Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Cutaneous Macroglobulinosis Presenting as Serpiginous Purpura, a Case Report and Literature Review
Authors Sukanjanapong S, Chanprapaph K , Rutnin S , Boonsakan P, Pukiat S
Received 8 July 2021
Accepted for publication 18 August 2021
Published 26 August 2021 Volume 2021:14 Pages 1057—1064
DOI https://doi.org/10.2147/CCID.S327647
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Siriorn Sukanjanapong,1 Kumutnart Chanprapaph,1 Suthinee Rutnin,1 Paisan Boonsakan,2 Sulada Pukiat3
1Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Department of Pathology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 3Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Kumutnart Chanprapaph
Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchathewi, Bangkok, 10400, Thailand
Tel +66-2-2011141
Fax +66-2-2011211
Email [email protected]
Abstract: Cutaneous macroglobulinosis is a rare manifestation of Waldenstrom macroglobulinemia when there is deposition of IgM in the dermis. The clinical presentation varies from skin colored to pink papules and ulcerative nodules on trunk, extensor surfaces of upper and lower limbs to hyperkeratotic lesions on the soles. We herein report a 78-year-old-male with Waldenstrom macroglobulinemia who presented with serpiginous purpura. The differential diagnoses included occlusion of vessel lumen by the lymphoplasmacytoid cells, high level of IgM or cryoglobulin, as well as small to medium vasculitis secondary to Waldenstrom macroglobulinemia. The histopathology revealed vasculopathy and vasculitis, while further immunohistochemistry highlighted deposition in the vessel lumen and vessel wall with IgM, suggesting the diagnosis of cutaneous macroglobulinosis. In this case report, we discuss this rare presentation and reviewed previous cases of cutaneous macroglobulinosis.
Keywords: Waldenstrom macroglobulinemia, lymphoplasmacytic lymphoma, monoclonal gammopathy, vasculitis, vasculopathy
Introduction
Waldenstrom macroglobulinemia (WM) is a low-grade B-cell lymphoma characterized by lymphoplasmacytic infiltration of the bone marrow of 10% or greater and immunoglobulin M (IgM) monoclonal gammopathy in the blood.1,2 Cutaneous involvement in WM can be specific, with either lymphoplasmacytic tumor cells or monoclonal IgM deposition in the skin, or non-specific, for example, lesions that are related to hyperviscosity or cryoglobulinemia.3 Monoclonal IgM infiltration in the skin has been termed cutaneous macroglobulinosis (CM) or IgM storage papule disease. The IgM deposition is well recognized to be in the dermis or superficial vascular lumen, producing a clinical picture of skin-colored to pink papules, nodules or plaques on trunk or extensor surfaces of the extremities.4 Cutaneous vasculitis has rarely been reported in the context of cutaneous macroglobulinosis. We hereby present a case with IgM deposition in the vascular lumens and vessel walls causing vasculopathy and vasculitis presenting as serpiginous purpura in a patient with WM.
