Back to Journals » Journal of Asthma and Allergy » Volume 13
Current Controversies and Future Prospects for Peanut Allergy Prevention, Diagnosis and Therapies
Authors Gray CL
Received 4 November 2019
Accepted for publication 18 December 2019
Published 16 January 2020 Volume 2020:13 Pages 51—66
DOI https://doi.org/10.2147/JAA.S196268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Claudia Liesel Gray 1, 2
1Red Cross Children’s Hospital and University of Cape Town, Cape Town, South Africa; 2Kidsallergy Centre, Vincent Pallotti Hospital, Cape Town, South Africa
Correspondence: Claudia Liesel Gray Email [email protected]
Abstract: Peanut allergy has increased substantially in the past few decades, both in developed and developing countries. Peanut allergy has become a major public health concern, affecting up to 1 in 50 children, with repercussions for school and airline policies. Recent research findings have shown that, contrary to the long-standing teaching of “delayed” introduction of allergens, early introduction of peanut protein is of benefit as an allergy prevention strategy, especially in high-risk cases. Ideal dose, frequency and duration of “proactive” peanut therapy for maximum protection remain to be determined in order for it to become acceptable and practical on a large scale. Logistics around widespread screening of high-risk patients remain complex. The correct diagnosis of peanut allergy is crucial and diagnostic tests have been fine-tuned in the past 2 decades in order to help differentiate true allergy from false-positive sensitization through cross-reactivity. Component-resolved diagnostics have become routinely available, and the use of basophil activation tests has increased, although standardization and availability remain issues. Future tests, including epitope testing and histamine-release assays, promise to be even more specific in ruling out false positives and reducing the need for incremental food challenges. Stringent peanut avoidance and prompt treatment of reactions remain the cornerstone of treatment. The concept of exposing the allergic body to small amounts of peanut protein in a cautious, orderly, escalating fashion in the form of desensitization has been widely applied in the past 10– 15 years, mainly in the research domain, but of late spilling over into every-day practice. However, desensitization does not equate to a cure, and has significant safety concerns and practical ramifications; probably requiring lifelong-controlled peanut ingestion for ongoing protection. Further strategies to enhance the safety and efficacy of immunotherapy are under exploration, many with a non-specific immune-modifying effect. Despite recent advances in peanut allergy, we still need to go back to basics with accurate diagnosis, nutritional counselling, well-organized allergy action plans and accessible emergency kits.
Keywords: peanut allergy, prevention, early introduction, component-resolved diagnostics, basophil activation test, peanut immunotherapy, policies in schools
Introduction
Within the food allergy “epidemic” of the past 2 decades, peanut allergy has become the poster girl of the food allergy world, with a tangible and documented increase in both developed1–4 and developing5 countries.
Peanut allergy is a major public health concern estimated to affect between 0.5% and 2% of the children.1,4,5 Peanut allergy can be severe or life-threatening and is one of the most common causes of food-related anaphylaxis.3 Moreover, peanut allergy begins early in life for most and is persistent in the vast majority of cases.6 Therefore, preventing peanut allergy would have a significant impact on the individual and potentially at a public health level.
Prevention is not always possible, but for those with a possible peanut allergy, accurate diagnosis is key in order to facilitate a targeted management plan. The gold standard diagnostic clincher of an incremental, supervised oral food challenge is not practical, available or financially viable for all- hence advances in diagnostic markers which more accurately discern the “real” from the “noise” are welcome.
The cornerstone of food allergy management has traditionally consisted of careful, targeted elimination of the allergy-provoking food, and timely, severity-driven treatment in the case of accidental ingestion. The concept of desensitization to peanut has arisen in the past 10–15 years with the hope of effecting more of a cure, and has recently been converted from a research concept into anticipated FDA approval of a peanut desensitization “drug.”8 Desensitization is not without complications and does not necessarily equate to tolerance development, so is an area of ongoing research and controversy.9
The very real threat of peanut allergy in a substantial proportion of young and school-going children, who cannot be expected to take full responsibility in avoidance of allergenic food, has led to recommendations and policy changes on a large-scale basis in schools.10 The peanut-allergic child has the right to a safe learning environment, with efforts required to reduce the risk of allergen exposure.11 Nut-free schools may not necessarily be the answer. Certainly, schools should be allergy aware, and equipped for allergy-related emergencies.
This article focuses on four major aspects of peanut allergy:
- Prevention
- Accurate diagnosis
- Treatment
- Peanut allergy in schools,
outlining recent advances, current challenges and future options in each of these categories.
Peanut Allergy Prevention
Prevention of peanut allergy would be first prize in reining in the peanut allergy epidemic and would have the greatest impact at the individual and public health levels.
Recent Advances in Peanut Allergy Prevention
Recent efforts in peanut allergy prevention have focused on the concept of early oral introduction of peanut protein, and preservation of the skin barrier to reduce the chance of epicutaneous sensitization.
Early Oral Introduction of Peanut Protein
Two large studies published in the past 5 years have demonstrated the potential protective effect of early peanut introduction. The most persuasive of these studies, albeit in a high-risk population, was the LEAP study (Learning Early about Peanut Allergy),12 a landmark trial in the realm of peanut allergy prevention.6 This study enrolled 640 children at high risk of peanut allergy, which was defined for this study as infants between 4 and 11 months of age with severe eczema and/or egg allergy. At study entry, LEAP participants were stratified according to skin prick test (SPT) result to peanut into those with a negative SPT response (n=530), a primary prevention group, and those with a measurable SPT response (1–4 mm), n=98, a secondary prevention group as they were considered sensitized but not allergic to peanut at study entry. Participants with an SPT of greater than or equal to 5 mm were excluded because of their high risk of established peanut allergy, although these patients did not undergo oral food challenges (OFC) to determine if they were truly peanut-allergic. Patients were randomized to consume at least 2 g of peanut protein thrice weekly or avoid peanut-containing food until 5 years of age, at which stage a peanut OFC was performed. Infants randomized to consume peanut ingested a median of 7.7 g peanut protein per week during the first 2 years of the trial.
Figure 1 demonstrates the outcome of the study, with a significant protective effect of early peanut introduction in high-risk infants.13
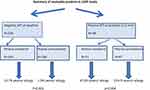 |
Figure 1 Summary of LEAP study outcome. |
The LEAP-ON study14 demonstrated the long-term persistence of oral tolerance to peanut achieved in the LEAP trial when peanut consumers subsequently avoided peanut for 1 year from 60 to 72 months. A further analysis on nutrition in the LEAP cohort15 showed that introduction of peanut did not affect the frequency or duration of breastfeeding and did not influence growth or nutrition.
