Back to Journals » Clinical Ophthalmology » Volume 9
Current and emerging treatment options for myopic choroidal neovascularization
Authors El Matri L, Chebil A, Kort F
Received 1 November 2014
Accepted for publication 9 February 2015
Published 24 April 2015 Volume 2015:9 Pages 733—744
DOI https://doi.org/10.2147/OPTH.S49437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Leila El Matri, Ahmed Chebil, Fedra Kort
Department B of Ophthalmology, Hedi Rais Institute of Ophthalmology, Faculty of Medicine of Tunis, University of El Manar, Tunis, Tunisia
Abstract: Choroidal neovascularization (CNV) is the main cause of visual impairment in highly myopic patients younger than 50 years of age. There are different treatments for myopic CNV (mCNV), with 5- to 10-year outcomes currently. Chorioretinal atrophy is still the most important determinant factor for visual outcome. The purpose of this study is to provide an overview of the current treatments for mCNV, including laser, surgical management, verteporfin photodynamic therapy, and mainly anti-vascular endothelial growth factor therapy. Emerging treatment options are also discussed.
Keywords: myopia, choroidal neovascularization, current treatment, emerging treatment
Introduction
Choroidal neovascularization (CNV) is the main cause of visual impairment in highly myopic patients, often affecting adults of working age, resulting in irreversible central vision loss due to progressive and irreversible central chorioretinal atrophy (CRA).1 CNV occurs in approximately 5%–10% of patients with pathological myopia1 – at any degree of myopia – and even in eyes without characteristic myopic fundus features.2
The overall prevalence of myopic CNV (mCNV) is therefore estimated to be approximately 0.04%–0.05% in the general population.1 Moreover, the natural history of mCNV is particularly variable, with a poor long-term visual outcome. Yoshida et al3 investigated the natural history of mCNV and studied the long-term progression pattern of myopic maculopathy. Macular atrophy was described in 90.1% untreated mCNV eyes, with a mean follow-up of 11.8 years. Best-corrected visual acuity (BCVA) in 96.3% eyes was 20/200 or worse at 10-year follow-up. The pathogenesis of mCNV remains unclear and different theories have been suggested: mechanical, heredodegenerative, and hemodynamic changes in choroidal circulation.4 A possible explanation includes, certainly, the induced hypoxia in the outer retina, which is a large source of vascular endothelial growth factor (VEGF) secretion. Chorioretinal stretching, lacquer crack formation, choroidal thinning, choroidal flow disturbance with reduced flow, choroidal filling delay, atrophy of the retinal pigment epithelium (RPE) and overlying retina, and loss of photoreceptors may all be involved in VEGF release and mCNV formation.4
Currently, with 5–10 years of follow-up, it is possible to determine the efficiency of the following different treatment options: laser photocoagulation, verteporfin photodynamic therapy (vPDT), surgical management, and mainly intravitreal anti-VEGF therapy.
The aim of this article is to provide a review of the literature related to mCNV and highlight the current and emerging treatment options.
Current treatment options for myopic choroidal neovascularization
Various approaches have been explored to treat myopic choroidal neovascularization (Table 1), which are as follows:
Laser photocoagulation
For a long time, laser photocoagulation was the only treatment for extrafoveal mCNV. However, laser is not indicated in subfoveal mCNV.5 Laser scar expansion and recurrence of CNV are the most important complications.5 Therefore, laser photocoagulation has been currently discontinued and replaced with more effective treatments.6
Surgical management
Surgical treatments for subfoveal CNV were tried before PDT or antiangiogenic drugs were introduced. They include CNV removal, limited macular translocation, or macular translocation with 360-degree retinectomy (MT360).
Surgical removal of mCNV
Myopic CNV is located under the retina but anterior to the RPE. Thus, the neovascular membrane could be removed with relative preservation of the underlying RPE and stabilization of vision. The integrity of the RPE and the choriocapillaris plays a fundamental role in determining the final visual prognosis following submacular surgery.7 Surgical excision of subfoveal CNV has been tried in a few studies.7,8 Short-term visual acuity (VA) improvement was observed in approximately 50% of the cases.
Macular translocation
Macular translocation (MT) consists of displacing the neurosensory retina of the fovea to a new location along with the normal RPE–Bruch’s membrane–choriocapillaris complex. Thus, the subfoveal lesion is converted to an extrafoveal one, allowing other modalities of treatment without damaging the fovea. Two techniques – limited MT and MT360 – have been performed in the past decade with controversial results.9,10 Better results have been reported for limited MT than for surgical removal but with a high rate of recurrences.8 Unlike surgical excision, MT surgery preserves damage to the RPE–choriocapillaris complex. Specific complications associated with MT include mainly retinal detachments, macular holes, choroidal hemorrhages, and recurrences. Binocular viewing altered by torsional deviations may limit the final visual outcome. The clinical efficacy of MT for mCNV remains uncertain because of the lack of randomized studies. This operation is rarely performed because of the difficult surgical procedures and complications. Nonetheless, in very select cases of mCNV refractory to anti-VEGF therapy, MT may be useful. Recently, Sakimoto et al11 have reported the long-term effects of MT in a large consecutive case series of 60 eyes with mCNV that underwent MT (mean follow-up period: 76.3 months). They showed that the logarithm of the minimum angle of resolution (logMAR) BCVA values at 1 year, 3 years, and 5 years postoperatively significantly (P<0.001) improved to 0.54 at 1 year and then remained stable. MT for mCNV maintained the improvement in VA for >5 years. However, postoperative complications and progression of CRA due to myopia still seem to limit the visual improvement after MT for mCNV.
