Back to Journals » Cancer Management and Research » Volume 11
CXCR7, a Prognostic Biomarker in Cervical Squamous Cell Carcinoma, May Be a Screening Index for Treatment Options at Stages IB1 and IIA1
Authors Zhao D, Qin W, Zhao C, Long J , Li M
Received 26 August 2019
Accepted for publication 21 November 2019
Published 9 December 2019 Volume 2019:11 Pages 10287—10296
DOI https://doi.org/10.2147/CMAR.S228684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Danyang Zhao,1,* Wenban Qin,2,* Chuanxiang Zhao,3 Jianxiong Long,4 Mujun Li1
1Department of Reproductive Center, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021, People’s Republic of China; 2Department of Oncology, Chongzuo People’s Hospital, Nanning 530021, Guangxi Province, People’s Republic of China; 3Department of General Surgery, The Second People’s Hospital of Shenzhen, Nanning 530021, Guangxi Province, People’s Republic of China; 4School of Public Health, Guangxi Medical University, Nanning 530021, Guangxi Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianxiong Long
School of Public Health, Guangxi Medical University, No. 22 Shuangyong Road, Nanning 530021, Guangxi Province, People’s Republic of China
Email [email protected]
Mujun Li
Department of Reproductive Center, The First Affiliated Hospital of Guangxi Medical University, No. 6 Shuangyong Road, Nanning, Guangxi 530021, People’s Republic of China
Email [email protected]
Purpose: Recent studies indicate that CXC chemokine receptor type 7 (CXCR7) is associated with tumorigenesis, progression, and metastasis of various cancers, but its roles and molecular mechanisms of action in cervical squamous cell carcinoma (CSCC) remain unclear. Our purpose was to explore the expression patterns of CXCR7 and epidermal growth factor receptor (EGFR) in CSCC and to identify possible correlations with clinical characteristics. We also tested whether CXCR7 can be a screening index for treatment options for CSCC stages IB1 and IIA1.
Methods: Expression of CXCR7 and EGFR in tumors from 165 patients with CSCC was evaluated by immunohistochemistry and compared with the clinical data including survival.
Results: Patients at CSCC stages IB1 and IIA1 received different treatment options, including radical hysterectomy, pelvic lymph node dissection, and para-aortic lymph node sampling (RH group, 67 patients) or pelvic external-beam radiation therapy with brachytherapy (EBRT group, 34 patients). Disease-free survival (DFS) and overall survival (OS) were compared between two groups at different CXCR7 expression levels. Immunohistochemical staining showed that CXCR7, EGFR, phospho-ERK, and phospho-AKT amounts increased from normal cervical epithelia and cervical intraepithelial neoplasia to CSCC, and CXCR7 was associated with the disease stage, lymph node metastasis, tumor size ≥40 mm, and EGFR expression. Kaplan–Meier analysis revealed that CXCR7 and EGFR expression was associated with shorter DFS and OS. Multivariate analysis suggested that CXCR7 was independently associated with DFS and OS. Prevalence of recurrence and distant metastasis was significantly lower in the EBRT group than in the RH group during CXCR7 expression. Besides, CXCR7 knockdown significantly decreased the proliferation and invasion of CSCC cells.
Conclusion: CXCR7 is coexpressed with EGFR, which may be involved in ERK or AKT pathway activation. CXCR7 may be a screening index for treatment options at CSCC stages IB1 and IIA1.
Keywords: CXC chemokine receptor type 7, cervical cancer, cancer treatment protocol
Introduction
Cervical carcinoma is one of the most common genital-system cancers. Among various malignant tumors, cervical cancer has shown the largest increase in incidence among women, especially in China, and continues an increasing trend of mortality as one of the 10 most prevalent cancers worldwide.1 Although many therapeutic options for cervical cancer are available (surgical treatments, chemotherapy, and radiotherapy), selection of an optimal treatment strategy for patients at different stages is yet to be confirmed by further studies. For example, radical hysterectomy and pelvic external-beam radiation therapy play major roles in the treatment of stages IB1 and IIA1 of cervical squamous cell carcinoma (CSCC). Nonetheless, which of the two treatments is a better option is still unclear.
