Back to Journals » Breast Cancer: Targets and Therapy » Volume 9
Current perspectives on CHEK2 mutations in breast cancer
Authors Apostolou P , Papasotiriou I
Received 21 January 2017
Accepted for publication 20 April 2017
Published 12 May 2017 Volume 2017:9 Pages 331—335
DOI https://doi.org/10.2147/BCTT.S111394
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Panagiotis Apostolou, Ioannis Papasotiriou
Department of Molecular Medicine, Research Genetic Cancer Centre S.A. (R.G.C.C. S.A.), Florina, Greece
Abstract: Checkpoint kinase 2 (CHEK2) is a serine/threonine kinase which is activated upon DNA damage and is implicated in pathways that govern DNA repair, cell cycle arrest or apoptosis in response to the initial damage. Loss of kinase function has been correlated with different types of cancer, mainly breast cancer. CHEK2 functionality is affected by different missense or deleterious mutations. CHEK2*1100delC and I157T are most studied in populations all over the world. Although these variants have been identified in patients with breast cancer, their frequency raises doubts about their importance as risk factors. The present article reviews the recent advances in research on CHEK2 mutations, focusing on breast cancer, based on the latest experimental data.
Keywords: CHEK2, breast cancer
Introduction
Breast cancer is one of the leading causes of death among women worldwide, and it has been reported as the topmost cause of mortality among female cancer patients in the US in 2016.1 According to recent estimations, by the year 2026, the number of breast cancer patients will be more than 4.5 million in the US, compared to 3.5 million recorded in 2016.2 Management and treatment of cancer are correlated with the diagnosis and specific characteristics of the tumor, such as hormone production and genetic mutations.3 The most well-studied genes that correlate with risk susceptibility are BRCA1/2, while other genes that correlate with an increased risk for breast cancer have been identified as well; one of these genes is CHEK2, a tumor suppressor gene that encodes a serine/threonine kinase, the CHK2. This gene is involved in pathways such as DNA repair, cell cycle regulation and apoptosis in response to DNA damage. Mutations of CHEK2 have been implicated in various types of cancer including breast cancer. The present article reviews the most recent advances in research on CHEK2 and breast cancer, focusing on mutations and how they could be correlated with diagnosis or prognosis of the disease.
CHEK2 gene
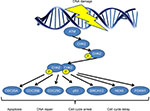
CHEK2 gene is activated by phosphorylation of Thr68 by ATM, which causes the dimerization of the gene enabling it to acquire kinase activity. CHEK2 then reacts with downstream phosphatase CDC25, serine/threonine protein kinase NEK6, transcription factor FOXM1, p53 protein and BRCA1 or BRCA2.4 CHEK2 regulates cell division by preventing cells from entering mitosis or arresting cell cycle in gap 1 phase (G1), in response to DNA damage (Figure 1). Therefore, CHEK2 is essential for cell cycle regulation, and its abnormal expression could lead to cancer. Besides gene and/or protein expression of CHK2, mutations in genomic DNA have been implicated in abnormal functioning of the CHK2 protein. Various aberrations in CHEK2 gene have been recorded, including 1100delC, I157T, R117G, I160M, G167R, G167A and so on.5 Among them, 1100delC and I157T are the most well-studied and have been correlated with risk susceptibility to cancer.
CHEK2 in cancer
CHEK2 germline mutations had been implicated in inherited cancer susceptibility a few years ago. Different mutations of CHEK2 were detected among patients with Li–Fraumeni syndrome.6 Furthermore, mutations of this gene were correlated with other types of cancer. Male carriers have a higher risk for prostate cancer, as CHEK2 overexpression decreases cell growth while its downregulation affects androgen receptor activity.7,8 The I157T variant is associated with other types of cancer, including breast (odds ratio [OR] 1.4; p=0.02), prostate (OR 1.7; p=0.002), kidney (OR 2.1; p=0.0006), colon (OR 2.0; p=0.001) and thyroid (OR 1.9; p=0.04) cancer.9 Results from a study by Havranek et al confirmed that truncated or missense variants of CHEK2 gene correlated with an increased risk of non-solid tumor types, because mutations were associated with a higher risk of developing non-Hodgkin lymphoma (OR 2.86; p=0.003).10 Table 1 represents the different mutations according to region.