Thus, surgical removal of mCNV may be beneficial in selected patients, but this technique is highly dependent on the surgeon’s ability.
Photodynamic therapy
PDT (Visudyne; QLT Inc, Vancouver, BC, Canada) is the only approved treatment for subfoveal mCNV. The verteporfin in photodynamic therapy (VIP) study, a randomized, double-masked, placebo-controlled clinical trial,12,13 demonstrated stabilization of VA in 72% of the eyes with subfoveal mCNV treated with PDT over a period of 12 months. Unfortunately, there was no statistically significant benefit in the visual outcome at 24 months.
Many other studies have reported similar outcomes, with BCVA remaining nearly unchanged at 2 and 3 years.14–16 Hayashi et al16 prospectively followed for 4 years 60 mCNV eyes treated with vPDT. CRA developed in 70% of the eyes at 4 years. The BCVA did not change significantly after PDT. Coutinho et al17 followed prospectively, for 5 years, 45 mCNV eyes treated with PDT. VA stabilized or improved in 65% of the eyes after 24 months, with no significant VA change between month 24 and month 60. Recently, Varano et al18 evaluated and compared the effects of PDT in eyes with subfoveal and juxtafoveal CNV with 10-year follow-up. Prevalence and extension of CRA were greater in eyes with subfoveal CNV compared to eyes with juxtafoveal CNV. These results confirm the limited long-term effectiveness of PDT in subfoveal mCNV.14,15,18 This fact may probably be explained by differences in the retinal structure of the central foveal and the juxtafoveal areas. Besides, PDT worsens CRA and, therefore, the long-term visual prognosis of mCNV.
Age at onset of mCNV is the most important factor affecting the final visual outcome following PDT treatment. In fact, PDT may induce greater damage in elderly subjects who already have choriocapillaris atrophy secondary to longer evolution of myopic retinopathy and/or to age-related choroidal vascular sclerosis.19
In conclusion, all studies show that visual improvement is significant at 1 year after PDT.18 There is no significant change thereafter. Better results are obtained in younger patients.20,21 However, PDT is still a convenient option, especially for patients with juxtafoveal mCNV and when anti-VEGF therapy is unsuitable or may not be feasible.22
Anti-VEGF therapy
Anti-VEGF drugs are currently the “gold standard” for the treatment of mCNV from different causes including high myopia. Intravitreal anti-VEGF is actually recommended as the first-line treatment for mCNV.
Treatment with intravitreal anti-VEGF injection in mCNV was first published in 2007.23 Thereafter, many publications were reported despite the widely “off-label” use of these drugs in mCNV. Nonetheless, all the studies universally demonstrated that intravitreal injections of anti-VEGF agents are efficacious for managing mCNV, improving the functional and anatomical outcomes.23,24 In 2014, ranibizumab was approved for treatment of subfoveal mCNV.
Treatment with bevacizumab
In 2005, Nguyen et al25 reported the results of eyes with subfoveal mCNV treated with systemic bevacizumab (Avastin®; Genentech/Roche). Then intravitreal bevacizumab (IVB) was tested, with no apparent retinal toxicity.26
Yamamoto et al23 and Sakaguchi et al24 were the first to report the treatment of subfoveal mCNV with IVB. Encouraging short-term results and no ocular or systemic side effects were reported in their small case series.
Several articles cited by Silva27 reported on the outcome of IVB at 1-year follow-up. The initial therapeutic protocol was three consecutive, monthly IVB injections for some authors, while for others, only a single IVB injection was used as initial treatment followed by pro re nata injections (PRN). In all studies, the statistically significant visual improvement of two lines or more was sustained at 12 months. Wang and Chen,28 in a systematic review and meta-analysis, compared two groups: three monthly injections (3+ PRN group) and a single injection (1+ PRN group). They did not find significant difference in retinal thickness between the two groups within 12 months. In addition, the 1+ PRN group required 1.37 fewer injections than the 3+ PRN group within 12 months. Thus, for treating mCNV, one single injection followed by PRN seems to be the best choice. This regimen may decrease the total number of injections and reduce the impact of pan inhibition of VEGF on retinal neuron survival.29
Moreover, a functional improvement was registered in microperimetry studies at 6 months and 12 months.30,31 Scupola et al showed improvement of macular sensitivity and fixation stability 1 year after IVB for mCNV, advocating a stable and progressive macular function recovery.31
Subsequently, studies with 2-year follow-up have reported favorable visual outcomes after IVB,32–37 with improvement of visual acuity by two Early Treatment Diabetic Retinopathy Study (ETDRS) lines. But the results of these studies were controverted because other authors have reported that the visual improvement became nonsignificant after 2 years of follow-up. Thus, Baba et al32 reported that the BCVA was significantly improved at 12 months and at 24 months. In contrast, El Matri et al38 reported that 12 months after starting treatment with bevacizumab, the gain in BCVA was no longer significant. Calvo-Gonzalez et al39 and Ruiz-Moreno and Montero34 also found that after 2 years, the gain in BCVA with anti-VEGF therapy was no longer significant.