Recent evidence suggests that CXC chemokine receptor type 7 (CXCR7), a novel receptor of stromal cell-derived factor 1 (CXCL12), is strongly expressed in different tumor types and tumor-associated vasculature but is only weakly expressed in many normal tissues.2–5 Furthermore, CXCR7 is involved in the initiation, progression, invasiveness, and distant metastasis of cancers of the skin, esophagus, lung, and colon. It performs crucial functions in the biological behavior of tumors. Nevertheless, studies on the functions and mechanisms of action of CXCR7 in CSCC are limited.
Some reports have shown that CXCR7, along with a signaling receptor of epidermal growth factor (EGFR), triggers intracellular signaling.6 EGFR and CXC chemokine receptor type 4 (CXCR4, a receptor of CXCL12) have been reported to be associated with distant metastases and disease-free survival (DFS) in cervical cancer. Accordingly, whether CXCR7 has a similar effect on CXCR4 should be determined. The present study was aimed at evaluating the protein expression of CXCR7 and of its putative coreceptor EGFR by immunohistochemical (IHC) analysis as well as at determining their relation with patient characteristics and survival in CSCC. Additionally, we evaluated the potential usefulness of CXCR7 as a prognostic factor and a screening index for treatment options at CSCC stages IB1 and IIA1. These data have not been reported previously.
Materials and Methods
Patients
Between March 2005 and March 2011, data on 106 cases with normal cervical epithelia (NCE), 120 cases of cervical intraepithelial neoplasia (CIN), and 165 cases of CSCC were retrieved from the Pathology Department in The First Affiliated Hospital of Guangxi Medical University and were analyzed retrospectively. All the tissue samples were fixed in formalin and embedded in paraffin. The patients with CSCC ranged in age from 29 to 77 years, with a mean age of 48 years. Among the 165 cases of CSCC, according to the International Federation of Gynecology and Obstetrics 2009 system (FIGO) criteria, 67 cases were FIGO stage IB1–IIA1 (40.6%), 40 cases were FIGO stage IIB–III (24.2%), and 58 cases were FIGO stage IV (35.2%). No subject underwent chemotherapy, radiotherapy, or other adjuvant therapeutic modalities before the tissues were sampled during the surgical operation. After surgical operation, all CSCC patients with high risk factors received radiotherapy.
A total of 106 NCE samples were obtained from patients with uterine myoma who underwent routine hysterectomy. Among the 120 cases of CIN and CIN I, the patients were treated with the surface damage method, whereas CIN II and III patients were treated with focus excision. Of 67 patients at FIGO stages IB1 and IIA1, 33 underwent radical hysterectomy, pelvic lymph node dissection, and para-aortic lymph node sampling (RH group), whereas 34 patients received pelvic external-beam radiation therapy with brachytherapy (EBRT group, total point A dose: 80–85 Gy).