  | Table 1 CHEK2 mutations according to region Note: N/A: data unavailable. Abbreviation: CHEK2, checkpoint kinase 2. |
CHEK2*1100delC
CHEK2*1100delC allele was first correlated with the Li–Fraumeni and Cowden syndromes. However, the above mutation cannot be associated with the risk for these syndromes because, in a study among women in Brazil, neither the mutation carriers nor their family members were reported to have these syndromes.11
According to Schmidt et al, who compared different studies that included more than 40,000 patients, carriers of the CHEK2*1100delC allele have a higher risk of developing breast cancer; however, this risk is lower in higher age groups. In addition, carriers acquire higher probability to develop estrogen receptor-positive breast cancer than noncarriers; however, there is no evidence that this risk is dependent on the status of progesterone receptor (PR) or human epidermal growth factor receptor 2.12
In a study of healthy volunteers and breast cancer patients in North America, the frequency of CHEK2*1100delC allele was lower (0.3%) than the respective frequency observed in European populations.13 Another study in the US confirmed the rare frequency of this allele in America (1.1%); however, this allele has been correlated with a risk of breast cancer.14 Similar results were obtained in South America, as the allele was also rarely observed in Brazil.15 In addition to America, the CHEK2*1100delC allele was rarely detected in breast cancer patients from Malaysia.16 The above aberration was found to be rare in Jewish Ashkenazi women with breast cancer as well as in an Australian population (0.68%).17,18 In contrast, a higher frequency (2.7%) of 1100delC allele was observed among women suffering from breast cancer in Russia.19 Among European populations, the frequency rates vary by country. In Spain, this variant is almost absent;20 however, in Germany, the frequency ranges from 1.4% to 2.3%, while in Sweden, the frequency of CHEK2*1100delC was found to range between 1.4% and 2.9%.21,22 Most recent experimental data demonstrated that this allele is also associated with an increased risk for other types of cancer apart from breast cancer.23
CHEK2*I157T
The substitution of isoleucine 157 by threonine affects several interactions at the interface of forkhead-associated and kinase domains, leading to problems in the homodimerization of CHEK2 which is required for its activation.24 Although I157T decreases protein activity, it cannot be considered as a risk factor for cancer in general populations because significant differences among the alleles concerning prognosis, metastasis, relapse and estrogen receptor or PR expression have not been recorded.25 According to Kilpivaara et al, the I157T variant may be associated with susceptibility to breast cancer, but in a lower frequency than the CHEK2*1100delC allele.26 However, the I157T allele may be associated with an increased risk for lobular breast cancer.27 In addition, the missense variant increases the risk for breast cancer if carriers already have a deleterious CHEK2 mutation.28
Other mutations
Besides the well-known and most studied mutations described previously, others have been reported, such as Q20X and E85X mutations at exons 1 and 2, respectively, which are novel and have been identified in breast cancer patients from Pakistan.29 Previous experimental data on the same area suggested that two additional missense mutations (H371Y and D438Y) are also associated with breast cancer in women.30 H371Y has been associated with moderate risk of breast cancer in Chinese women.31 In the UACC812 cell line, derived from the mammary gland, another nonsense truncating mutation, L303X, was detected.32
CHEK2 and therapy
CHEK2 mutations are not only correlated with risk for breast cancer but have also been involved in response to therapy. Mutations of CHEK2 or TP53 have been associated with resistance to anthracycline-based chemotherapy in patients with breast cancer.33 Another study in Chinese women with breast cancer demonstrated that H371Y carriers may have better response to neoadjuvant chemotherapy (p=0.031).34 In contrast, there was no difference observed in response to adjuvant chemotherapy or endocrine therapy.35,36 Furthermore, CHEK2 variants have been associated with response to epirubicin, because different allele carriers respond differently to this chemotherapeutic drug.37 On comparing healthy controls with CHEK2*1100delC carriers, there was no difference observed with regard to chromosomal radiosensitivity.38
CHEK2 and breast cancer risk
Although CHEK2 mutations are rare, the risk of developing breast cancer is higher in carriers of truncating mutations. This risk is correlated with family history and increases when the carriers have first- and second-degree relatives who are affected. In carriers with no affected relative, the risk is approximately 20%, and it increases up to 44% when both first- and second-degree relatives are affected.39 In an earlier study where 2000 samples were screened, it was found that only the 1100delC allele could contribute to breast cancer susceptibility.40
According to Meijers-Heijboer et al, the same mutation results in an increased risk of breast cancer both in women and men; however, the risk does not alter when these individuals are carriers of BRCA1 or BRCA2 mutations. The last may be caused due to interactions among BRCA1, BRCA2, and CHEK2 because they are involved in the same signaling pathways.41 Another study, which included a large number of samples (more than 50,000 individuals) also demonstrated that 1100delC heterozygotes have a higher risk of breast cancer.42
Conclusion
CHEK2 gene is activated upon DNA damage, and also activates specific genes related to basic cellular activities, such as apoptosis, repair, cell cycle arrest and so on. Although different mutations have been associated with increased risk for breast cancer and response to chemotherapy, the low frequency rate of the allele is a complicating factor. Experimental data until now are encouraging; however, studies evaluating the function of CHEK2 along with other genes might be more useful in providing more accurate and reliable data.