The significant reduction in peanut allergy in the early consumption group led to international effort to develop practical clinical recommendations on peanut allergy prevention.16 Although many existing infant feeding guidelines prior to 2015 already suggested the introduction of allergenic foods from 4 to 6 months onwards, these did not specifically emphasize that avoidance may be harmful. A consensus statement regarding the implementation of LEAP findings was published after the LEAP findings in 2015 on behalf of several international professional societies.17 In addition, the National Institute of Allergy and Infectious Diseases (NIAID) published addendum guidelines for the prevention of peanut allergy in the United States in 2017,18 an addendum to the 2010 “Guidelines for the diagnosis and management of food allergy in the United States.”
Table 1 summarises the general recommendations for peanut introduction in infants according to risk level for peanut allergy,17 and Figure 2 demonstrates the recommended pathway of peanut introduction in high-risk infants.13
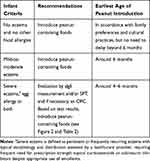 |
Table 1 Recommendations for Peanut Introduction in Infants According to Risk Stratification |
 |
Table 2 Main Peanut Components |
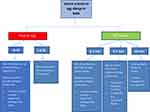 |
Figure 2 Peanut protein introduction in infants at high risk of peanut allergy. ©2018. Allergy Society of South Africa. Adapted from Gray CL, Venter C, Emanuel S, Fleischer D. Peanut introduction and the prevention of peanut allergy: evidence and practical implications. Curr Allergy Clin Immunol. 2018;31:28–30.13 Data from Fleischer et al.17 |
Health and economic benefit modelling found that early peanut introduction is cost-effective when compared to delaying peanut introduction beyond 12 months.19
The Enquiring about tolerance study (EAT study) is another recent study looking into the introduction of a variety of allergenic foods in a more unselected population of infants.20 This study enrolled 1303 exclusively breastfed infants at 3 months of age and randomized half to exclusively breastfeed till about 6 months of age and then introduce solids according to family preference (the standard introduction group); and half to consume six allergenic foods (peanut, cow’s milk, egg, wheat, fish, sesame) twice weekly from study enrolment (early introduction group). Per protocol analysis found peanut allergy prevalence to be significantly lower in the early intervention group (0% v 2.5%, p=0.003). There was a trend towards an effect in the intention to treat analysis. A secondary intention-to-treat analysis showed that early introduction was effective in preventing the development of food allergy in specific groups of infants at increased risk of food allergy: those sensitized to any food at enrolment, and those with moderate eczema at enrolment.21 A follow-up of EAT children at 8 years of age will be performed to study a more long-term outcome of early allergen introduction.
Preservation of the Skin Barrier to Minimize Transcutaneous Entry of Allergens
Skin barrier dysfunction has been shown to play a role in food allergy. The dual allergen hypothesis proposes that allergic sensitization may occur through the skin, but tolerance may be induced via the gut.22
There is evidence that environmental peanut exposure increases the risk of peanut sensitization and peanut allergy, particularly in those with atopic dermatitis and filaggrin loss of function mutations.23–27 Studies have shown that early emollient therapy may reduce the chances of developing eczema.28,29
Studies are now underway to examine whether early emollient application can prevent food allergy sensitization. The PEBBLES pilot study showed a trend towards reduced food sensitization with regular emollient therapy.30 Larger and robust studies on skin barrier preservation and food allergy prevention are underway.
The LEAP study demonstrated that Staphylococcus aureus on the skin is associated with food sensitization and allergy, independent of eczema severity. Prevention and prompt treatment of Staphylococcus aureus in children with eczema may therefore be another means of enhancing the integrity of the skin barrier and maintaining tolerance to allergens.31
Current Challenges in Peanut Allergy Prevention
With a documented protective effect of early peanut introduction, a population programme aiming to identify and screen all infants at risk of peanut allergy would be ideal. However, it would pose major logistic and economic challenges, hence a more pragmatic approach needs to be taken for patients.32 Other challenges and controversies in early peanut introduction are the following:
- Is the recommended amount of regular peanut intake realistic?
The LEAP study suggests that the first dose of peanut protein should be a cumulative dose of around 2 g of peanut protein. Thereafter, the total minimum amount of peanut protein should be 6–7 g per week, consumed over three or more feedings per week.12 Figure 3 demonstrates pictorially that this is a substantial amount of peanut protein for young infants to eat in one sitting. However, it is not yet known if other amounts and frequencies of ingesting peanut would have the same results.
 |
Figure 3 Typical peanut-containing foods and portion sizes. |
Future Prospects in Peanut Allergy Prevention
Further research trials that explore real-life application of early peanut introduction guidelines are needed, as well as the potential role of early peanut introduction in the general population.
Some prospective ideas on how to implement prevention strategies are discussed below:
- The message about potential advantages of early peanut protein introduction needs to be relayed at all tiers of the healthcare system. Baby (immunization) clinics can play an important role in spreading the message when discussing solids with parents. Posters explaining the introduction of solids and allergenic solids could be designed and displayed in baby clinics and day-care centres. The child’s “health” book could contain a page on allergen introduction and warning signs of “high risk” infants who should be screened.
- A practical consideration for applying this guideline at 4–6 months of age is that infants visit their health-care provider for routine health-care visits during this time. This provides a fortuitous opportunity for the evaluation of risk factors, such as eczema and/or reported milk or egg allergy, and a prompt referral to a specialist for peanut allergy evaluation and introduction advice.
- Consideration should be given to having screening opportunities at primary health-care centres or family doctors for peanut allergy screening. Opportunities to pre-screen with a simple skin prick test at routine immunization visits around the 4- to 6-month mark could provide an exciting opportunity which would require prospective studies to check for feasibility. Perhaps the facility for “corridor” challenges in lower risk cases should be more widely available in the health-care setting at many levels.
- Routine encouragement of the use of suitable emollients to the infant skin to reduce eczema rates and perhaps transcutaneous sensitization can be implemented on a large-scale basis. Discouraging the use of harsh soapy products in young babies is key. Local infant skincare guidelines for countries should include advice on the maintenance of an intact skin barrier.
Diagnosis of Peanut Allergy
Accurate diagnosis of peanut allergy is critical. Sensitization to peanut does not always equate to allergy: cross-reactivity with peanut proteins can lead to false positives, with overdiagnosis leading to unnecessary dietary elimination, stress and reduced quality of life. On the other hand, it is imperative to recognise a true peanut allergy in order to be able to institute the correct management process and equip the patient for unintentional exposures.
First-line treatment in peanut allergy diagnosis remains a detailed history coupled with skin prick tests and/or specific IgE to peanut: both skin prick tests and specific IgE to peanut are highly sensitive (95%) but specificity is poor (around 60%).34 A negative test is useful for excluding peanut allergy, whereas a high positive result coupled with a positive history has a high likelihood ratio for peanut allergy. However, for those with intermediate results, further specialized tests such as food challenges may be required to differentiate between asymptomatically sensitized and truly allergic patients. Food challenges are time-consuming, labour-intensive and potentially hazardous, requiring expertise narrowed to certain centres.
Therefore, the quest remains to find accurate diagnostic means to clarify true diagnosis versus false positivity, with aim of reducing (but not abating) the need for food challenges.