The variability of results at 2-year follow-up may be explained by the small sample sizes and the CNV location. In fact, the great majority of studies have included both subfoveal and juxtafoveal mCNV. About 30% of eyes had nonsubfoveal location at presentation, and the natural history of juxtafoveal CNV is not well known. For Hayashi et al40 the results of IVB are different in eyes with a subfoveal CNV from that of eyes with nonsubfoveal CNV. The BCVA was significantly improved after 2 years of follow-up in eyes with nonsubfoveal CNV, while there was no significant difference in eyes with subfoveal CNV. Thus, for eyes with nonsubfoveal CNV, IVB might be a good treatment option. Parodi et al6 obtained similar results on extrafoveal mCNV and, interestingly, no patient experienced a foveal involvement during the follow-up.
More recently, Peiretti et al41 reported the results of a 4-year study of IVB for the treatment of mCNV. This study showed maintenance or improvement of vision in about 85% of the eyes with mCNV. These results are consistent with those reported in previous short-term studies. The best number of IVB injections needed to achieve mCNV closure is not predictable; however, 2–15 IVB injections were applied per eye. Moreover, Ruiz-Moreno and Montero34 clearly demonstrated the efficacy of intravitreal injections of anti-VEGF in their relatively large case series and showed that VA gain was maintained at 4 years through a mean total number of 4.9 IVB (range: 1–29).
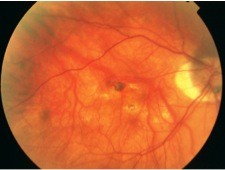
For Oishi et al42 also, IVB for mCNV is effective for vision improvement in the long term. However, the effect rather declined to slightly nonsignificant levels after 4 years. In fact, visual improvement is restricted by the frequent development or enlargement of CRA. Yoshida et al reported that in the natural course of mCNV, 3 years after disease onset, VA levels had deteriorated continuously and continued to do so for at least another 7 years. This visual deterioration may be due to the development of myopic CRA, which also might decrease the efficacy of anti-VEGF agents for improving VA (Figure 1).
Because of scleral thinning in high-myopic patients, especially at the posterior pole,43 it was hypothesized that a posterior sub-Tenon injection of bevacizumab (PSTB) may be an effective alternative route of delivery due to the significant penetration by bevacizumab through the sclera to reach the CNV zone. Liang et al44 evaluated the effectiveness of PSTB treatment for mCNV: 88.89% of eyes remained at baseline VA or had visual improvement, 66.7% of eyes improved by at least two lines, and 44.4% of eyes showed improvement of at least three lines. Their results are comparable with, or even better than, those reported previously for IVB injections, showing that the effect of PSTB in mCNV is promising.
Treatment with ranibizumab
Ranibizumab (Lucentis™; Novartis Pharma AG, Basel, Switzerland, and Genentech Inc, South San Francisco, CA, USA) has been specifically designed for ocular use. It is the only licensed anti-VEGF therapy for treatment of mCNV.
Intravitreous ranibizumab (IVR) showed apparently superior results when compared with PDT for juxtafoveal and subfoveal mCNV. Silva et al45 reported the first results of IVR for mCNV following a PRN regimen since the first injection. At 3 months after IVR injection, VA improved by one or more lines in 65% of eyes and all eyes had stable or improved vision at 3 months.45 These short-term results seemed to be similar to those of other studies, cited by Silva which used IVB for mCNV.27 Many authors46,47 have reported the results of IVR at 1-year follow-up with a VA improvement of three or more lines in 24%–46.8% of cases. Angiographic closure occurred at 3 months in 93.8% of the eyes.45 During the 12-month follow-up, a mean of 3.6 intravitreal injections was performed.
Treatment regimens with and without loading dose were evaluated in different studies. Lai et al46 obtained a significant VA gain at 1 year using a loading dose of three monthly injections. Of the 16 patients, 75% gained vision and only one patient needed retreatment.
Other authors reported, in prospective studies, similar good results with no loading dose, achieving a significant VA improvement from baseline.39,47,48
Actually, the use of ranibizumab for treatment of mCNV is supported by data from phase II (Ranibizumab for treatment of CNV secondary to Pathological myopia: An Individualized Regimen or REPAIR) and phase III (RADIANCE) trials.49–51
REPAIR is a large, phase II, open-label, single-arm, multicenter, nonrandomized study of 65 patients from the UK. It indicated that ranibizumab was effective in improving vision and preventing vision loss with a median of 3 injections over 12 months.50
RADIANCE, a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia, compared the efficacy and safety of ranibizumab in two groups treated by IVR with two different PRN schedules to a third group treated with vPDT (N=227).49
The first group treated with PRN ranibizumab was guided by VA stabilization criteria and the second group was guided by disease activity criteria. RADIANCE showed that patients treated with both PRN regimens of ranibizumab provided superior BCVA gains in comparison with patients treated with vPDT at 3 months. Retreatment criteria did not affect the 6-month results in the ranibizumab treatment groups. At 12 months, irrespective of retreatment criteria, ranibizumab was effective in improving and sustaining BCVA and was generally well tolerated in patients with mCNV.49 Moreover, patients who were previously treated by vPDT could still gain vision when they were switched to ranibizumab. Nevertheless, patients initially treated by vPDT and later switched to IVR did not achieve the same visual gains as those treated initially with IVR. Anatomical outcome improvements were observed with both IVR and vPDT.49 RADIANCE also revealed significant improvements in several quality-of-life parameters for patients treated with ranibizumab compared with vPDT, which were maintained through to 12 months.52
Franqueira et al53 published the 3-year follow-up of a prospective study that included 40 eyes with mCNV treated with ranibuzimab. The mean VA improved significantly from 55.4 ETDRS letters at baseline to 63.4 letters at 36 months. Thirty-five percent of the patients gained ≥3 lines at 36 months. A mean of 4.1 injections was used in the first year, 2.4 in the second year, and 1.1 in the third year.
