Back to Journals » Drug Design, Development and Therapy » Volume 14
Curcumin Combined with Thalidomide Reduces Expression of STAT3 and Bcl-xL, Leading to Apoptosis in Acute Myeloid Leukemia Cell Lines
Authors Mohammadi Kian M, Salemi M, Bahadoran M, Haghi A , Dashti N, Mohammadi S, Rostami S, Chahardouli B, Babakhani D, Nikbakht M
Received 25 August 2019
Accepted for publication 16 December 2019
Published 15 January 2020 Volume 2020:14 Pages 185—194
DOI https://doi.org/10.2147/DDDT.S228610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Mahnaz Mohammadi Kian, 1, 2 Mahdieh Salemi, 1, 2 Mohammad Bahadoran, 3 Atousa Haghi, 1, 4 Nasrin Dashti, 5 Saeed Mohammadi, 1, 2 Shahrbano Rostami, 1, 2 Bahram Chahardouli, 1, 2 Davood Babakhani, 1 Mohsen Nikbakht 1, 2
1Hematology Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran; 2Hematologic Malignancies Research Center, Tehran University of Medical Sciences, Tehran, Iran; 3Department of Biochemistry, Institute of Biochemistry and Biophysics, University of Tehran, Tehran, Iran; 4Young Researchers & Elite Club Tehran Medical Sciences, Islamic Azad University, Tehran, Iran; 5Department of Medical Laboratory Sciences, School of Allied Health Sciences, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Mohsen Nikbakht
Hematology, Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran
Tel +982184902639
Fax +982188004140
Email [email protected]
Nasrin Dashti
Department of Medical Laboratory Sciences, School of Allied Health Sciences, Tehran University of Medical Sciences, Tehran, Iran
Tel +989123583690
Email [email protected]
Introduction: Acute myeloid leukemia (AML) is a type of blood disorder that exhibits uncontrolled growth and reduced ability to undergo apoptosis. Signal transducer and activator of transcription 3 (STAT3) is a family member of transcription factors which promotes carcinogenesis in most human cancers. This effect on AML is accomplished through deregulation of several critical genes, such as B cell lymphoma-extra-large (BCL-XL) which is anti-apoptotic protein. The aim of this study was to evaluate the effect of curcumin (CUR) and thalidomide (THAL) on apoptosis induction and also the alteration of the mRNA expression level of STAT3 and BCL-XL mRNA on AML cell line compounds.
Methods: The growth inhibitory effects of CUR and THAL and their combination were measured by MTT assay in U937 and KG-1 cell lines. The rates of apoptosis induction and cell cycle analysis were measured by concurrent staining with Annexin V and PI. The mRNA expression level of STAT3 and BCL-XL was evaluated by Real-Time PCR.
Results: CUR inhibited proliferation and induced apoptosis in both KG-1 and U937 cells and this effect increased by combination with THAL. The expression level of STAT3 and BCL-XL was significantly down-regulated in KG-1 cells after treatment by CUR and THAL and their combination.
Conclusion: Overall, our findings suggested that down-regulation of STAT3 and BCL-XL mRNA expression in response to CUR and THAL treatment lead to inhibition of cell growth and induction of apoptosis.
Keywords: acute myeloid leukemia, curcumin, thalidomide, STAT3, Bcl-xL
Introduction
AML is a heterogeneous malignant disorder, which is characterized by the accumulation of clonal leukemic blasts in the bone marrow.1,2 Despite high-dose chemotherapy, the total survival rate of AML patients was around 30–40%, so, there remains a need for innovative, effective, minimally toxic, therapies for AML.3–6 STAT plays a fundamental role in the regulation of growth, survival, and differentiation of diverse cells. STAT3 modulates the transcription of a variety of signaling pathways involved in the regulation of critical functions, including apoptosis, angiogenesis, metastasis, cell differentiation, proliferation and immune responses. STAT3 plays a critical role in progress and carcinogenesis.7 Aberrant STAT3 activation promotes tumor growth and development through deregulation of some gene expression such as CYCLIN D1, MYC, BCL-XL, BCL-2, survivin and VEGF that involve in cell growth, survival, angiogenesis and invasion.8–11 Aberrant activation of signal transduction and transcription of STAT3 proteins have been reported in leukemia.12,13 Inhibition of STAT3 signaling cascades causes cell apoptosis and ultimately leukemia remission.14,15
BCL-XL is a member of BCL-2 families.16 BCL-XL has an anti-apoptotic effect, controlled by several mechanisms. BCL-XL acts as an anti-apoptotic protein by preventing the release of mitochondrial contents such as cytochrome c, which leads to caspase activation and ultimately programmed cell death.17 STAT3 and BCL-XL play crucial roles in cellular proliferation (Figure 1). Studies showed that STAT3 signaling provides survival signals that suppress apoptosis in malignant cells, this effect was done by BCL-XL as a target gene.18
 |
Figure 1 Schematic mechanisms of CUR/THAL on STAT3 and BCL-XL and contributing to carcinogenesis. |
Recently, the use of natural dietary agents recommended as an authentic option for the treatment of cancers.19 Among these natural agents, CUR is a product from a perennial herb (Curcuma longa), that has an anti-cancer,20,21 antioxidant,22 and anti-inflammatory effect.23,24 Several studies showed that CUR inhibited carcinogenesis by the effect on cell cycle and up-regulation of pro-apoptotic proteins (such as BAX, BAD) and downregulation of anti-apoptotic proteins (such as BCL2 and BCL-XL).25 THAL inhibits angiogenesis by inhibiting basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF).26–29 The aim of the present study was to investigate the effects of CUR and THAL on proliferation and apoptosis and examine the effect of these compounds on mRNA expression levels of STAT3 and BCL-XL on U937 and KG-1 as indicated AML cell lines.