Recent Advances and Current Controversies in Peanut Allergy Diagnosis
95% Positive Predictive Values
Ninety-five percent positive predictive values (95% PPVs) have been established to predict food allergies more reliably.
Several studies in different populations have postulated that a skin prick test to whole peanut extract of 8 mm or more provides high specificity and PPV for peanut allergy.35,36 Studies endeavouring to define a 95% PPV for specific IgE to peanut in diagnosing peanut allergy have suggested a threshold of 15 kU/L.37,38
However, these PPVs may be population and age specific. Age-specificity of cut-off values was demonstrated by the Health Nuts™ study in Australia, which showed a 95% PPV of 34 kU/L at 12 months of age, with a corresponding PPV of 2.1 kU/L at 4 years.39
Population-specificity was demonstrated by a recent study in South Africa: Commonly used 95% PPVs for SPTs and Ara h 2 levels fared sub-optimally in this population. Maximum PPVs for this study population were found at SPT 11 mm, and ImmunoCAP Ara h 2 of 8 kU/L.40
Component-Resolved Diagnostics
The use of molecular allergology using component-resolved diagnostics has become commonplace in peanut allergy diagnosis in the past decade.
Component-resolved diagnostics are useful in refining reactive peanut-specific IgE tests to differentiate true peanut allergy from in vitro cross-reactivity (possible false positives).41,42 Peanut components are prefixed “Ara” after the name for peanut, Arachis hypogaea. Component testing for peanut proteins helps differentiate between the more cross-reactive components such as Ara h 5, 8 and 9 and specific peanut components such as Ara h 1, 2, 3 and 6, which are heat-resistant storage proteins (Table 2). In Mediterranean countries, the lipid transfer factor Ara h 9 is an important peanut allergen.43 Ara h 8, in the PR10 protein group of labile food allergens, is more prominent in those exposed to certain pollens such as birch and alder.44 The pattern and relevance of peanut components may therefore vary between geographical areas and possibly between ethnic groups.
Ara h 2 (2S albumin storage protein) has been shown to be the most important component in prediction of true food allergy in several countries, including the UK,46 France,47 Japan,48 the USA,49 and South Africa.50 A systemic review51 of 22 studies concluded that Ara h 2 was the most accurate in predicting true peanut allergy versus false positivity, and may be useful as a second step investigation in patients with a positive SPT or specific IgE to peanut to clarify further and reduce the number of challenges.
Cut-off values for Arah2 predicting 95% likelihood of reactivity vary between studies and region, and the initially suggested reactivity level of 0.35 kU/L has been recently been shown to lead to significant false positivity in many populations. A South African study showed optimal PPV of >90% at ImmunoCAP Ara h 2 of 8 kU/L.50
A more recent systemic review52 suggested that Ara h 6 may have a higher diagnostic accuracy for peanut allergy compared with Ara h 2, but more research is required to determine clinically appropriate cut-offs.
Basophil Activation Test
The Basophil Activation Test (BAT) is an in vitro functional assay that assesses the expression of activation markers (such as CD63 and CD203c) on the surface of live basophils in whole fresh blood by flow cytometry following stimulation with peanut antigen.
The use of BAT in peanut allergy diagnosis has been assessed in multiple studies with overall high sensitivity (83–92%) and specificity (77–100%).53,54
Similar to blood tests for specific IgE, the BAT can be performed on patients with active eczema and patients can continue antihistamine treatment. BAT requires fresh whole blood within 4 hrs of sampling with no previous storage; hence, proximity to a suitable laboratory is needed, and sampling can only be performed during “office” hours. Currently, it is only offered by certain specialist laboratories.
However, BAT essentially remains a research tool for now with limited availability. More work needs to be performed on standardizing laboratory methods and analysis. Availability and cost-effectiveness are further concerns.
Future Prospects in Peanut Allergy Diagnosis
The aims of “future” diagnostic tests in peanut allergy would include
- Accurate differentiation between allergic and tolerant patients with a superior specificity to currently available tests.
- Tests which predict severity of peanut allergy
- Tests which more accurately predict the likelihood of allergy persistence versus tolerance development
- Tests that aid in identifying patients most suitable for peanut immunotherapy.
Currently, emerging diagnostic tests include
- Mast cell activation tests – In this test, the ability of the patient’s allergen-specific IgE antibodies to elicit mast cell degranulation is assessed. Mast cell activation tests are currently undergoing validation.55,56
- Histamine-release assays – These measure the amount of histamine released from activated basophils57
- Specific epitope binding – This refines component-resolved diagnostics one step further to look at specific peanut epitope binding is under investigation.58
Treatment of Peanut Allergy
Recent Advances in Peanut Allergy Treatment
Currently, stringent avoidance, and quick and correct emergency response to reactions are the mainstay of treatment for peanut allergy.
A hope of a more curative approach to peanut allergy was born in the concept of peanut immunotherapy. Peanut oral immunotherapy and more recently sublingual and epicutaneous immunotherapy have been studied extensively in the past decade or so, culminating in the anticipated FDA approval of the first peanut oral immunotherapy programme in the near future.8
The idea of immunotherapy is initially to raise the threshold of reactivity and protect the patient against small hidden exposure; ultimately permanent resolution of the allergy would be first prize but not routinely achievable.7
Desensitization is a transient state of reduced reactivity, measured as an increased dose that triggers a reaction in a post-treatment DBPCFC, with the aim of protection from accidental exposure. Interruption of desensitization may lead to the loss of the protective effect.
Sustained unresponsiveness is a more lasting clinical outcome that leaves the treated patient clinically protected for weeks or months, even when not consuming the allergen regularly. This can be measured by intentional interruption of immunotherapy dosing for at least 4–12 weeks followed by a “tolerance” food challenge59 (see Figure 4).
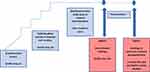 |
Figure 4 Overview of the peanut oral immunotherapy process. |
Full tolerance is a permanent resolution of the allergy, with unresponsiveness to the allergen even after prolonged avoidance.
Oral Immunotherapy (Specific Oral Tolerance Induction or SOTI)
Over the past decade, hundreds of patients have participated in randomized trials of peanut oral immunotherapy (OIT).
There has been much heterogeneity between OIT trials with different dosing regimens and endpoints, making results difficult to compare and interpret.60–62
In an attempt to pool available results, a recently published meta-analysis analyzed 12 clinical trials of peanut oral immunotherapy involving 1041 patients followed up for a median of 12 months; comparing peanut OIT versus placebo or peanut avoidance.9 Oral immunotherapy outperformed no oral immunotherapy on the standard primary endpoint: an estimated 40% of the treatment group passed a supervised oral food challenge at the end of the regular treatment period compared with 3% of the control group. However, many more serious adverse events were observed in the treatment group: There was a pooled prevalence of 22% of the anaphylaxis in oral immunotherapy group, versus 7% in those without active peanut OIT (placebo or avoidance). This equated to an additional 15 anaphylactic events per 100 treated patients.