Iacono et al54 reported the results of one of the largest randomized studies of IVB versus IVR in treatment-naïve mCNV. VA improved significantly after anti-VEGF therapy and improvement remained statistically significant at 18 months. Moreover, there was no significant difference in VA between eyes treated with bevacizumab or ranibizumab at all time points.
Ruiz-Moreno et al55 demonstrated the long-term efficacy of intravitreal injections of both bevacizumab and ranibizumab. They showed that the mean number of letters read was 46.1 at baseline, 55.5 at 1 year, 50.1 at 2 years, 54.2 at 3 years, and 53.1 at 4 years.
Recently, Freitas-da-Costa et al56 reported the long-term results of anti-VEGF therapy in mCNV. The mean change from baseline BCVA was significant at 2 years (+8.6 letters; P<0.001) and this gain remained significantly stable for a period of 5 years. The mean number of injections performed during the first year was 5.2, becoming lower in subsequent years (P<0.001).
In conclusion, when antiangiogenic drugs are used to treat mCNV, the suggested protocol could be an initial injection of 0.05 mL of either 1.25 mg bevacizumab or 0.5 mg ranibizumab, followed by a monthly evaluation of VA using optical coherence tomography (OCT), with additional treatments in case of loss of BCVA, evidence of CNV activity on OCT, or persistent leakage of CNV shown on fluorescein angiography.55 A favorable long-term outcome can be achieved with a relatively small number of injections in cases of mCNV.
Treatment with pegaptanib
Intravitreal pegaptanib (IVP) was also evaluated in mCNV. The first case treated successfully with IVP injections, a young patient with mCNV refractive to laser photocoagulation and PTD, was reported by Benett and Yee57 in 2007. Then, Rinaldi et al58 and Kitagawa and Yuzawa59 reported 1 year or less outcome of intravitreal pegaptanib. The initial treatment protocol consisted of three monthly consecutive injections. The findings demonstrate that the selective inhibition of VEGF-165 isoform by IVP injections is an effective treatment for mCNV.
One-year treatment outcomes of intravitreal injections of pegaptanib sodium and bevacizumab for mCNV were compared.60 Although changes in VA at 1 year did not differ significantly between the two groups, bevacizumab improved mean retinal sensitivity with fewer injections than pegaptanib, suggesting that bevacizumab may be more effective than pegaptanib for mCNV.
Treatment with aflibercept (VEGF trap-eye)
Aflibercept (Eylea®; Regeneron Pharmaceutical Inc and Bayer) is the most recent member in the anti-VEGF arsenal. Its efficacy and safety for mCNV was evaluated in the ongoing phase III, multicenter, randomized, sham-controlled, 12-month MYRROR study in Asian patients (N=121; NCT01249664). A single intravitreal aflibercept injection is administered, followed by a PRN regimen. After 6-month follow-up, a 12.1-letter improvement in BCVA is reported, compared with a 2-letter loss in those receiving sham injections. BCVA gains are maintained up to 12 months, as mentioned by Wong et al.52
In conclusion, once mCNV is diagnosed, prompt treatment is suggested with a single intravitreal injection of anti-VEGF, followed by PRN dosing. Then, patients should be followed monthly to monitor the signs of activity of mCNV, seeking by questioning a decrease in vision, new or persistent metamorphopsia, and detecting disease activity by both clinical evaluation such as new hemorrhage on fundus examination and relevant imaging: OCT and/or angiography showing intraretinal or subretinal fluid or persistent leakage. Spectral domain OCT is actually the most useful tool for evaluating neovascular activity. A decrease in BCVA alone is not sufficient. It could indicate additional pathologies, such as myopic traction maculopathy (foveoschisis), macular hole, retinal tears, and rhegmatogenous detachments.59
If there is a disease activity, another intravitreal injection of anti-VEGF is performed. A favorable long-term outcome can be achieved with a notably lower number of injections in cases of mCNV compared to other conditions such as neovascular age-related macular degeneration (AMD). As the long-term safety and efficacy of anti-VEGF treatment of mCNV are unknown, we must not forget that, because VEGF has an important role in neuroprotection and retinal development also,36,37 anti-VEGF treatment may produce retinal impairment after a long period of administration. Moreover, we must not forget the likely risk of retinal detachment in myopic eyes submitted to repeated intravitreal injections. Intravitreal injection of anti-VEGF should be given only in case of resumption of activity of the mCNV. If there is no disease activity, patients should be controlled monthly the first 3 months. Then, visits will be considered every 3 months for the first year.
Whether ranibizumab, bevacizumab, or aflibercept should be chosen is unknown. They are all effective. The availability of intravitreal bevacizumab, an inexpensive drug, provides an opportunity to prevent VA loss in low-income countries where the cost of ranibizumab or aflibercept is excessively high.
Therefore, regardless of the selected treatment, the development of CRA around the mCNV is a specific complication threatening vision, requiring prevention for maintaining the long-term vision. In fact, it is still unknown whether anti-VEGF treatment could slow down the atrophy development in myopic eyes.
Recently, Farinha et al61 analyzed the long-term progression of myopic maculopathy and functional outcome in eyes treated for mCNV and in eyes without CNV. Three different therapeutic approaches – PDT, IVR, and PDT + IVR – were compared. There were no significant differences in morphological and functional outcomes among the groups. The morphological changes in all treated eyes were more likely to be linked with the natural progression of the myopic maculopathy than with the type of treatment performed.