Case Report
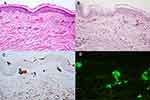
A 78-year-old man with underlying type II diabetes, hypertension, dyslipidemia and coronary artery disease presented at the hematologist clinic with malaise for 2 months. The initial laboratory findings revealed normochromic anemia (Hb 9.40 g/dL, Hct 30%, Plt 175,000/cumm), hyperglobulinemia, hypercalcemia (corrected calcium 12.1 mg/dL) and elevated creatinine (1.88 mg/dL). His serum electrophoresis exhibited a monoclonal peak at IgM region, while IgM kappa was detected on immunofixation and serum IgM protein level exceeded 58.5 g/l. A bone marrow aspiration and biopsy later showed infiltration by a small B-cell neoplasm with plasma cell differentiation (20–25%), consistent with lymphoplasmacytic lymphoma (LPL). Therefore, the diagnosis of WM was established. Dermatologic consultation was made due to an asymptomatic progressive erythematous rash on the abdomen and both thighs for 4 days. The patient had no symptoms of hyperviscosity and had not received any treatment for WM when the rash first appeared. On examination, there were multiple linear and serpiginous non-blanchable erythematous patches on abdomen and both thighs (Figure 1A–C). The lesions were consistent with purpura along vessel territory and the clinical differential diagnosis included both causes of vasculopathy and small to medium vessel vasculitis. A skin biopsy of the lesion on his abdomen revealed dense superficial perivascular infiltration with lymphocytes, neutrophils, nuclear dusts and extravasated red blood cells, along with some superficial vascular lumen occlusions (Figure 2A). There was no evidence of intravascular neoplastic cell infiltration. Further staining with Periodic acid–Schiff (PAS) showed eosinophilic homogenous materials in vascular lumens and vascular walls (Figure 2B), which were negative for Congo red. Immunohistochemistry also highlighted deposition in the vessel lumens and vessel walls with IgM (Figure 2C), but not with IgG, IgA and IgD. On direct immunofluorescence, homogenous deposition in the vessel lumen was detected with multiple immunoglobulins, including IgM, IgA and C3. With C3, both homogenous deposition in the vessel walls and granular deposition on the perivascular areas were detected (Figure 2D). The patient was diagnosed with vasculopathy and vasculitis, in association with cutaneous macroglobulinosis. Further investigations for secondary vasculitis, including viral hepatitis B and C profile, cryoglobulin, antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (C- and P- ANCA) and anti-HIV, were all negative. To prevent a pseudonegative result, the blood sample taken for cryoglobulinemia was transported and allowed to clot at 37 degrees Celsius for 1 hour. After warm centrifugation, the serum was then stored at 4 degrees Celsius for observation of cryoprecipitate. In addition, the patient denied any use of medications that can cause purpura.
 |
Figure 1 Clinical presentation: (A) multiple linear and serpiginous purpura on the abdomen, (B) back, (C) left thigh. |
After receiving dexamethasone (5mg IV every 8 hours) and the first cycle of rituximab (375 mg/m2) and bendamustine, the skin lesions disappeared in approximately one week (Figure 3).
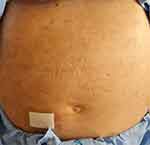 |
Figure 3 Resolution of lesions on the abdomen. |
Discussion
WM, defined as LPL with monoclonal IgM, contributes to approximately 2% of all hematologic malignancies.1 MYD88 L265P somatic mutation was found in more than 90% of WM patients.1 Non-specific cutaneous involvement is more common and includes lesions that are related to hyperviscosity, cryoglobulinemia or miscellaneous causes, eg, Schnitzler’s syndrome.3 The rare specific involvement can be caused by infiltration of the LPL or deposition of IgM in the dermis. Malignant LPL cell infiltration presents as plaques, papules and nodules on the face, trunk and extremities. MYD88 L265P mutation can be detected from skin biopsy and they can have large cell transformation, which gives a poorer prognosis.5
Deposition of IgM in the dermis was first termed macroglobulinemia cutis in 1978 by Tichenor et al.6 Since then, numerous case reports have identified the condition as cutaneous macroglobulinosis or IgM storage papule disease (Table 1). The clinical presentation varies from skin colored to pink papules and ulcerative nodules on trunk, extensor surfaces of upper and lower limbs to hyperkeratotic lesions on the soles. On histopathology, they uniformly showed abundant homogenous eosinophilic deposition in the dermis and occasionally within the vessel lumen. Transepidermal elimination was also observed in necrotic and crusted lesions. The material is stained positive for PAS and negative for all amyloid-specific stains. Evidence of IgM deposition can be demonstrated through immunohistochemical staining with IgM or direct immunofluorescence. Apart from CM, IgM in WM can be directed against the basement membrane zone, leading to IgM bullous disease, with various clinical pictures including pruritic papules, vesiculobullous lesions or urticaria.7