Collection of Clinical Data
All the data including age, histological grade, disease stage, resection margins, vasoinvasion status, parametrial infiltration depth, lymph node metastasis status, and tumor size were retrieved from a combination of clinical and histopathological data, outpatient-clinic medical records, and communication with the patients. According to multidetector-row computed tomography, round or irregular nodes of >10 mm in the major axis in the abdomen and pelvis were clinically diagnosed as lymph node metastasis. The tumor depth was determined as the vertical distance from the epidermis (granular layer) to the deepest lower margin of the SCC. The parametrial infiltration depth was categorized as <15 mm or ≥15 mm because some studies have shown that cervical cancer more than 15 mm deep is likely to metastasize.7
IHC Staining
Expression levels of CXCR7 and EGFR in the resected and biopsy specimens from 391 patients were analyzed by IHC staining. In this procedure, tissue blocks were sectioned (at 4 µm) and deparaffinized with xylene prior to rehydration in a gradient of ethanol solutions. Antigens were retrieved with ethylenediamine tetraacetic acid via heating of the tissue samples in a thermostat-controlled water bath at 95 °C for 15 min, followed by incubation with 3% H2O2 for 10 min to block endogenous peroxidase. After incubation with anti-CXCR7 (monoclonal mouse IgG1 clone No. 11G8, 1:100, R&D Systems, Minneapolis, MN, USA), anti-EGFR (monoclonal rabbit IgG1 clone No. 1902–1, 1:100, Epitomics, Burlingame, CA, USA), anti-phosphorylated-ERK (anti-p-ERK; monoclonal mouse IgG1 clone No. sc-136521, 1:100, Santa Cruz Biotechnology, Dallas, TX, USA), anti-ERK (monoclonal mouse IgG2a clone No. sc-514302, 1:100, Santa Cruz Biotechnology), anti-p-AKT (monoclonal mouse IgG2b clone No. sc-514032, 1:100, Santa Cruz Biotechnology), and anti-AKT (monoclonal rabbit IgG clone No. sc-8312, 1:100, Santa Cruz Biotechnology) antibodies at 4 °C overnight, every section was probed with an appropriate secondary antibody for 50 min at room temperature. The tissue sections were stained with 3,3-diaminobenzidine tetrahydrochloride for 5 min. A negative control involved PBS instead of the primary antibodies, and a positive control included breast cancer tissue in lieu of cervical tissue.
The sections were analyzed by two pathologists without knowledge of the patients’ clinical data. Tumor cells with a brown cytoplasm or membrane were considered positively stained. The staining intensity was scored on a four-point scale: 0, none; 1, weak brown; 2, moderate brown; and 3, strong brown. The percentage of positive tumor cells was classified into four categories: 0, <10%; 1, 10%–49%; 2, 50%–74%; and 3, >75%. A staining index (SI), which was calculated by multiplying the staining intensity by the percentage of positive cells, was assigned to all cases. The SI of 0–3 was classified as negative staining, whereas an SI of more than 3 was classified as positive staining.
Survival Indices
DFS and overall survival (OS) were evaluated to determine the time point of disease recurrence and death from any cause, respectively. DFS was assessed from the date of treatment until the date of either regional recurrence or distant metastasis. The end point for OS was either the date of death or last follow-up examination. We obtained follow-up information in March 2018 through regular outpatient reviews and telephone interviews with the patients or their relatives.
Cell Culture and Transfection
Two human CSCC cell lines (HeLa and SiHa) were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China), and were grown in Dulbecco’s modified Eagle’s medium (DMEM) that was supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin mixture (all from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All cells were kept at 37°C in a humidified incubator containing 5% CO2.
Small interfering (si)RNA targeting CXCR7 (si-CXCR7) and non-targeting siRNA (si-NC) were chemically synthesized by GenePharma Co., Ltd (Shanghai, China). For transfection, cells were seeded into 6-well plates, and were incubated at 37°C with 5% CO2 for about 12 h. Si-CXCR7 or si-NC was transfected into cells using Lipofectamine 2000™ reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer’s protocol.
Reverse-Transcription Quantitative PCR (RT-qPCR)
The isolation of total RNA was performed from si-CXCR7 or si-NC-transfected HeLa and SiHa cells by means of a TRIzol reagent (Thermo Fisher Scientific, Waltham, MA). For the determination of CXCR7 mRNA expression, reverse transcription was conducted using a Prime Script RT reagent Kit (Takara, Tokyo, Japan). The synthesized cDNA was then subjected to amplification through a SYBR Premix Ex Taq (Takara). GAPDH acted as a endogenous control for normalizing the CXCR7 mRNA expression. Relative CXCR7 mRNA was analyzed using the 2–ΔΔCq method.