Calvo-Gonzalez et al39 also found that after 2 years of treatment with IVR for mCNV, 70.2% of the eyes developed CRA around the regressed CNV. Oishi et al42 reported, 4 years after IVB, development or enlargement of CRA in 72.7% of the eyes. Moreover, Hayashi et al40 reported that 74.1% of untreated eyes developed macular atrophy, and after 5 years, the prevalence was 96.3%. In eyes treated for mCNV, the area of macular atrophy increased significantly until the final evaluation. So, this confirms that CRA can develop and enlarge long after CNV has regressed not only in untreated eyes but also in eyes treated with PDT and/or anti-VEGF and may be responsible for the progressive reduction of treatment efficacy in the long term.29,39,45
Multivariate regression analysis showed that age, degree of myopia, presence of staphyloma, and a greater baseline central area of macular atrophy were predictive of greater areas of macular CRA in the long term.12,30,61,62
Emerging treatments
Promising investigations that are being conducted in AMD–CNV could be extrapolated to mCNV management.63 Emerging treatments may include new molecules acting on different parts of the VEGF cascade, antiangiogenic molecules of greater duration of action, sustained-release ophthalmic drug delivery systems, new surgical approaches such as cell transplantation (retinal pigment epithelial cell transplantation) and transplantation of retina and choroid, or the use of radiation in combination with or without intravitreal therapies.64
New molecules acting on different parts of the VEGF cascade can be classified as follows:
- Small interfering RNA (siRNA):65,66 siRNA 027 (AGN211745) targets VEGF receptor (VEGFR)-1 on the endothelial cells and siRNA PF-04523655 (REDD14) inhibits the expression of hypoxia-induced gene RTP801.
- Anti-VEGF agents: other antagonists to the VEGF pathway are also being evaluated. 1) Sphingosine-1-phosphate (S1P) antibody: The RPE cells are a major source of S1P in the retina and S1P is in charge of the pathological angiogenesis, vascular permeability, inflammatory responses, and fibrosis associated with neovascular AMD.67 2) Squalamine lactate.68 3) Palomid 529 is an investigational medication involving the immune Akt/mTOR pathway and unique in dissociating both targets of rapamycin complexes TORC1 and TORC2.69 4) KH902 has similar properties as aflibercept.70 5) Intravitreal injection of adeno-associated virus-2 vector is being used to deliver an anti-VEGF molecule, sFLT01.71 6) MP0112 is a designed ankyrin repeat protein that specifically binds all VEGF-A isoforms.72 7) Tyrosine kinase inhibitors: vatalanib with activity against the platelet-derived growth factor receptor (PDGFR) and c-Kit receptor kinases;73 and oral pazopanib with activity against VEGFR, PDGFR, and c-kit.74 8) PDGF antagonists, eg, E10030.
Antiangiogenic molecules with greater duration of action
Drug formulations of greater duration of action are being considered to reduce the frequency of intravitreal injection as well as to reduce the rate of complications. Liposomes are one of the most advanced drug nanocarriers.75
Sustained-release ophthalmic drug delivery systems
In situ injectable polysaccharide cross-linked hydrogel was developed for ocular drug delivery of bevacizumab.76 Intravitreal, liquid, sustained drug delivery system formulated with triamcinolone acetonide in combination with ranibizumab has been evaluated in AMD and has resulted in fewer ranibizumab retreatments. Besides, intraocular injection of triamcinolone acetonide nanoparticles incorporated in thermoreversible gels seems to reduce VEGF expression in neovascular AMD.77
Radiation therapies
The following therapies are explored: proton therapy, stereotactic radiotherapy, and epimacular brachytherapy.
Recently, Chen et al78 reported the 2-year results of a randomized clinical trial on proton beam irradiation for non-AMD–CNV. Myopic CNV constituted the most common diagnosis (52.9% of 51 eyes) among their cases. The authors found that at 24 months, proton beam radiation therapy with either 16 or 24 cobalt gray equivalent is safe, may prevent vision loss, and, in some cases, improve vision in patients with mCNV. The principle of therapy is that radiation can inhibit endothelial cell proliferation, decrease angiogenic cytokine-producing inflammatory cells in CNV complexes, and reduce proliferation of fibroblasts involved in scar formation. Thus, this treatment modality may be considered as an alternative therapy in cases where PDT or intravitreal anti-VEGF treatment may not be feasible. Proton therapy has mainly the benefit of a single cure and the possibility for more selectivity due to the relative resistance of neural tissue to radiation damages and it may be used as an adjunct to anti-VEGF therapy.78
Epimacular brachytherapy was developed to deliver intraocular radiation. The source of beta radiation is placed close to the CNV complex in the macular region and the radiation (24 Gy) is delivered via a pars plana vitrectomy positioning the probe over the CNV lesion (VideON system). This treatment can stabilize neovascular AMD, thus decreasing the requirement for intravitreal anti-VEGF therapy.79
Stereotactic radiotherapy (SRT; IRay system) uses a low-voltage X-ray system with a great advantage of not requiring invasive surgical procedures. The X-ray is collimated into a narrow beam that enables precise targeting limited to the macula. A single dose of SRT significantly reduces intravitreal injections of anti-VEGF in AMD, over a period of 2 years.80
Besides, the role of genetics in the development of myopia and pathological myopia is now obvious based on scientific evidence. It can therefore be assumed that there is a genetic role in the pathogenesis of mCNV. To date, there are no publications on genes associated with mCNV.81 Miyake et al82 reported that VEGF polymorphism influences VA prognosis in highly myopic eyes with CNV within 1 year after anti-VEGF treatment. This association was still observed after removing its confounding effect through CNV size. The rs2010963 polymorphism was not associated with CNV recurrence or CRA progression, which indicates that these changes are not tied to intrinsic factors and may be controllable by improving treatment methods.