Materials and Methods
Reagents
For this experimental study, curcumin and Annexin V-FITC apoptosis detection kit and dimethyl sulfoxide (DMSO) and 5-diphenyltetrazolium bromide (MTT) dye were obtained from the Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA), Thalidomide was purchased from the Santa Cruz Company (Santa Cruz, Dallas, TX, USA), RPMI 1640 medium and fetal bovine serum (FBS) were obtained from Gibco (Gibco, Carlsbad, CA). The cDNA synthesis kit and SYBR Premix Ex Taq™ were purchased from Takara Biotechnology Co (Otsu, Japan).
Cell Lines and Cell Culture
U937 and KG-1 were obtained from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). U937 cells were cultured in RPMI 1640 medium supplemented with 10% FBS and KG-1 cultured in DMEM medium supplemented with 20% FBS, then cells incubated at 37°C in 5% CO2. CUR was dissolved in DMSO to prepare a 50 mM concentration. Culture media with 0.1% of DMSO were used as a control. THAL was dissolved in DMSO to make 20 mM concentration and then dissolved in sterile double-distilled water.
MTT Assay
MTT assay was carried out to estimate cell viability after treatment with CUR and THAL. Briefly, the cells were cultured in 96-well plates at a density of 5000 cells per well in the presence of the above-mentioned compounds. After 24, 48 and 72 hrs, 50 μL MTT solution (0.5 mg/mL) (Sigma-Aldrich, St. Louis, MO) was added to each well and then incubated at 37°C and 5% CO2 for 2 hrs. The precipitated formazan was dissolved in 100 μL of DMSO, and the optical wavelength was 570 nm in an ELISA reader (Microplate Reader; Bio-Rad, Hercules, CA, USA).
Flow Cytometry Analysis
U937 and KG-1 cell lines were seeded at a density of 3 × 105 cell/well and incubated for 24 hrs in the absence and presence of CUR, THAL and their combinations. To assess the percentage of apoptosis induction by treated compounds, Annexin V-FITC staining assay was done based on protocol.
Cell Cycle Analysis
Cells were treated with different concentrations of THAL and CUR; after 48 hrs, cells were washed with cold PBS and fixed with 70% ethanol overnight. Cells again were washed with PBS and incubated at 37° C for 30 mins with RNase I and PI. Data analyzed by Flowjo software(Tree Star Inc., version 9.6.3, USA).
RNA Isolation and Real-Time PCR
Total RNA was isolated with the Tripure Isolation Reagent (Roche Applied Science, Peuzberg, Germany) according to the manufacturer’s directions. Complementary DNA (cDNA) was reverse transcribed by using cDNA synthesis kit (Takara Bio Inc., Otsu, Japan). Real-time PCR was performed with Step One Plus™ (Applied Biosystems, Foster City, CA, USA) using SYBR green technology (Takara Bio Inc.). HPRT1 mRNA expression level as a housekeeping gene used for estimating the relative expression levels in comparison with STAT3 and BCL-XL and the relative expression was calculated according to the 2−ΔΔCT method. The primer sequences and their amplicon size are listed in Table 1.
 |
Table 1 The Primer Used for qRT –PCR |
Statistical Analysis
All experiments were performed in triplicate, and results have been expressed as the mean ± SE. Student’s t-test and one-way ANOVA used to determine the statistical significance of difference. ANOVA was used to compare groups. Since the groups were different at the 0.05 level of error, Duncan’s post hoc test was used to further compare each group with the control group and to explain which one had a significance level of 0.05 and 0.01.