Cell Counting Kit-8 Assay
Cellular proliferation was assessed through CCK-8 assay. In detail, si-CXCR7 or si-NC-transfected HeLa and SiHa cells were collected, resuspended and then inoculated into 96-well plates with a density of 2,000 cells/well. subsequent to different time incubation, each well was supplemented with 10 µL of the CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan), after which cells were incubated at 37 °C in 5% CO2 incubator for additional 2 h. The absorbance of each well was read at 450 nm wavelength using a microplate reader (BioTek, Winooski, VT, USA).
Transwell Invasion Assay
HeLa and SiHa cells transfected with si-CXCR7 or si-NC were harvested after 48 h culture, washed with DMFM and resuspended in serum-free DMEM. A total of 200 µL cell suspension containing 5×104 cells was inoculated into the upper compartments of Transwell inserts that were precoated with Matrigel (BD Biosciences, Franklin Lakes, New Jersey, USA). The lower compartments were placed with 600 µL DMEM containing 10% FBS. After 24 h culture, the invasive cells were fixed with methanol and stained with 0.5% crystal violet, and photographed using an inverted light microscope (Olympus, Tokyo, Japan). Five visual fields of each insert insert were randomly selected and the average number of invasive cells was counted.
Statistical Analysis
Continuous variables were evaluated by Student’s t test, whereas enumeration data were analyzed using the χ2 test. One-way ANOVA followed by the Student–Newman–Keuls post hoc test was performed to analyze the differences among multiple groups (Figure 1). To investigate whether the expression of CXCR7 in CSCC is associated with EGFR expression, Spearman’s rank correlation test was conducted. Univariate survival curves were constructed by the Kaplan–Meier method, and their differences were analyzed by the logrank test. Univariate and multivariate Cox regression models were employed to process the data by means of SPSS 18.0 software (Chicago, IL, USA). Statistical significance was set to P < 0.05.
Results
Quantitation of CXCR7, EGFR, ERK, p-ERK, AKT, And p-AKT in NCE, CIN, and CSCC
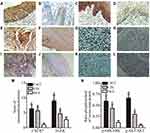
IHC staining of CXCR7 in CSCC tissue sections was detected in the cytoplasm (Figure 1A), and negative CXCR7 staining is shown in Figure 1B. The expression of CXCR7 was significantly higher in CSCC than in NCE and CIN (P < 0.05; Table 1). Staining for EGFR was the strongest in the membrane and cytoplasm in the CSCC group (Figure 1C), with Figure 1D illustrating negative EGFR staining. The positivity prevalence of EGFR in CSCC was 64.2% (106 of 165 cases). EGFR was expressed in 62 out of 120 CIN cases (51.7%). Of the 106 NCE tissue samples, only 39 (36.8%) yielded uniformly detectable EGFR immunostaining. The positivity prevalence rates of EGFR were significantly higher among the CSCC and CIN tissue samples than among NCE tissue samples (P < 0.05). In addition, we determined the amounts of ERK, p-ERK, AKT, and p-AKT in the same groups of tissue samples. Similar to the expression status of EGFR, the amounts of ERK (Figure 1E and F; P < 0.05), p-ERK (Figure 1G and H; P < 0.05), AKT (Figure 1I and J; P < 0.05), and p-AKT (Figure 1K and L; P < 0.05) were all higher in CSCC and CIN tissue samples than in the NCE tissue samples. The statistical analysis of the IHC results on CXCR7 and EGFR is presented in Figure 1M. The ratio of phosphorylated to total protein for ERK and AKT was analyzed and is shown in Figure 1N.
 |
Table 1 Correlation Between CXCR7 Expression and Clinical Data |
Clinical Characteristics in Relation to the Expression of CXCR7 in the Tumors of Patients with CSCC
Clinical characteristics of the 165 patients with CSCC are summarized in Table 1. The mean age at diagnosis was 48 years (range, 29–77 years). The expression of CXCR7 was significantly associated with the stage of CSCC, and strong staining for CXCR7 corresponded to more advanced stages (P < 0.05; Table 1). Moreover, CXCR7 status was associated with lymph node metastasis and tumor size ≥40 mm in cervical cancer (both P < 0.05; Table 1). CXCR7 expression significantly correlated with EGFR expression (r = 0.880, P < 0.05). No other associations between CXCR7 and clinical characteristics were found.