 |  |  |
Table 1 Review of Case Reports and Case Series of Cutaneous Macroglobulinosis (CM) |
In our patient, the IgM deposition was found in both vessel lumens and vessel walls, as highlighted by PAS staining and IgM immunohistochemical staining. We believe the presence of IgM in these locations has led to occlusive vasculopathy and secondary vasculitis. However, it was difficult to determine which pathology occurred first, as IgM infiltration in vessel wall could have caused the vasculitis, and consequently secondary vasculopathy. As mentioned above, the presence of cutaneous IgM deposition in the context of WM is termed CM. To our knowledge, this is the first case of CM clinically presenting as serpiginous purpura and histologically confirmed vasculopathy and vasculitis. In the past, IgM deposition in vascular lumen or vessel wall has been described,8,9 but the clinical presentations were papules, nodules and plaques, unlike serpiginous purpura like in our patient. Most of CM presented with clinical presentation of papules and nodules and was mainly due to the infiltration in dermis. In addition, two previous cases have reported leukocytoclastic vasculitis in WM patients who later developed CM, but not within the same lesion.10
There is an extensive list of differential diagnoses which must be excluded in this patient, mainly divided into conditions that cause vessel lumen occlusion and vessel wall damage. Given the diagnosis of WM, vessel lumen occlusion can be caused by numerous lymphoplasmacytoid cells or from high level of IgM, both leading to hyperviscosity syndrome. Cryoglobulinemia type I can also be associated with WM, with vessel occlusion being the predominant presentation. However, our biopsy result has failed to demonstrate any infiltration of lymphoplasmacytoid cells and cryoglobulinemia was negative in this patient. As mentioned earlier, other causes of small vessel vasculitis were excluded via blood test and we believed the vasculitis was most likely secondary to the vasculopathy. Finally, drug-induced purpura can be excluded as the patient denied any use of medications that can cause purpura.
Symptoms of hyperviscosity are common in WM and can manifest as acral purpura, mucosal bleeding and peripheral edema.3 In our case, hyperviscosity was assumed to contribute to the occlusive vasculopathy. Despite having no symptoms of hyperviscosity, eg, visual impairment or headaches, his screening eye examination revealed dotted hemorrhages, Roth spots and vein dilatation which were consistent with hyperviscosity syndrome.
CM can occur before, concurrent or after the diagnosis of WM. The cutaneous manifestation can predict a latent plasma cell dyscrasia before any other clinical or pathologic evidence.4 Overall, CM may be linked to the underlying WM, as improvement of WM often leads to resolution of CM. Rituximab, cyclophosphamide and chlorambucil had successfully cleared the lesion. In consistent with previous case reports, treatment with rituximab in our case had led to complete resolution of the cutaneous lesions. We assume that the dexamethasone administration has resulted in a partial response. When rituximab and bendamustine were then given to the patient, their synergistic effects may decrease blood viscosity leading to a rapid clearance of lesions in our case. Nevertheless, there was no solid evidence of improvement of vasculopathy as we did not re-biopsy the patient. Although most studies have agreed that CM reflects disease activity, a study had found no correlation between IgM levels and progression of cutaneous lesions.11 Moreover, CM is not a prognosis marker of WM as lesion can occur in both WM cases with good treatment response and refractory cases with poor outcome.10
The accumulation of IgM in the skin can occur at the same time as accumulation of IgM in the myelin sheath.9 Previous studies have hypothesized that CM may be related to peripheral neuropathy, with symptoms of paresthesia of the legs and detection of anti–myelin-associated glycoprotein (MAG) antibodies.10 However, in our patient there were no neurological symptoms and test for anti-MAG antibodies was not performed.
In conclusion, we present a 78-year-old male with WM who developed linear and serpiginous purpura on abdomen. Serpiginous purpura along blood vessel territories has different causes such as vessel lumen occlusion by hyperviscosity syndrome, hypercoagulable state or malignant cells, or a wide range of conditions with small to medium vessel vasculitis. However, cutaneous macroglobulinosis should always be considered in patients with WM. In our patient, histopathology and special stains have demonstrated IgM deposition in the vessel lumen and vessel wall, leading to the diagnosis of cutaneous macroglobulinosis presenting as vasculopathy and vasculitis.
Consent Statement
Written informed consent was provided by the patient to have the case details and any accompanying images published. Institutional approval was not required to publish the case details.