Disclosure
The authors report no conflicts of interests in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. | ||
Carlson RW, Allred DC, Anderson BO, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7(2):122–192. | ||
Magni M, Ruscica V, Buscemi G, et al. Chk2 and REGgamma-dependent DBC1 regulation in DNA damage induced apoptosis. Nucleic Acids Res. 2014;42(21):13150–13160. | ||
Dufault MR, Betz B, Wappenschmidt B, et al. Limited relevance of the CHEK2 gene in hereditary breast cancer. Int J Cancer. 2004;110(3):320–325. | ||
Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999;286(5449):2528–2531. | ||
Cybulski C, Huzarski T, Gorski B, et al. A novel founder CHEK2 mutation is associated with increased prostate cancer risk. Cancer Res. 2004;64(8):2677–2679. | ||
Ta HQ, Ivey ML, Frierson HF Jr, et al. Checkpoint kinase 2 negatively regulates androgen sensitivity and prostate cancer cell growth. Cancer Res. 2015;75(23):5093–5105. | ||
Cybulski C, Gorski B, Huzarski T, et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004;75(6):1131–1135. | ||
Havranek O, Kleiblova P, Hojny J, et al. Association of germline CHEK2 gene variants with risk and prognosis of non-Hodgkin lymphoma. PLoS One. 2015;10(10):e0140819. | ||
Palmero EI, Alemar B, Schuler-Faccini L, et al. Screening for germline BRCA1, BRCA2, TP53 and CHEK2 mutations in families at-risk for hereditary breast cancer identified in a population-based study from Southern Brazil. Genet Mol Biol. 2016;39(2):210–222. | ||
Schmidt MK, Hogervorst F, van Hien R, et al. Age- and tumor subtype-specific breast cancer risk estimates for CHEK2*1100delC carriers. J Clin Oncol. 2016;34(23):2750–2760. | ||
Offit K, Pierce H, Kirchhoff T, et al. Frequency of CHEK2*1100delC in New York breast cancer cases and controls. BMC Med Genet. 2003;4:1. | ||
Mateus Pereira LH, Sigurdson AJ, Doody MM, et al. CHEK2:1100delC and female breast cancer in the United States. Int J Cancer. 2004;112(3):541–543. | ||
Abud J, Koehler-Santos P, Ashton-Prolla P, Prolla JC. CHEK2 1100DELC germline mutation: a frequency study in hereditary breast and colon cancer Brazilian families. Arq Gastroenterol. 2012;49(4):273–278. | ||
Thirthagiri E, Cheong LS, Yip CH, Teo SH. CHEK2*1100delC does not contribute to risk to breast cancer among Malay, Chinese and Indians in Malaysia. Fam Cancer. 2009;8(4):355–358. | ||
Laitman Y, Kaufman B, Lahad EL, Papa MZ, Friedman E. Germline CHEK2 mutations in Jewish Ashkenazi women at high risk for breast cancer. Isr Med Assoc J. 2007;9(11):791–796. | ||
Jekimovs CR, Chen X, Arnold J, et al. Low frequency of CHEK2 1100delC allele in Australian multiple-case breast cancer families: functional analysis in heterozygous individuals. Br J Cancer. 2005;92(4):784–790. | ||
Chekmariova EV, Sokolenko AP, Buslov KG, et al. CHEK2 1100delC mutation is frequent among Russian breast cancer patients. Breast Cancer Res Treat. 2006;100(1):99–102. | ||
Osorio A, Rodriguez-Lopez R, Diez O, et al. The breast cancer low-penetrance allele 1100delC in the CHEK2 gene is not present in Spanish familial breast cancer population. Int J Cancer. 2004;108(1):54–56. | ||
Rashid MU, Jakubowska A, Justenhoven C, et al. German populations with infrequent CHEK2*1100delC and minor associations with early-onset and familial breast cancer. Eur J Cancer. 2005;41(18):2896–2903. | ||
Margolin S, Eiberg H, Lindblom A, Bisgaard ML. CHEK2 1100delC is prevalent in Swedish early onset familial breast cancer. BMC Cancer. 2007;7:163. | ||
Naslund-Koch C, Nordestgaard BG, Bojesen SE. Increased risk for other cancers in addition to breast cancer for CHEK2*1100delC heterozygotes estimated from the Copenhagen General Population Study. J Clin Oncol. 2016;34(11):1208–1216. | ||