The conclusion of this meta-analysis is that there is strong evidence that peanut OIT results in desensitization, but the process carries a significant risk, albeit a “controlled risk” to a large degree.
The first Phase 3 clinical trial of OIT enrolled 554 peanut-allergic patients, aged 4–55 years, with reactivity at a maximum clinical dose of 100 mg peanut protein.63 Patients were randomized in a ratio of active to placebo of 3:1. The primary endpoint was tolerating, with no or mild symptoms, greater than or equal to 600 mg single dose peanut protein in a DBPCFC (cumulative dose 1043 mg as DBPCFC was done per modified PRACTALL guidelines), conducted 6 months after achieving a 300 mg maintenance dose. Results showed that 67% of the actively treated group tolerated greater than or equal to 600 mg peanut protein at the exit challenge, versus 4% of the placebo group (p<0.0001). The incidence of mild-to-moderate adverse events was high in both the active and placebo groups, but serious adverse events were significantly higher in the active group: 4.3% of the active group had a serious adverse event v 0.8% of the placebo group. There was one case of eosinophilic oesophagitis in the active group.
The significant and convincing rate of achieving desensitization at the exit peanut challenge led to FDA consideration for approval of the peanut protein desensitization “drug,” in September 2019 as Palforzia in the USA.8 The FDA has stipulated that during this treatment, the patient needs to carry an adrenaline autoinjector, and that initial dosing and updosing have to be performed at a facility capable of treating severe allergic reactions.
Sublingual Immunotherapy to Peanut
Recent studies show that sublingual immunotherapy may be a safe and effective way for peanut allergy sufferers to protect themselves from severe allergic reactions.
Because peanut protein avoids digestion when given sublingually, patients are given far smaller amounts of peanut protein, ranging from 0.0002 mg to 2 mg.64 SLIT poses smaller risks of side effects but efficacy remains to be established in larger studies. Kim et al followed 48 patients on a SLIT programme of 2 mg daily for 5 years; at the oral challenge after 5 years of maintenance, 67% were able to tolerate at least 750 mg of peanut protein without serious side effects, and 25% could tolerate 5000 mg.65
Several smaller studies66–68 have been published on SLIT showed a statistically significant increase in rate of desensitization in comparison with placebo. However, with SLIT the median threshold dose increased approximately 20-fold, in comparison with more than 300-fold with OIT, hence OIT seems more effective. A major advantage of SLIT is improved safety profile over OIT.
Pharma companies are currently working on a sublingual immunotherapy for possible commercialization.
Epicutaneous Immunotherapy
Epicutaneous application of peanut patches that release small amounts of peanut protein via the skin on a daily basis to effect desensitization represents a potentially safe and “easy” form of desensitization. Epicutaneous allergen-specific immunotherapy requires application of the patch to intact skin to ensure a tolerogenic effect, thus avoiding the transcutaneous sensitization potential of eczematous skin.69 A patch applied to intact skin leads to solubilization of the allergen and direct uptake by antigen-presenting cells, with transport to lymph nodes without entering the bloodstream.
A published study with a 250-µg epicutaneous peanut patch showed a 25% increase above placebo in the primary endpoint (primary endpoint was a 10-fold increase in the reaction-eliciting dose, or tolerating 1000 mg of peanut protein).70 In a phase 3 study, 356 peanut-allergic children were randomised 2:1 to receive a daily active patch of 250-µg peanut protein or placebo, for 12 months. In the exit challenge, participants were considered responders if they tolerated at least 100 mg peanut protein (143 mg cumulative) if their entry eliciting dose was 10 mg or less; or at least 300 mg peanut protein (443 mg cumulative) if their entry eliciting dose was >10 mg. Using these criteria, the response rate was 35.3% in active group v 13.6% in the placebo group.71 Based on this latter study, the pharma company initiating the trials has submitted its “Viaskin” patch for Biologics License Application to the FDA for review.
Current Controversies in Peanut Allergy Treatment
Whilst the excitement of the potential large-scale benefit of peanut immunotherapy is almost tangible, clinicians should take the following controversies into consideration when selecting patients for peanut immunotherapy:
Safety
The large meta-analysis of 12 clinical trials (n=1041) showed that OIT versus no OIT increased anaphylaxis risk (risk ratio 3.12 (1.75–5.55)) and adrenaline use (risk ratio 2.21 (1.27–3.83)).9
In a large Phase III oral immunotherapy study, adverse events led to the withdrawal of 12.4% of the active group versus only 1.6% of the placebo group.63
Eosinophilic oesophagitis has been flagged as a potential longer-term side effect of peanut OIT.72
Real-Life Representativity of Trials
Peanut doses given under supervision in clinical trials may not be representative of dosing under real-life conditions. In most studies, significant lifestyle limitations are applied, including no dosing on an empty stomach and no exercise for 2–3 hrs after ingestion. Protection can fluctuate under “real-life” conditions, with reactions even at doses previously well tolerated. Augmentation factors such as viral infection, exercise, use of NSAIDs, menstruation or even sleep deprivation could trigger reactions to the previously tolerated maintenance dose.73
Peanut Protein in Non-Food Forms May Not Allow for Early Warning Signs of Reactions
An advantage of oral immunotherapy using native, unencapsulated forms of peanut, such as peanut flour, and eventually actual nuts or peanut butter in the maintenance stage, is that it represents real-life taste, oral sensation and absorption. Sublingual/epicutaneous forms may lead to missing out of some warning signs such as oral itch, delaying the recognition and treatment of adverse events.
Cost
With commercialization of peanut desensitisation products, cost may become prohibitive for many. Cost-effective options do exist, for example, in the author’s allergy centre in the private setting in South Africa, a simple desensitization programme with peanut flour measured out sequentially for incremental doses over 20 weeks, has a total cost of equivalent to under US $700 for the entire 6 months escalation period. Thereafter, patients convert to peanut butter or whole peanuts which carries minimal cost. This programme is likely far more cost-effective to the patient (albeit more labour-intensive for the physician) than commercialized products.
Low Levels of Sustained Unresponsiveness and Tolerance
Whilst typically around 60–80% of the patients undergoing peanut OIT are able to reach maintenance dose and therefore desensitization, a far smaller proportion demonstrates sustained unresponsiveness after cessation of regular peanut intake. In a real-world experience retrospective analysis of 270 children who had undergone peanut desensitization, 79% completed the escalation phase and maintained desensitization with continued daily dosing, but only 6.5% were able to achieve sustained unresponsiveness.74
A recently published study of 120 patients who built up to and maintained 4000mg peanut protein to week 104, then randomized patients to either stop peanut, continue consumption at 300 mg, or continue on a placebo flour, showed that discontinuation and even reduction of peanut protein intake increased the likelihood of regaining clinical reactivity to peanut within 13 weeks.75
At the biochemical level, a recent paper by Santos et al demonstrates that protection during desensitization is afforded by blocking antibodies, produced in response to regular peanut consumption, but that there seem to be few permanent changes in reactivity at mast cell-level.76 Once again, this demonstrates the transient state of desensitization.