Finally, as the best treatment of myopic CNV is still unknown and the long-term visual prognosis is unclear, prevention should be considered. Research investigating and identifying risk factors for the development and progression of high myopia and mCNV must be conducted.
In the future, it will be mandatory both to assess the mechanism underlying chorioretinal atrophy development and enlargement and to establish the best treatment modalities that may prevent VA loss.
Disclosure
The authors report that they have no proprietary interest and have received no grants, funds, or financial support in relation to this study.
References
Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984;91(12):1573–1581. | ||
Leveziel N, Yu Y, Reynolds R, et al. Genetic factors for choroidal neovascularization associated with high myopia. Invest Ophthalmol Vis Sci. 2012;53:5004–5009. | ||
Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology. 2003;110(7):1297–1305. | ||
Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503. | ||
Virgili G, Menchini F. Laser photocoagulation for choroidal neovascularisation in pathologic myopia. Cochrane Database Syst Rev. 2005;4:CD004765. | ||
Parodi MB, Iacono P, Papayannis A, Sheth S, Bandello F. Laser photocoagulation, photodynamic therapy, and intravitreal bevacizumab for the treatment of juxtafoveal choroidal neovascularization secondary to pathologic myopia. Arch Ophthalmol. 2010;128:437–442. | ||
Adelberg DA, Del Priore LV, Kaplan HJ. Surgery for subfoveal membranes in myopia, angiod streaks, and other disorders. Retina. 1995;15:198–205. | ||
Hamelin N, Glacet-Bernard A, Brindeau C, Mimoun G, Coscas G, Soubrane G. Surgical treatment of subfoveal neovascularization in myopia: macular translocation vs surgical removal. Am J Ophthalmol. 2002;133(4):530–536. | ||
Fujikado T, Ohji M, Kusaka S, et al. Visual function after foveal translocation with 360-degree retinotomy and simultaneous torsional muscle surgery in patients with myopic neovascular maculopathy. Am J Ophthalmol. 2001;131(1):101–110. | ||
Yamada Y, Miyamura N, Suzuma K, Kitaoka T. Long-term follow-up of full macular translocation for choroidal neovascularization. Am J Ophthalmol. 2010;149(3):453–457. | ||
Sakimoto S, Sakaguchi H, Ohji M, et al. Consecutive case series with long-term follow-up of full macular translocation for myopic choroidal neovascularisation. Br J Ophthalmol. 2014;98(9):1221–1225. | ||
Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1-year results of a randomized clinical trial – VIP report no 1. Ophthalmology. 2001;108:841–852. | ||
Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial – VIP report no 3. Ophthalmology. 2003;110:667–672. | ||
Hayashi K, Ohno-Matsui K, Teramukai S, et al. Photodynamic therapy with verteporfin for choroidal neovascularization of pathologic myopia in Japanese patients: comparison with nontreated controls. Am J Ophthalmol. 2008;145(3):518–526. | ||
Chen YS, Lin JY, Tseng SY, Yow SG, Hsu WJ, Tsai SC. Photodynamic therapy for Taiwanese patients with pathologic myopia: a 2-year follow up. Retina. 2007;27:839–845. | ||
Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term results of photodynamic therapy for choroidal neovascularization in Japanese patients with pathologic myopia. Am J Ophthalmol. 2011;151(1):137–147. | ||
Coutinho AM, Silva RM, Nunes SG, Cachulo ML, Figueira JP, Murta JN. Photodynamic therapy in highly myopic eyes with choroidal neovascularization: 5 years of follow-up. Retina. 2011;31(6):1089–1094. | ||
Varano M, Iacono P, Giorno P, Chiaravalloti A, Parravano M. Photodynamic therapy in subfoveal and juxtafoveal myopic choroidal neovascularization: a 10-year retrospective analysis. Ophthalmologica. 2014;231(4):204–210. | ||
Ramrattan RS, Van der Schaft TL, Mooy CM. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in ageing. Invest Ophthalmol Vis Sci. 1994;35(6):2857–2864. | ||
Pece A, Isola V, Vadalà M, Matranga D. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization secondary to pathologic myopia: long-term study. Retina. 2006;26(7):746–751. | ||
Ruiz-Moreno JM, Amat P, Montero JA, Lugo F. Photodynamic therapy to treat choroidal neovascularisation in highly myopic patients: 4 years’ outcome. Br J Ophthalmol. 2008;92(6):792–794. | ||
Chew MC, Tan CS. Treatment options for myopic CNV–is photodynamic therapy still relevant? Indian J Ophthalmol. 2014;62(7):834–835. | ||
Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol. 2007;91(2):157–160. | ||
Sakaguchi H, Ikuno Y, Gomi F, et al. Intravitreal injection of bevacizumab for choroidal neovascularisation associated with pathological myopia. Br J Ophthalmol. 2007;91(2):161–165. | ||
Nguyen QD, Shah S, Tatlipinar S, Do DV, Anden EV, Campochiaro PA. Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol. 2005;89(10):1368–1370. | ||
Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 2006;26(3):257–261. | ||
Silva R. Myopic Maculopathy: a Review. Ophthalmologica. 2012;228(4):197–213. | ||