DFS and OS in Relation to CXCR7 and EGFR Expression in CSCC
To determine whether the expression levels of CXCR7 and EGFR correlate with DFS or OS in CSCC, patients were classified into CXCR7-negative cases (n = 69), CXCR7-positive cases (n = 96), EGFR-negative cases (n = 59) without CXCR7 expression (n = 53), and EGFR-positive cases (n = 106) with CXCR7 expression (n = 96). Follow-up information revealed that the median survival time was 57 months (range, 5–156 months), and 52 patients (31.5%) died of CSCC. Kaplan–Meier analysis suggested that patients with the expression of CXCR7 and EGFR in the tumor had significantly shorter DFS and OS than those without this expression pattern (P < 0.05; Figure 2A–F).
Univariate Cox regression analysis of all the clinical characteristics indicated that CXCR7 (hazard ratio [HR] = 0.260, 95% confidence interval [CI] 0.117–0.376, P = 0.0002), EGFR (HR = 0.394, 95% CI 0.226–0.687, P = 0.001), tumor size (HR = 0.468, 95% CI 0.268–0.816, P = 0.007), and lymph node metastasis (HR = 0.484, 95% CI 0.277–0.845, P = 0.011) were independently associated with DFS (Table 2). Univariate analysis revealed that the expression levels of CXCR7 and EGFR and tumor size were prognostic factors of OS (HR = 0.300 with P = 0.001; HR = 0.450 with P = 0.004; and HR = 0.533 with P = 0.024, respectively). Lymph node metastasis was also found to be a prognostic factor of OS (HR = 0.549; P = 0.032; Table 2).
 |
Table 2 Univariate and Multivariate Analyses of Clinical and IHC Factors Among the 165 Patients with CSCC |
Multivariate Cox regression analysis revealed that only CXCR7 (HR = 0.351, 95% CI 0.200–0.614, P = 0.0007) was an independent predictor of DFS after adjustment for EGFR, tumor size, and lymph node metastasis status. In multivariate Cox proportional hazards analysis, CXCR7 was a significant prognostic factor of OS in CSCC (HR = 0.405, 95% CI 0.233–0.702, P = 0.001; Table 2).
Survival Analysis for the Two Therapeutic Options (RH and EBRT) at Different CXCR7 Expression Levels
To test whether CXCR7 expression is useful as a screening index for treatment options at stages IB1 and IIA1 of CSCC, groups were compiled of CXCR7-negative cases (n = 37), including the RH group (n = 16) and EBRT group (n = 21), and CXCR7-positive cases (n = 30), including the RH group (n = 17) and EBRT group (n = 13). The median follow-up duration among patients with stage IB1 or IIA1 CSCC was 45 months (95% CI 41.4–55.3). The median follow-up duration was 43 and 48.5 months, for the RH group (95% CI 34.5–55.5) and EBRT group (95% CI 42.1–61.2), respectively. Furthermore, 19 patients (28.4%) died of stage IB1 or IIA1 CSCC. Kaplan–Meier analysis showed that cases with CXCR7 expression manifested no significant difference in OS and DFS between groups RH and EBRT (P = 0.141). Kaplan–Meier analysis of the cases of negative CXCR7 expression yielded similar results (P = 0.299).
