
Shepherd G, Mohorn P, Yacoub K, May DW. Adverse drug reaction deaths reported in United States vital statistics, 1999–2006. Ann Pharmacother. 2012;46(2):169–175. | ||
Matic M, de Wildt SN, Tibboel D, van Schaik RHN. Analgesia and opioids: a pharmacogenetics shortlist for implementation in clinical practice. Clin Chem. 2017;63(7):1204–1213. | ||
Ko TM, Wong CS, Wu JY, Chen YT. Pharmacogenomics for personalized pain medicine. Acta Anaesthesiol Taiwan. 2016;54(1):24–30. | ||
Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95(4):376–382. | ||
Bernard S, Neville KA, Nguyen AT, Flockhart DA. Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implications. Oncologist. 2006;11(2):126–135. | ||
Webster LR, Belfer I. Pharmacogenetics and personalized medicine in pain management. Clin Lab Med. 2016;36(3):493–506. | ||
Gregori M, Gregori S, Ranzani GN, Allegri M, Govoni S, Regazzi M. Individualizing pain therapy with opioids: the rational approach based on pharmacogenetics and pharmacokinetics. Eur J Pain Suppl. 2010;4(S4):245–250. | ||
Somogyi AA, Coller JK, Barratt DT. Pharmacogenetics of opioid response. Clin Pharmacol Ther. 2015;97(2):125–127. | ||
Solhaug V, Molden E. Individual variability in clinical effect and tolerability of opioid analgesics – importance of drug interactions and pharmacogenetics. Scand J Pain. 2017;17:193–200. | ||
Lloyd RA, Hotham E, Hall C, Williams M, Suppiah V. Pharmacogenomics and patient treatment parameters to opioid treatment in chronic pain: a focus on morphine, oxycodone, tramadol, and fentanyl. Pain Med. 2017;18(12):2369–2387. | ||
Linares OA, Daly D, Linares AD, Stefanovski D, Boston RC. Personalized oxycodone dosing: using pharmacogenetic testing and clinical pharmacokinetics to reduce toxicity risk and increase effectiveness. Pain Med. 2014;15(5):791–806. |
Supplementary material
  | Table S1 Phenotypes set for the various combinations of CYP2D6 alleles detected by xTAG CYP2D6 Kit v3 Abbreviations: EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.