Wang E, Chen Y. Intravitreal anti-vascular endothelial growth factor for choroidal neovascularization secondary to pathologic myopia: systematic review and meta-analysis. Retina. 2013;33(7):1375–1392. | ||
Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. | ||
Yodoi Y, Tsujikawa A, Nakanishi H, et al. Central retinal sensitivity after intravitreal injection of bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol. 2009;147(5):816–824. | ||
Scupola A, Tiberti AC, Sasso P, et al. Macular functional changes evaluated with MP-1 microperimetry after intravitreal bevacizumab for subfoveal myopic choroidal neovascularization: one-year results. Retina. 2010;30(5):739–747. | ||
Baba T, Kubota-Taniai M, Kitahashi M, Okada K, Mitamura Y, Yamamoto S. Two-year comparison of photodynamic therapy and intravitreal bevacizumab for treatment of myopic choroidal neovascularisation. Br J Ophthalmol. 2010;94(7):864–870. | ||
Ikuno Y, Nagai Y, Matsuda S, et al. Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol. 2010;149(1):140–146. | ||
Ruiz-Moreno JM, Montero JA. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-year outcome. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):937–941. | ||
Voykov B, Gelisken F, Inhoffen W, Voelker M, Bartz-Schmidt KU, Ziemssen F. Bevacizumab for choroidal neovascularization secondary to pathologic myopia: is there a decline of the treatment efficacy after 2 years? Graefes Arch Clin Exp Ophthalmol. 2010;248(4):543–550. | ||
Nakanishi H, Tsujikawa A, Yodoi Y, et al. Prognostic factors for visual outcomes 2-years after intravitreal bevacizumab for myopic choroidal neovascularization. Eye. 2011;25(3):375–381. | ||
Gharbiya M, Allievi F, Conflitti S, et al. Intravitreal bevacizumab for treatment of myopic choroidal neovascularization: the second year of a prospective study. Clin Ter. 2010;161(3):87–93. | ||
El Matri L, Kort F, Chebil A, Bouraoui R, Merdassi A, Bouladi M. Intravitreal bevacizumab versus photodynamic therapy for myopic choroidal neovascularization in a North-African population. Graefes Arch Clin Exp Ophthalmol. 2011;249(9):1287–1293. | ||
Calvo-Gonzalez C, Reche-Frutos J, Donate J, Fernandez-Perez C, Garcia-Feijoo J. Intravitreal ranibizumab for myopic choroidal neo- vascularization: factors predictive of visual outcome and need for retreatment. Am J Ophthalmol. 2011;151(3):529–534. | ||
Hayashi K, Shimada N, Moriyama M, Hayashi W, Tokoro T, Ohno-Matsui K. Two-year out-comes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathologic myopia. Retina. 2012;32(4):687–695. | ||
Peiretti E, Vinci M, Fossarello M. Intravitreal bevacizumab as a treatment for choroidal neovascularisation secondary to myopia: 4-year study results. Can J Ophthalmol. 2012;47(1):28–33. | ||
Oishi A, Yamashiro K, Tsujikawa A, et al. Long-term effect of intravitreal injection of anti-VEGF agent for visual acuity and chorioretinal atrophy progression in myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):1–7. | ||
McBrien NA, Norton TT. Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Exp Eye Res. 1994;59(4):475–486. | ||
Liang IC, Chang YY, Lee TS, Lin YR, Liu KR. Treatment of myopic choroidal neovascularization with posterior sub-Tenon’s bevacizumab injection (Avastin). Int Ophthalmol. 2014;34(4):971–977. | ||
Silva RM, Ruiz-Moreno JM, Nascimento J, et al. Short-term efficacy and safety of intravitreal ranibizumab for myopic choroidal neovascularization. Retina. 2008;28(8):1117–1123. | ||
Lai TY, Chan WM, Liu DT, Lam DS. Intravitreal ranibizumab for the primary treatment of choroidal neovascularization secondary to pathologic myopia. Retina. 2009;29(6):750–756. | ||
Silva RM, Ruiz-Moreno JM, Rosa P, et al. Intravitreal ranibizumab for myopic choroidal neovascularization: 12-month results. Retina. 2010;30(3):407–412. | ||
Vadalà M, Pece A, Cipolla S, et al. Is ranibizumab effective in stopping the loss of vision for choroidal neovascularisation in pathologic myopia? A long-term follow-up study. Br J Ophthalmol. 2011;95(5):657–661. | ||
Wolf S, Balciuniene VJ, Laganovska G. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121(3):682–692. | ||
Tufail A, Narendran N, Patel PJ, et al. Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology. 2013;120:1944–1945. | ||
Tufail A, Patel PJ, Sivaprasad S, et al. Ranibizumab for the treatment of choroidal neovascularisation secondary to pathological myopia: interim analysis of the REPAIR study. Eye. 2013;27:709–715. | ||
Wong TY, Ohno-Matsui K, Leveziel N, et al. Myopic choroidal neovascularisation: current concepts and update on clinical management. Br J Ophthalmol. 2015;99(3):289–296. | ||
Franqueira N, Cachulo ML, Pires I, et al. Long-term follow-up of myopic choroidal neovascularization treated with ranibizumab. Ophthalmologica. 2012;227(1):39–44. | ||
Iacono P, Parodi MB, Papayannis A, et al. Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina. 2012;32(8):1539–1546. | ||