Diligent life-long intake of controlled amounts of peanut protein on a regular basis may well be an unrealistic goal, especially as many patients have an aversion to the taste of peanut.
Quality of Life During Peanut Immunotherapy
In the large meta-analysis of 12 clinical trials (n=1041),9 the quality of life was not significantly different between active and placebo (combined parent and self-report RR1.21 (0.87–1.69)), mainly because of the high risk of side effects during desensitization. However, a real-world experience study of 270 patients who had undergone peanut OIT demonstrated that the vast majority of patients reported an enhanced quality of life during the desensitization process.74 Certainly in the author’s experience, most patients express an enhanced quality of life, with comments such as “life-changing”, “I’m much more relaxed” and even “I can go to restaurants again.” However, long-term quality of life remains to be studied.
Specificity of Peanut OIT
Peanut OIT, whilst providing a measure of protection against peanut protein, does not affect reactivity in co-existing allergies such as tree nut allergies. With multiple allergies in mind, the OUtMATCH study (Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen Oral Immunotherapy in Food Allergic Children and Adults) has recently been initiated.77 It will test the ability of biweekly or monthly injections of omalizumab, alone or together with multi-allergen oral immunotherapy, to increase allergen tolerance.
Future Prospects for the Treatment of Peanut Allergy
Future prospects in peanut allergy treatment include personalizing immunotherapy with tailored regimes, enhancing immunotherapy with immune treatments with a non-allergen specific impact and reducing the allergenicity of peanut.
Personalizing Immunotherapy
More accurate diagnostic tests, including molecular allergy diagnostic tests, may help identify patients who are unlikely to outgrow their peanut allergy as well as those who may favourably respond to OIT.78
Once immunotherapy has been chosen as an option, the type of immunotherapy will need to be adapted to the patient, taking into account factors such as age, likely compliance, risk for side effects, and cost.
Starting immunotherapy at a younger age may well have a better outcome and a greater chance of sustained unresponsiveness.79 In the author’s experience, starting at around 3–4 years of age is feasible from a practical point of view, and produces generally favourable desensitization results.
It may not be necessary for all to achieve large doses in OIT; lower doses may well achieve the aims of improved quality of life and reduced reactivity to trace amounts without inducing significant side effects.
A recent study on low-dose placebo-controlled peanut oral immunotherapy in 62 children aged 3–17 with proven IgE-mediated peanut allergy, with a maintenance dose of 125–250 mg of peanut protein, showed that 74% of the active group tolerated 300 mg peanut protein and 42% tolerated 4.5 g peanut protein versus 16.1% and 3.2% respectively in the placebo group, after 16 months of OIT. P<0.001.80
A further study showed that a lower maintenance dose (1200 mg daily vs 3000 mg daily of peanut protein) increased adherence to treatment and maintained desensitization.81
It may also be possible to improve the rate of tolerance with a longer duration of OIT.
In a long-term study of peanut OIT, 24 subjects were treated up to 5 years with maintenance daily dose 4000 mg peanut protein.59 Twelve (50%) of 24 passed a peanut challenge to 5000 mg of peanut protein 1 month after stopping OIT and were considered to have achieved sustained unresponsiveness, and added unrestricted peanut to their diet. This is in contrast with lower figures of sustained unresponsiveness of around 7–30% in previous studies.74
Strategies to Enhance or Optimize Oral Immunotherapy
The role of the microbiome in immune modulation has prompted the addition of probiotics as an adjunct to enhance desensitization or sustained unresponsiveness. In a placebo-controlled randomised trial in Australia in peanut-allergic children aged 1–10 years, the active treatment was peanut OIT combined with the probiotic lactobacillus rhamnosus daily for 18 months.82 At the end of this period, the desensitization rate in the active group was 82.1% versus 3.6% in the placebo group. The sustained unresponsiveness rate may also be enhanced with this approach,82,83 which deserves further study.
Omalizumab has been studied as a monotherapy to raise the threshold of reactivity in peanut allergy with some success.84,85
In conjunction with OIT, several studies have now demonstrated a more rapid and safe escalation of immunotherapy under the protection of omalizumab.86,87 Ideal duration of therapy, and possible effect on sustained unresponsiveness require further study. In the near future, anti-IL5 and anti- IL4ra as well as anti-IL13 and anti-IL 33 are likely to be involved in clinical trials as an adjunct to OIT, with anti IL4ra (dupilumab) in active planning stage.64
Food allergy herbal formula-2 (FAHF2) is a formulation currently under study for immune-modulatory effects. Although in vitro immune-modulatory effects have been demonstrated,88 efficacy was not significant in a Phase 2 clinical trial.89 Additional studies as an adjunct to immunotherapy are currently underway.
Using immunotherapy in which the allergenic proteins have been modified aims to induce immune tolerance and efficacy without the risk of severe side effects. Several are currently entering clinical trials. These include:
- recombinant peanut allergens modified by amino acid substitution at major IgE binding epitopes, administered rectally
- chemically modified aluminium hydroxide-adsorbed peanut extract, administered subcutaneously
- synthetic peptides representing T cell epitope sequences from Ara h 1 and 2, given intradermally.
- peanut extract adjuvanted with Glucopyranosyl Lipid A (GLA), administered subcutaneously
The first DNA “vaccine”, a product known as ASP0892, is undergoing early trials in peanut-allergic adults.90 DNA encoding peanut allergens are inserted in a single plasmid containing the coding sequence for lysosomal-associated membrane fusion protein. The hypothesis is that the vaccine will stimulate a protective Th1 response, with little chance IgE-mediated reactions.
Reducing the Allergenicity of Peanut
Many methods have been proposed to modify peanut allergens to make peanut-derived products less allergenic. Products with reduced allergenicity could be used for immunotherapy, food products and food ingredients, potentially reducing the severity of allergic reactions. Potential methods to reduce allergenicity include genetic modification, gamma irradiation, pulsed UV treatment, chemical modification, enzymatic cross-linking and enzymatic hydrolysis.91
Peanut Allergy in Schools
Going to school is an extremely stressful time for food-allergic children and their families, as they move from a “controlled” to a relatively “uncontrolled” environment.
With the current prevalence of peanut allergy having increased to 1–2% of the childhood population,1–5 this means that up to 1 in 50 children has a peanut allergy. In reality, in most average-sized schools, with 3–4 classes per grade, this equates to 1–2 children per grade. With a child having a right to being safe in the school environment, this has led to a requirement for policy changes in several schools.
Is a Total Ban of Nuts in Schools the Answer?
A total ban of nuts is an option, but not necessarily the answer for all schools. Further research is needed regarding the pros and cons of a total nut ban in schools. Potential disadvantages of a total nut ban include11,92,93
- A false sense of security. In reality, an allergic child and their teachers should always be prepared for a reaction and should not let their guard down.