Cai Z, Chehab NH, Pavletich NP. Structure and activation mechanism of the CHK2 DNA damage checkpoint kinase. Mol Cell. 2009;35(6):818–829. | ||
Muranen TA, Blomqvist C, Dork T, et al. Patient survival and tumor characteristics associated with CHEK2:p.I157T – findings from the Breast Cancer Association Consortium. Breast Cancer Res. 2016;18(1):98. | ||
Kilpivaara O, Vahteristo P, Falck J, et al. CHEK2 variant I157T may be associated with increased breast cancer risk. Int J Cancer. 2004;111(4):543–547. | ||
Liu C, Wang Y, Wang QS, Wang YJ. The CHEK2 I157T variant and breast cancer susceptibility: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2012;13(4):1355–1360. | ||
Cybulski C, Gorski B, Huzarski T, et al. Effect of CHEK2 missense variant I157T on the risk of breast cancer in carriers of other CHEK2 or BRCA1 mutations. J Med Genet. 2009;46(2):132–135. | ||
Baloch AH, Khosa AN, Bangulzai N, et al. Novel nonsense variants c.58C>T (p.Q20X) and c.256G>T (p.E85X) in the CHEK2 gene identified in breast cancer patients from Balochistan. Asian Pac J Cancer Prev. 2016;17(3):1089–1092. | ||
Baloch AH, Daud S, Raheem N, et al. Missense mutations (p.H371Y, p.D438Y) in gene CHEK2 are associated with breast cancer risk in women of Balochistan origin. Mol Biol Rep. 2014;41(2):1103–1107. | ||
Liu Y, Liao J, Xu Y, et al. A recurrent CHEK2 p.H371Y mutation is associated with breast cancer risk in Chinese women. Hum Mutat. 2011;32(9):1000–1003. | ||
Wasielewski M, Hanifi-Moghaddam P, Hollestelle A, et al. Deleterious CHEK2 1100delC and L303X mutants identified among 38 human breast cancer cell lines. Breast Cancer Res Treat. 2009;113(2):285–291. | ||
Knappskog S, Chrisanthar R, Lokkevik E, et al. Low expression levels of ATM may substitute for CHEK2/TP53 mutations predicting resistance towards anthracycline and mitomycin chemotherapy in breast cancer. Breast Cancer Res. 2012;14(2):R47. | ||
Liu Y, Xu Y, Ouyang T, et al. Association between CHEK2 H371Y mutation and response to neoadjuvant chemotherapy in women with breast cancer. BMC Cancer. 2015;15:194. | ||
Kriege M, Hollestelle A, Jager A, et al. Survival and contralateral breast cancer in CHEK2 1100delC breast cancer patients: impact of adjuvant chemotherapy. Br J Cancer. 2014;111(5):1004–1013. | ||
Kriege M, Jager A, Hollestelle A, et al. Sensitivity to systemic therapy for metastatic breast cancer in CHEK2 1100delC mutation carriers. J Cancer Res Clin Oncol. 2015;141(10):1879–1887. | ||
Chrisanthar R, Knappskog S, Lokkevik E, et al. CHEK2 mutations affecting kinase activity together with mutations in TP53 indicate a functional pathway associated with resistance to epirubicin in primary breast cancer. PLoS One. 2008;3(8):e3062. | ||
Baeyens A, Claes K, Willems P, De Ruyck K, Thierens H, Vral A. Chromosomal radiosensitivity of breast cancer with a CHEK2 mutation. Cancer Genet Cytogenet. 2005;163(2):106–112. | ||
Cybulski C, Wokolorczyk D, Jakubowska A, et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol. 2011;29(28):3747–3752. | ||
Schutte M, Seal S, Barfoot R, et al. Variants in CHEK2 other than 1100delC do not make a major contribution to breast cancer susceptibility. Am J Hum Genet. 2003;72(4):1023–1028. | ||
Meijers-Heijboer H, van den Ouweland A, Klijn J, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31(1):55–59. | ||
Weischer M, Bojesen SE, Ellervik C, Tybjaerg-Hansen A, Nordestgaard BG. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol. 2008;26(4):542–548. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.