Funding
Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yun S, Johnson AC, Okolo ON, et al. Waldenström Macroglobulinemia: review of pathogenesis and management. Clin Lymphoma Myeloma Leuk. 2017;17(5):252–262. doi:10.1016/j.clml.2017.02.028
2. Vijay A, Gertz MA. Waldenström macroglobulinemia. Blood. 2007;109(12):5096–5103. doi:10.1182/blood-2006-11-055012
3. Libow LF, Mawhinney JP, Bessinger GT. Cutaneous Waldenström’s macroglobulinemia: report of a case and overview of the spectrum of cutaneous disease. J Am Acad Dermatol. 2001;45(6 Suppl):S202–6. doi:10.1067/mjd.2001.103262
4. Hassab-El-Naby HMM, El-Khalawany M, Rageh MA. Cutaneous macroglobulinosis with Waldenström macroglobulinemia. JAAD Case Rep. 2020;6(8):771–775. doi:10.1016/j.jdcr.2020.06.024
5. Stien S, Durot E, Durlach A, et al. Cutaneous involvement in Waldenström’s macroglobulinaemia. Acta Derm Venereol. 2020;100(15):adv00225. doi:10.2340/00015555-3535
6. Tichenor RE, Rau JM, Mantz FA. Macroglobulinemia cutis. Arch Dermatol. 1978;114(2):280–281. doi:10.1001/archderm.1978.01640140090024
7. Cobb MW, Domloge-Hultsch N, Frame JN, Yancey KB. Waldenström macroglobulinemia with an IgM-kappa antiepidermal basement membrane zone antibody. Arch Dermatol. 1992;128(3):372–376. doi:10.1001/archderm.1992.01680130086011
8. Oshio-Yoshii A, Fujimoto N, Shiba Y, et al. Cutaneous macroglobulinosis: successful treatment with rituximab. J Eur Acad Dermatol Venereol. 2017;31(1):e30–e31. doi:10.1111/jdv.13613
9. Camp BJ, Magro CM. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012;39(10):962–970. PMID: 22882527. doi:10.1111/j.1600-0560.2012.01983.x
10. Gressier L, Hotz C, Lelievre JD, et al. Cutaneous macroglobulinosis: a report of 2 cases. Arch Dermatol. 2010;146(2):165–169. doi:10.1001/archdermatol.2009.359
11. Bernstein LE, Shea CR, Baron JM. Erythematous papules on the legs. IgM storage papules in association with Waldenstrom macroglobulinemia. Arch Dermatol. 2009;145:77.
12. Mascaro JM, Montserrat E, Estrach T, et al. Specific cutaneous manifestations of Waldenström’s macroglobulinaemia: a report of two cases. Br J Dermatol. 1982;106(2):217–222. doi:10.1111/j.1365-2133.1982.tb00932.x
13. Harnalikar M, Pande S, Kharkar V, Khopkar U. Keratotic vascular papules over the feet: a case of Waldenström’s macroglobulinaemia-associated cutaneous macroglobulinosis. Clin Exp Dermatol. 2010;35(3):278–281. doi:10.1111/j.1365-2230.2009.03651.x
14. Lüftl M, Sauter-Jenne B, Gramatzki M, Eckert F, Jenne L. Cutaneous macroglobulinosis deposits in a patient with IgM paraproteinemia/incipient Waldenström macroglobulinemia. J Dtsch Dermatol Ges. 2010;8(12):1000–1003.
15. Marchand T, Tas P, Houot R, et al. Cutaneous macroglobulinosis treated with bortezomib and rituximab. Eur J Haematol. 2011;87(1):98. doi:10.1111/j.1600-0609.2011.01610.x
16. D’Acunto C, Nigrisoli E, Liardo EV, et al. Painful plantar nodules: a specific manifestation of cutaneous macroglobulinosis. J Am Acad Dermatol. 2014;71(6):e251–e252. doi:10.1016/j.jaad.2014.08.041
17. Roupie AL, Battistella M, Talbot A, et al. Coexisting cutaneous macroglobulinosis and scleredema of Buschke in a patient with a Waldenstrom macroglobulinemia. J Eur Acad Dermatol Venereol. 2019;33(3):e104–e106. doi:10.1111/jdv.15268
18. Fayne R, Rosenberg M, White K, et al. Disseminated cutaneous immunoglobulin M macroglobulinosis associated with cryoglobulinemia and minimal residual disease of Waldenstrom macroglobulinemia. JAAD Case Rep. 2019;5(10):918–922. doi:10.1016/j.jdcr.2019.06.037
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.