- A total nut ban is not representative of the “real” world and the child may not be equipped to deal with the reality of allergens outside the school environment, on outings, at birthday parties, etc.
- It may make the school lax about other protective measures, eg handwashing, which may become an issue if there is a “lapse” in the nut-free status, eg a child is given a nut-containing sandwich by a caregiver in error, and no further nut-reduction techniques may be in place if the school is “nut free.”
- A nut-free school may discount other food allergies, for example, egg and milk allergies may be just as severe, but the school lets its guard down under the auspices of being “allergen free.”
A recently published study from the United States provided the first large dataset (over 2000 schools over a 5-year period) exploring the effects of school peanut-free policies on clinical outcomes. Interestingly, this study identified higher rates of adrenaline usage in schools with peanut-free policies compared with schools without such policies.94
Other Options for Allergen Restriction in the School Environment Include:
- Nut-free classrooms or zones if there is a child in a class with a nut allergy, particularly if it is a severe allergy.
- A nut-free table or protected eating area, which is cleaned regularly.
- An integrated eating area but a strict policy of no lunch box sharing.
- Close supervision of eating times if there is an allergic child in the class.
- Strict handwashing after meal times to minimize the transmission of allergens on to work surfaces.
The school should decide on which allergy-aware options they will adopt and make specific plans accordingly. This should always be coupled with accurate documentation of children’s allergies, an accessible emergency action plan and emergency kit, and training of teachers in allergy recognition and management.
A further advance in the school environment has been legislation in some countries to allow the use of an “unnamed” adrenaline autoinjector for emergency use. Recent legislation in the United Kingdom allows schools to obtain an adrenaline autoinjector, without a named prescription, to be kept on campus for emergencies.10
Similar to the school environment, much discussion is taking place around allergen restriction on airlines, and availability of emergency adrenaline on aeroplanes.
Conclusion
Peanut allergy has increased in prevalence, can cause severe allergic reactions and is rarely outgrown, placing a significant burden on the peanut-allergy sufferer. Peanut allergy has enjoyed being a focus of food allergy research from many perspectives in the past decade: prevention, diagnosis, treatment and public health policies. Many advances have been made in these spheres, but many controversies and practical frustrations remain, which need to be addressed by future research. The advantage of early peanut introduction in the infant at risk of peanut allergy has been underpinned by high-quality research, but wide-scale application of this principle faces many hurdles. The benefits of component-resolved diagnostics, basophil activation tests and even microarray tests have been demonstrated, but they are not fail-proof. Immunotherapy to peanut is an exciting treatment prospect which has grown in acceptance outside of the research realm – but desensitization does not equate to tolerance development.
Despite recent advances, we still need to go back to basics with accurate diagnosis, including food challenges in equivocal cases, nutritional counselling, well-organized allergy action plans and accessible emergency kits. Refining labelling laws for “may contain” products and instituting policies for schools and public transport are vital for increased protection of the food-allergic population.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–e17. doi:10.1542/peds.2011-0204
2. Venter C, Maslin K, Patil V, et al. The prevalence, natural history and time trends of peanut allergy over the first 10 years of life in two cohorts born in the same geographical location 12 years apart. Pediatr Allergy Immunol. 2016;27:804–811. doi:10.1111/pai.2016.27.issue-8
3. Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–1207. doi:10.1016/S0091-6749(03)02026-8
4. Osborne NJ, Koplin JJ, Martin PE, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–676. doi:10.1016/j.jaci.2011.01.039
5. Basera W, Botha M, Gray CL, et al. The South African food sensitisation and food allergy population-based study of IgE-mediated food allergy: validity, safety, and acceptability. Ann Allergy Asthma Immunol. 2015;115:113–119. doi:10.1016/j.anai.2015.06.003
6. Skolnick HS, Conover-Walker MK, Barnes C, et al. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107:367–374. doi:10.1067/mai.2001.112129
7. Anna Nowak-Węgrzyn A, Albin E. Oral immunotherapy for food allergy. Curr Allergy Clin Immunol. 2016;29(2):90–99.
8. Queeney E For children with peanut allergies, FDA experts recommend a new treatment. The New York times online. Available from: www.nytimes.com.
9. Chu DK, Wood RA, French A, et al. Oral immunotherapy for peanut allergy (PACE): a systemic review and meta-analysis of efficacy and safety. Lancet. 2019;3293(10187):2222–2232.
10. Available from: www.anaphylaxis.org.uk.
11. Gray CL, Levin ME. Food allergies-should we be going peanut-free in South African schools? Curr Allergy Clin Immunol. 2018;31:28–30.
12. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Eng J Med. 2015;372:803–813. doi:10.1056/NEJMoa1414850
13. Gray CL, Venter C, Emanuel S, Fleischer D. Peanut introduction and the prevention of peanut allergy: evidence and practical implications. Curr Allergy Clin Immunol. 2018;31:28–30.