Survival Outcomes
The details of failure patterns of the two therapeutic options at different expression levels of CXCR7 are listed in Table 3. A total of 33 patients experienced locoregional failure, and the most common failure site was the vagina. Approximately 36 cases experienced distant metastasis, and the most common failure site was the lungs. Among the 37 CXCR7-negative cases, no significant difference was found in the failure pattern between the RH group and EBRT group (recurrence, P = 0.104; locoregional failure, P = 0.508; and distant metastasis, P = 0.743). Among the 30 CXCR7-positive cases, the prevalence rates of recurrence and distant metastasis were significantly lower in the EBRT group than in the RH group (P = 0.007 and P = 0.025, respectively; Table 3). By contrast, no significant difference between the RH group and EBRT group was found in terms of locoregional failure (P = 0.484).
 |
Table 3 Failure Patterns of the Two Treatment Options at Different Expression Levels of CXCR7 |
Roles of CXCR7 Knockdown in CSCC
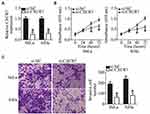
To test the functions of CXCR7 in CSCC progression, we silenced its expression in HeLa and SiHa cells via transfecting with si-CXCR7. The HeLa and SiHa cells that were treated with si-NC served as the control. RT-qPCR analysis confirmed the successful knockdown of CXCR7 in HeLa and SiHa cells following si-CXCR7 transfection (Figure 3A, P < 0.05). CCK-8 and Transwell invasion assays were respectively applied to examine the impacts of CXCR7 knockdown on HeLa and SiHa cells. The proliferative (Figure 3B, P < 0.05) and invasive (Figure 3C, P < 0.05) capacities of HeLa and SiHa cells were turned out to be obviously hindered after CXCR7 silencing. These results collectively demonstrated that CXCR7 plays a cancer-promoting part in CSCC progression.
Discussion
Aside from CXCR4, CXCR7 was recently identified as a novel G protein–coupled seven-span transmembrane receptor of CXCL12, which is strongly expressed in many cancers, such as esophageal squamous cell carcinoma, bronchogenic carcinoma, pancreatic cancer, and bladder cancer.8–10 In the present study, IHC staining proved that CXCR7 is frequently expressed in the cytoplasm or membrane of cervical epithelial cells, suggesting that the source of CXCR7 in the tumor is tumor cells and that CXCR7 may play a part in tumorigenesis.
Furthermore, this study addressed CXCR7 expression in NCE, CIN, and CSCC and revealed that the highest expression of CXCR7 among the three cervical epithelial tissue types is present in CSCC lesions. Similar results have been reported on bladder cancer.10 Besides, our study shows that CXCR7 manifests stage-dependent expression in CSCC. High CXCR7 expression indicated more lymph node metastases and greater tumor size in patients with CSCC. These results suggest that CXCR7 expression may be conducive to metastasis and aggressive tumor growth, for the following reasons. First, rapid growth of tumor tissue results in a microenvironment featuring local hypoxia. Hypoxic preconditions induce the expression of CXCR7 mRNA in squamous epithelial cervical carcinoma cells, which resemble bone marrow–derived mesenchymal stem cells. Moreover, the high expression of CXCR7 in malignant tumors may be due to hypoxia; this finding has been confirmed by several studies.3 Second, CXCR7 might participate in the formation of (and angiogenesis in) CSCC because CXCR7 is strongly expressed in tumor-associated blood vessels in breast and prostate cancer tissues.11,12 Other researchers have reported that selective CXCR7 upregulation in (or application of CXCR7 to) hypoxic lungs has a significant effect on pulmonary vascular-cell proliferation and vascular remodeling. Third, CXCR7 can promote the migration and adhesion of squamous epithelial cervical carcinoma cells, and a decrease in CXCR7 expression reduces the number of tumor cells.13
Our data also mean that CSCC tumors overexpress EGFR, which was more strongly expressed in cervical cancer tissues than in the other two types of tissue. Of note, CXCR7 was found to be statistically significantly coexpressed with EGFR in the tumors; both were associated with DFS and OS in CSCC. The high expression of CXCR7 was related to shorter survival, and survival was poor among the patients with CSCC and positive EGFR staining in the tumor, indicating that CXCR7 may form heterodimers with EGFR for optimal intracellular signaling.14,15 Although our present study supports this hypothesis, functional studies on cervical cancer cell lines should be conducted to confirm this conclusion. Accordingly, a series of functional experiments were performed to determine the functions of CXCR7 on CSCC progression. The results indicated that interference of CXCR7 expression resulted in an obvious decrease of CSCC cell proliferation and invasion. Hence, CXCR7 worked as an oncogene in the malignancy of CSCC, and might be a potential target for treating patients with this disease.