Ruiz-Moreno JM, Arias L, Montero JA, Carneiro A, Silva R. Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br J Ophthalmol. 2013;97(11):1447–1450. | ||
Freitas-da-Costa P, Pinheiro-Costa J, Carvalho B, et al. Anti-VEGF therapy in myopic choroidal neovascularization: long-term results. Ophthalmologica. 2014;232(1):57–63. | ||
Bennett MD, Yee W. Pegaptanib for myopic choroidal neovascularization in a young patient. Graefes Arch Clin Exp Ophthalmol. 2007;245(6):903–905. | ||
Rinaldi M, Chiosi F, Dell’Omo R, et al. Intravitreal pegaptanib sodium (macugen) for treatment of myopic choroidal neovascularization: a morphologic and functional study. Retina. 2013;33(2):397–402. | ||
Kitagawa T, Yuzawa M. Intravitreal pegaptanib sodium, for myopic choroidal neovascularisation: 1 year results of a prospective pilot study. Nihon Ganka Gakkai Zasshi. 2013;117(4):344–350. | ||
Kitagawa T, Yuzawa M. Comparison of 1-year treatment outcome of intravitreal pegaptanib sodium versus bevacizumab for myopic choroidal neovascularization. Nihon Ganka Gakkai Zasshi. 2013;117(9):727–734. | ||
Farinha CL, Baltar AS, Nunes SG, et al. Progression of myopic maculopathy after treatment of choroidal neovascularization. Ophthalmologica. 2014;231:211–220. | ||
Neelam K, Cheung CM, Ohno-Matsui K, Lai TY, Wong TY. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res. 2012;31(5):495–525. | ||
Kaiser PK. Emerging therapies for neovascular age-related macular degeneration: drugs in the pipeline. Ophthalmology. 2013;120(5):S11–S15. | ||
Joussem AM, Kirchhof B. Surgery for age-related macular degeneration. Still an option in the age of pharmacotherapy? Klin Monbl Augenheilkd. 2014;231(9):874–882. | ||
Kaiser PK, Symons RC, Shah SM, et al; Sirna-027 Study Investigators. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol. 2010;150(1):33–39. | ||
Nguyen QD, Schachar RA, Nduaka CI, et al; MONET Clinical Study Group. Evaluation of the siRNA PF-04523655 versus ranibizumab for the treatment of neovascular age-related macular degeneration (MONET Study). Ophthalmology. 2012;119(9):1867–1873. | ||
Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol. 2011;162(6):1225–1238. | ||
Ohr Pharmaceutical Inc. Efficacy and Safety of Squalamine Lactate Eye Drops in Subjects with Neovascular (Wet) Age-Related Macular Degeneration (AMD). Bethesda, MD: National Library of Medicine (US). | ||
Dalal M, Jacobs-El N, Nicholson B, et al. Subconjunctival Palomid 529 in the treatment of neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2013;251(12):2705–2709. | ||
Zhang M, Zhang J, Yan M, et al; KH902 Phase 1 Study Group. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology. 2011;118(4):672–678. | ||
Maclachlan TK, Lukason M, Collins M, et al. Preclinical safety evaluation of AAV2-sFLT01 a gene therapy for age-related macular degeneration. Mol Ther. 2011;19(2):326–334. | ||
Molecular Partners AG. A Phase I/II, Open-Label, Non-Controlled, Escalating Dose, Multicentre Clinical Trial Evaluating the Safety, Preliminary Efficacy, and Pharmacokinetics of MP0112 Injected Intravitreally in Patients with Wet Age Related Macular Degeneration (AMD). Bethesda, MD: National Library of Medicine (US); 2010. | ||
Novartis NCI. Safety and Efficacy of Oral PTK787 in Patients with Subfoveal Choroidal Neovascularization Secondary to Age-Related Macular Degeneration (AMD) (ADVANCE). Bethesda, MD: National Library of Medicine (US). | ||
McLaughlin MM, Paglione MG, Slakter J, et al. Initial exploration of oral pazopanib in healthy participants and patients with age-related macular degeneration. JAMA Ophthalmol. 2013;131(12):1595–1601. | ||
Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. | ||
Xu X, Weng Y, Xu L, Chen H. Sustained release of Avastin® from polysaccharides cross-linked hydrogels for ocular drug delivery. Int J Biol Macromol. 2013;60:272–276. | ||
Hirani A, Grover A, Lee YW, Pathak Y, Sutariya V. Triamcinolone acetonide nanoparticles incorporated in thermoreversible gels for age-related macular degeneration. Pharm Dev Technol. 2014;26:1–7. | ||
Chen L, Kim IK, Lane AM. Proton beam irradiation for non-AMD CNV: 2-year results of a randomised clinical trial. Br J Ophthalmol. 2014;98(9):1212–1217. | ||
Casaroli-Marano RP, Alforja S, Giralt J, Farah ME. Epimacular brachytherapy for wet AMD: current perspectives. Clin Ophthalmol. 2014;30(8):1661–1670. | ||
Jackson TL, Chakravarthy U, Slakter JS, et al; INTREPID Study Group. Stereotactic radiotherapy for neovascular age-related macular degeneration: year 2 results of the INTREPID study. Ophthalmology. 2015;122(1):138–145. | ||
Li YJ, Goh L, Khor CC, et al. Genome-wide association studies reveal genetic variants in CTNND2 for high myopia in Singapore Chinese. Ophthalmology. 2011;118(2):368–375. | ||
Miyake M, Yamashiro K, Akagi-Kurashige Y, et al. Vascular endothelial growth factor gene and the response to anti-vascular endothelial growth factor treatment for choroidal neovascularization in high myopia. Ophthalmology. 2014;121(1):225–233. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.