14. du Toit G, Sayre PH, Roberts G, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med. 2016;374:1435–1443. doi:10.1056/NEJMoa1514209
15. Feeney M, du Toit G, Roberts G, et al. Impact of peanut consumption in the LEAP Study: feasibility, growth, and nutrition. J Allergy Clin Immunol. 2016;138:1108–1118. doi:10.1016/j.jaci.2016.04.016
16. Koplin JJ, Peters RL, Dharmage SC, et al. Understanding the feasibility and implications of implementing early peanut introduction for prevention of peanut allergy. J Allergy Clin Immunol. 2016;138:1131–1141. doi:10.1016/j.jaci.2016.04.011
17. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and prevention of peanut allergy in high-risk infants. J Allergy Clin Immunol. 2015;136:258–261. doi:10.1016/j.jaci.2015.06.001
18. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29–44. doi:10.1016/j.jaci.2016.10.010
19. Shaker M, Stukus D, Chan ES, Fleischer DM, Spergel JM, Greenhawt M. “To screen or not to screen”: comparing the health and economic benefits of early peanut introduction strategies in five countries. Allergy. 2018;73:1707–1714. doi:10.1111/all.2018.73.issue-8
20. Perkin MR, Logan KL, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Eng J Med. 2016;374:1733–1743. doi:10.1056/NEJMoa1514210
21. Perkin MR, Logan KL, Bahnson HT, et al. Efficacy of the Enquiring About Tolerance (EAT) study among infants at high risk of developing food allergy. J Allergy Clin Immunol. 2019;144:1606–1614. doi:10.1016/j.jaci.2019.06.045
22. du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. doi:10.1016/j.jaci.2008.08.039
23. Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Eng J Med. 2003;348:977–985. doi:10.1056/NEJMoa013536
24. Hourihane JOB, Kilburn SA, Dean P, Warner JO. Clinical characteristics of peanut allergy. Clin Exp Allergy. 1997;27:634–639. doi:10.1111/cea.1997.27.issue-6
25. Matsumoto K, Mori R, Miyazaki C, Ohya Y, Saito H. Are both early egg introduction and eczema treatment necessary for primary prevention of egg allergy? J Allergy Clin Immunol. 2018;141:1997–2001. doi:10.1016/j.jaci.2018.02.033
26. Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–423. doi:10.1016/j.jaci.2008.12.014
27. Brough HA, Liu AH, Sicherer S, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015;135:164–170. doi:10.1016/j.jaci.2014.10.007
28. Horimukai K, Morita K, Narita M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol. 2014;134:824–830. doi:10.1016/j.jaci.2014.07.060
29. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818–823. doi:10.1016/j.jaci.2014.08.005
30. Lowe AJ, Su JC, Allen KJ, et al. A randomised trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitisation: the PEBBLES pilot study. Br J Dermatol. 2018;178:e19–e21. doi:10.1111/bjd.15747
31. Tsilochristou O, du Toit G, Sayre PH, et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J Allergy Clin Immunol. 2019;144:494–503. doi:10.1016/j.jaci.2019.04.025
32. Fisher HR, Keet CA, Lack G, du Toit G.Preventing peanut allergy: where are we now? J Allergy Clin Immunol Pract. 2019;7:367–373. doi:10.1016/j.jaip.2018.11.005
33. Soriano VX, Peters RL, Ponsonby AL, et al. Earlier ingestion of peanut after changes to infant feeding guidelines: the earlyNuts study. J Allergy Clin Immunol. 2019;144:1327–1335. doi:10.1016/j.jaci.2019.07.032
34. Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systemic review and meta-analysis. Allergy. 2014;69:76–86. doi:10.1111/all.12333
35. Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy. 2000;30(11):1540–1546. doi:10.1046/j.1365-2222.2000.00928.x
36. Peters RL, Allen KJ, Dharmage SC, et al. Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg and sesame allergy in children. J Allergy Clin Immunol. 2013;132:874–880. doi:10.1016/j.jaci.2013.05.038
37. Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444–451. doi:10.1016/S0091-6749(97)70133-7
38. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi:10.1067/mai.2001.114708
39. Peters RL, Allen KJ, Dharmage SC, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: a population-based assessment. J Allergy Clin Immunol. 2015;135:1257–1262. doi:10.1016/j.jaci.2015.01.002
40. Gray CL, Levin ME, Du Toit G. Ethnic differences in peanut allergy patterns in South African children with atopic dermatitis. Pediatr Allergy Immunol. 2015;26(8):721–730. doi:10.1111/pai.12459
41. Sastre J. Molecular diagnosis in allergy. J Brit Soc Allergy Clin Immunol. 2010;40(10):1442–1460. doi:10.1111/j.1365-2222.2010.03585.x
42. Borres MP, Ebisawa M, Eigenmann PA. Use of allergen components begins a new era in pediatric allergology. Ped Allergy Immunol. 2011;20:454–461. doi:10.1111/j.1399-3038.2011.01197.x
43. Krause S, Reese G, Randow S, et al. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009;124(4):771–778. doi:10.1016/j.jaci.2009.06.008
44. Maeda Y, Ono E, Fukutomi Y, et al. Correlations between alder specific IgE and alder-related tree pollen specific IgE by RAST method. Allergol Int. 2008;57(1):79–81. doi:10.2332/allergolint.O-07-496
45. Van Rooyen C, van den Berg S. Advances in the laboratory diagnosis of food allergy. Curr Allergy Clin Immunol. 2016;29(2):77–82.
46. Nicolaou N, Murray C, Belgrave D, et al. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127(3):684–685. doi:10.1016/j.jaci.2010.12.012
47. Codreanu F, Collignon O, Roitel O, et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011;154(3):216–226. doi:10.1159/000321108
48. Ebisawa M, Moverare R, Sato S, et al. Measurement of Arah1-,2-, and 3-specific antibodies is useful in diagnosis of peanut allergy in Japanese children. Pediatr Allergy Immunol. 2012;23(6):573–581. doi:10.1111/j.1399-3038.2012.01332.x
49. Hong X, Caruso D, Kumar R, et al. IgE, but not IgG4, antibodies to Ara h 2 distinguish peanut allergy from asymptomatic peanut sensitization. Allergy. 2012;67(12):1538–1546. doi:10.1111/all.12047
50. Gray CL, Levin ME, Du Toit G. Which test is best for diagnosing peanut allergy in South African children with atopic dermatitis. SAMJ. 2016;106(2):214–220. doi:10.7196/SAMJ.2016.v106i2.10125
51. Klemans RJB, van Os-medendorp H, Blankestijn M, Bruijnzeel-Koomen CAFM, Knol EF, Knulst AC. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy; a systemic review. Clin Exp Allergy. 2015;45:720–730. doi:10.1111/cea.12412
52. Flores Kim J, McCleary N, Nwaru BI, Stoddart A, Sheikh A. Diagnostic accuracy, risk assessment, and cost-effectiveness of component-resolved diagnostics for food allergy: a systemic review. Allergy. 2018;73:1609–1621. doi:10.1111/all.13399
53. Santos AF, Douiri A, Becares N, et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. 2014;134:645–652. doi:10.1016/j.jaci.2014.04.039
54. Glaumann S, Nopp A, Johansson SGO, Rudengren M, Borres MP, Nilsson C. Basophil allergen threshold sensitivity, CD-sens, IgE-sensitization and DBPCFC in peanut-sensitized children. Allergy. 2012;67:242–247. doi:10.1111/all.2012.67.issue-2
55. Santos AF, Couto-Francisco N, Becares N, Kwok M, Bahnson HT, Lack G. A novel human mast cell activation test for peanut allergy. J Allergy Clin Immunol. 2018;142:689–691. doi:10.1016/j.jaci.2018.03.011
56. Bahri R, Custovic A, Korosec P, et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J Allergy Clin Immunol. 2018;142:485–496. doi:10.1016/j.jaci.2018.01.043
57. Larsen LF, Juel-Berg N, Hansen KS, et al. A comparative study on basophil activation test, histamine release assay, and passive sensitization histamine release assay in the diagnosis of peanut allergy. Allergy. 2017;73:137–144. doi:10.1111/all.13243
58. Beyer KL, Ellman-Grunther L, Jarvinen KM, Wood RA, Hourihane J, Sampson HA. Measurement of peptide-specific IgE as an additional tool in identifying patients with clinical reactivity to peanuts. J Allergy Clin Immunol. 2003;112:202–207. doi:10.1067/mai.2003.1621
59. Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–475. doi:10.1016/j.jaci.2013.11.007
60. Nurmatov U, Devereux G, Worth A, Healy L, Sheikh A. Effectiveness and safety of orally administered immunotherapy for food allergies: a systematic review and meta-analysis. British J Nutrition. 2014;111:12–22. doi:10.1017/S0007114513002353
61. Nurmatov U, Venderbosch I, Devereux G, Simons FE, Sheikh A. Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Sys Rev. 2012;9:CD009014.