Additionally, we noted that CXCR7, EGFR, tumor size, and lymph node metastasis were prognostic risk factors of DFS and OS in our univariate analysis. Only CXCR7 was an independent prognostic factor in our multivariate analysis, which suggested that tumor CXCR7 expression may represent a subset of “high-risk” patients or could serve as a prognostic biomarker of CSCC. On the other hand, our results are inconsistent with some studies.8,16 These discrepancies might be due to different histological sources or different patient enrollment conditions leading to variation in prognoses. CSCC is a squamous epithelial malignant tumor, whereas hepatic cellular cancer (in the other studies) is an epithelioglandular tumor. Therefore, the secretion of CXCR7 may be affected by different histological sources, thereby leading to the discrepancy in results between this and other studies. In addition, factors such as age, medical history, and clinical stage possibly affect the DFS and OS of patients with malignant tumors.
A number of treatment options are available for the FIGO stages IB1 and IIA1 of CSCC. Nonetheless, selecting a feasible therapeutic option is an issue that clinicians must address. Our study shows for the first time that external-beam radiation therapy with brachytherapy is a feasible therapeutic regimen for the FIGO stages IB1 and IIA1 of CSCC with high expression of CXCR7 in the tumor.
This study revealed no survival advantage between groups RH and EBRT during either the presence or absence of CXCR7 expression. The 37 patients with negative CXCR7 staining exhibited no significant difference between the RH group and EBRT group in terms of failure patterns. Among the 30 patients with CXCR7 expression, the failure patterns of RH and EBRT for CSCC stages IB1 and IIA1 were compared, and the data showed that pelvic external-beam radiation therapy with brachytherapy significantly reduced the probability of recurrence and distant metastasis. Therefore, to clinicians, when high CXCR7 expression is present, pelvic external-beam radiation therapy with brachytherapy may be recommended to minimize recurrence and promote local control. Nevertheless, clinical trials are needed to analyze the postoperative adverse reactions and side effects of pelvic external-beam radiation therapy with brachytherapy; the resultant data should make the screening index based on CXCR7 highly clinically relevant and convincing.
In conclusion, the findings of this retrospective study suggest that CXCR7 may cause aggressive tumor growth and may be a valuable prognostic biomarker in CSCC. CXCR7 and EGFR turned out to be related to cervical carcinogenesis and associated with CSCC progression, which may involve the activation of the ERK or AKT pathway. These observations could offer new molecular targets for treating CSCC. Nevertheless, further studies are necessary to identify the exact mechanisms of CXCR7 and EGFR actions on CSCC progression. Moreover, CXCR7 may serve as a screening index for treatment options (RH and EBRT) among patients with stage IB1 or IIA1 CSCC. Randomized controlled trials are necessary to find the optimal therapeutic regimen in the case of CXCR7 overexpression owing to the limitations of the retrospective design, our small sample size, and a lack of patients receiving pelvic external-beam radiation therapy and brachytherapy with or without concurrent chemotherapy.
Abbreviations
CI, confidence interval; CIN, cervical intraepithelial neoplasia; CSCC, cervical squamous cell carcinoma; DFS, disease-free survival; EBRT group, pelvic external-beam radiation therapy with brachytherapy; FIGO, International Federation of Gynecology and Obstetrics 2009 system; HR, hazard ratio; IHC, immunohistochemical; NCE, normal cervical epithelia; OS, overall survival; p-AKT, phospho-AKT; RH group, radical hysterectomy, pelvic lymph node dissection, and para-aortic lymph node sampling; SI, staining index.
Consent for Publication
All the patients approved the publication of the images associated with this study.
Ethics Approval and Consent to Participate
The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (Guangxi, China). Every patient provided verbal informed consent because of the absence of an existing standard for written consent in our department.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding authors on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Tang T, Xia Q, Xi M. CXC chemokine receptor 7 expression in cervical intraepithelial neoplasia. Biomed Rep. 2016;4(1):63–67. doi:10.3892/br.2015.529
3. Tang T, Xia QJ, Qiao X, Xi M. Expression of C-X-C chemokine receptor type 7 in otorhinolaryngologic neoplasms. Singapore Med J. 2016;57(3):157–160. doi:10.11622/smedj.2016057
4. Wu K, Cui L, Yang Y, et al. Silencing of CXCR2 and CXCR7 protects against esophageal cancer. Am J Transl Res. 2016;8(8):3398–3408.
5. Xia J, Wang J, Chen N, et al. Expressions of CXCR7/ligands may be involved in oral carcinogenesis. J Mol Histol. 2011;42(2):175–180. doi:10.1007/s10735-011-9322-x
6. Tachezy M, Zander H, Gebauer F, et al. CXCR7 expression in esophageal cancer. J Transl Med. 2013;11:238. doi:10.1186/1479-5876-11-238
7. Schrevel M, Karim R, Ter Haar NT, et al. CXCR7 expression is associated with disease-free and disease-specific survival in cervical cancer patients. Br J Cancer. 2012;106(9):1520–1525. doi:10.1038/bjc.2012.110
8. Goto M, Yoshida T, Yamamoto Y, et al. CXCR4 expression is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2017;24(3):832–840. doi:10.1245/s10434-015-4974-5
9. Roy I, Zimmerman NP, Mackinnon AC, Tsai S, Evans DB, Dwinell MB. CXCL12 chemokine expression suppresses human pancreatic cancer growth and metastasis. PLoS One. 2014;9(3):e90400. doi:10.1371/journal.pone.0090400
10. Nazari A, Khorramdelazad H, Hassanshahi G. Biological/pathological functions of the CXCL12/CXCR4/CXCR7 axes in the pathogenesis of bladder cancer. Int J Clin Oncol. 2017;22(6):991–1000. doi:10.1007/s10147-017-1187-x
11. Hattermann K, Holzenburg E, Hans F, Lucius R, Held-Feindt J, Mentlein R. Effects of the chemokine CXCL12 and combined internalization of its receptors CXCR4 and CXCR7 in human MCF-7 breast cancer cells. Cell Tissue Res. 2014;357(1):253–266. doi:10.1007/s00441-014-1823-y
12. Zheng J, Wang J, Sun X, et al. HIC1 modulates prostate cancer progression by epigenetic modification. Clin Cancer Res. 2013;19(6):1400–1410. doi:10.1158/1078-0432.CCR-12-2888
13. Xu D, Li R, Wu J, Jiang L, Zhong HA. Drug design targeting the CXCR4/CXCR7/CXCL12 pathway. Curr Top Med Chem. 2016;16(13):1441–1451. doi:10.2174/1568026615666150915120218
14. Luo Y, Azad AK, Karanika S, et al. Enzalutamide and CXCR7 inhibitor combination treatment suppresses cell growth and angiogenic signaling in castration-resistant prostate cancer models. Int J Cancer. 2018;142(10):2163–2174. doi:10.1002/ijc.v142.10
15. Hoy JJ, Kallifatidis G, Smith DK, Lokeshwar BL. Inhibition of androgen receptor promotes CXC-chemokine receptor 7-mediated prostate cancer cell survival. Sci Rep. 2017;7(1):3058. doi:10.1038/s41598-017-02918-3
16. Semaan A, Dietrich D, Bergheim D, et al. CXCL12 expression and PD-L1 expression serve as prognostic biomarkers in HCC and are induced by hypoxia. Virchows Arch. 2017;470(2):185–196. doi:10.1007/s00428-016-2051-5
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.