62. Yee CS, Rachid R. The heterogeneity of oral immunotherapy clinical trials: implications and future directions. Curr Allergy Asthma Rep. 2016;16:25. doi:10.1007/s11882-016-0602-0
63. Vickery BP, Vereda A, Casale TB, et al.; PALISADE Group of Clinical Investigators. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991–2001.
64. Vickery BP, Ebisawa M, Shreffler WG, Wood RA. Current and future treatment of peanut allergy. J Allergy Clin Immunol Pract. 2019;7:357–365. doi:10.1016/j.jaip.2018.11.049
65. EH K, Yang L, Ping Y, et al. Long-term sublingual immunotherapy for peanut allergy in children: clinical and immunological evidence of desensitization. J Allergy Clin Immunol. 2019. e-published ahead of print.
66. Fleischer DM, Burks AW, Vickery BP, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multi-center trial. J Allergy Clin Immunol. 2013;131:119–127. doi:10.1016/j.jaci.2012.11.011
67. Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135:1275–1282. doi:10.1016/j.jaci.2014.11.005
68. Burks AW, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: long-term follow-up of a randomized multicentre trial. J Allergy Clin Immunol. 2015;135:1240–1248. doi:10.1016/j.jaci.2014.12.1917
69. Mondoulet L, Dioszeghy V, Puteaux E, et al. Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy. 2012;2:22. doi:10.1186/2045-7022-2-22
70. Sampson HA, Shreffler WG, Yang WH, et al. Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: a randomized clinical trial. JAMA. 2017;318:1798–1809. doi:10.1001/jama.2017.16591
71. Fleisher DM, Sussman GL, Begin P. Effects of epicutaneous immunotherapy on inducing peanut desensitization in peanut-allergic children: topline Peanut Epicutaneous Immunotherapy Efficacy and Safety (PEPITES) randomized clinical trial results. J Allergy Clin Immunol. 2018;141:AB410. doi:10.1016/j.jaci.2017.12.967
72. Semancik E, Sayej W. Oral immunotherapy for peanut allergy induces eosinophilic oesophagitis: three paediatric case reports. Pediatr Allergy Immunol. 2016;27(5):539–541. doi:10.1111/pai.12554
73. Dua S, Ruiz-Garcia M, Bond S, et al. Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: A randomised, controlled study. J Allergy Clin Immunol. 2019. doi:10.1016/j.jaci.2019.06.038
74. Wasserman RL, Hague AR, Pence DM, et al. Real-world experience with peanut oral immunotherapy: lessons learned from 270 patients. J Allergy Clin Immunol Pract. 2019;7(2):418–426. doi:10.1016/j.jaip.2018.05.023
75. Chinthrajah RS, Purington N, Andorf S, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED Study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019;394:1437–1449. doi:10.1016/S0140-6736(19)31793-3
76. Santos AF, James LK, Kwok M, et al. Peanut oral immunotherapy induces blocking antibodies but does not change the functional characteristics of peanut-specific IgE. J Allergy Clin Immunol. 2019. doi:10.1016/j.jaci.2019.09.005
77. Wood RA, Chinthrajah S (Study chairs). Omalizumab as monotherapy and as adjunct therapy to multi-allergen OIT in food allergic participants (OUtMATCH Study). ClinicalTrials.gov, posted March 2019.
78. Koplin JJ, Perrett KP, Sampson HA. Diagnosing peanut allergy with fewer oral challenges. J Allergy Clin Immunol Pract. 2019;7(2):375–380. doi:10.1016/j.jaip.2018.11.010
79. Vickery BP, Berglund JP, Burk CM, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. 2017;139:173–181. doi:10.1016/j.jaci.2016.05.027
80. Blumchen K, Trendelenburg V, Ahrens F, et al. Efficacy, safety, and quality of life in a multicentre, randomized, placebo controlled trial of low-dose peanut oral immunotherapy in children with peanut allergy. JACI Pract. 2019;7:479–491.
81. Nachshon L, Goldberg MR, Katz Y, et al. Long-term outcome of peanut oral immunotherapy -real-life experience. Pediatr Allergy Immunol. 2018;29:519–526. doi:10.1111/pai.12914
82. Tang ML, Ponsonby AL, Orsini F, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. 2015;135:737–744. doi:10.1016/j.jaci.2014.11.034
83. Dunn Galvin A, McMahon S, Ponsoby AL, Hsiao KC, Tang MLK; PPOIT study team. The longitudinal impact of probiotic and peanut oral immunotherapy on health-related quality of life. Allergy. 2018;73:560–568. doi:10.1111/all.13330
84. Leung DY, Sampson HA, Yunginger JW, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Eng J Med. 2003;348:986–993. doi:10.1056/NEJMoa022613
85. Sampson HA, Leung DY, Burks AW, et al. A Phase II, randomized, double-blind, parallel-group, placebo-controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127:1309–1310. doi:10.1016/j.jaci.2011.01.051
86. MacGinnitie AJ, Rachid R, Gragg H, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. 2017;139:873–881. doi:10.1016/j.jaci.2016.08.010
87. Lin C, Lee IT, Sampath V, et al. Combining anti-IgE with oral immunotherapy. Pediatr Allergy Immunol. 2017;28:619–627. doi:10.1111/pai.2017.28.issue-7
88. Patil SP, Wang J, Song Y, et al. Clinical safety of food allergy Herbal Formula-2 (FAHF-2) and inhibitory effect on basophils from patients with food allergy: extended Phase I study. J Allergy Clin Immunol. 2011;128:1259–1265. doi:10.1016/j.jaci.2011.06.015
89. Wang J, Jones SM, Pongracic JA, et al. Safety, clinical, and immunological efficacy of a Chinese herbal medicine (Food Allergy Herbal Formula-2) for food allergy. J Allergy Clin Immunol. 2015;136:962–970. doi:10.1016/j.jaci.2015.04.029
90. Various for Astella Pharma Inc. A study to evaluate safety, tolerability and immune response in adolescents allergic to peanut after receiving intradermal administration of ASP0892, a single multivalent peanut (Ara h1, h2, h3) lysosomal associated membrane protein DNA plasmid vaccine. ClinicalTrials.gov Identifier NCT03755713, November 2018.
91. Yu J. Methods for reducing allergenicity of peanuts and peanut derived products. J Food Sci Edu. 2016;1:1012–1020.
92. Wang J, Fleischer DM. Should peanut be banned in schools? J Allergy Clin Immunol Pract. 2017;5:290–294. doi:10.1016/j.jaip.2017.01.006
93. Stukus DR. Peanut-free schools: what does it really mean, and are they necessary? J Allergy Clin Immunol. 2017;140:391–392. doi:10.1016/j.jaci.2017.03.037
94. Bartnikas LM, Huffaker MF, Sheehan WJ, et al. Impact of school peanut-free policies on epinephrine administration. J Allergy Clin Immunol. 2017;140:465–473. doi:10.1016/j.jaci.2017.01.040
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