Results
CUR Inhibits Cell Proliferation
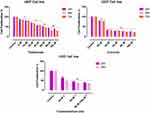
The effect of CUR and THAL on cell viability was evaluated in KG1 and U937 cell lines. After treatment with different concentrations of compounds for 24, 48 and 72 hrs, proliferation assessed by MTT assay (Figures 1 and 2). Results showed that CUR and THAL inhibited cell proliferation. IC50 of CUR was 40 μM in KG-1 and U937 and IC50 of THAL were 80 μM and 60 μM in KG-1 and U937 cells, respectively. Results indicated that CUR and THAL had a significant effect on both cell lines in dose- and time-dependent manners. To investigate the synergic activity of CUR in combination with THAL, the proliferation of treated cells was also assessed 24 and 48 hrs post-treatments (72 hrs had no significant difference when compared with 48 hrs). Furthermore, a combination of CUR and THAL when compared with single-agent treatment showed a significant decrease in cell proliferation on both cells (Figures 2 and 3).
CUR Enhances Apoptosis in Combination with THAL
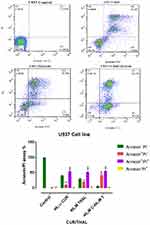
Flow cytometry analysis was performed to assess the effect of CUR/THAL on apoptosis induction. Results revealed that the percentage of annexin V/PI increased gradually after 48 h of treatment. These results indicated that the CUR and THAL and their combinations induced apoptosis in U937 and KG-1 cells (Figures 4 and 5). We observed a significant increase in early apoptotic cells when cells were treated with concentration THAL 60µM, CUR 40µM and CUR 40µM/THAL 60µM in U937 and THAL 80µM, CUR 40µM and CUR 40µM/THAL 80µM in KG-1 cells as compared with control in both cell lines. A significant increase of apoptotic cells (68.8% in KG-1 and 94.5% in U937) was seen in combination of CUR and THAL. In histograms the lower left quadrant in every panel represents viable cells, upper left represents necrotic cells which excluded PI and were negative for annexin V-FITC binding. The upper right quadrant contains nonviable, late apoptotic cells, positive for annexin V-FITC/PI uptake. Lower right quadrant contains early apoptotic cells, annexin V-FITC positive and PI negative.
Cell Cycle Analysis
DNA content of KG-1 and U937 cells was evaluated by flow cytometry (PI). Results of cell cycle analysis demonstrated that cell population of U937 at subG1 phase increased especially in combination of CUR 40µM/THAL 60µM, subG1 phase increased from 1.44% to 36.87. Moreover, the cell population of KG-1 arrested at the subG1 phase especially in combination of CUR 40µM/THAL 80µM, subG1 phase increased up to 48.7% (Figure 6).
STAT3 and BCL-XL Expression Level Were Decreased by CUR/THAL
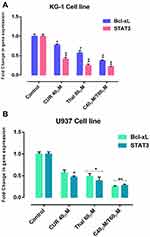
KG-1 and U937 cells were treated with certain concentrations of CUR and THAL for 48 hrs and then examined for expression of STAT3 and BCL-XL by Real-Time PCR. The mRNA expression level of STAT3 and BCL-XL was decreased in a certain concentration of CUR and THAL and their combination in KG-1 and U937 cells (Figure 7).
Discussion
AML is a kind of hematological malignancies characterized by the infiltration of blasts to the bone marrow, blood, and other tissues.19,30 Constitutive activation of STAT3 has a critical impact on the carcinogenesis,15 via deregulation of critical genes like the BCL-2 family (the best characterized group of apoptosis-mediating factors) including BCL-2, BCL-XL, BAX and BAK, which control cell growth and angiogenesis, survival, migration, invasion and metastasis. [10, 26-28-32]. BCL-XL has anti-apoptotic effect.16 According to this observation, blockage of STAT3 gene expression could inhibit cell survival by blockage of BCL-XL gene expression as an anti-apoptotic protein. Various studies demonstrated that CUR (the active principle of turmeric) as a phytochemical inhibits proliferation in different types of cancer cells via targeting multiple cellular signaling pathways such as NF-kB, the mitogen-activated protein kinase, Wnt, PI3K/AKT/mTOR and Notch-mediated signaling pathways.31,32 During the last few years, several studies have investigated the potential impact of CUR (alone or in combination with other anticancer agents) on cancer stem cells.33 Curcumin has been shown to inhibit neoplastic initiation, promotion, and progression in several cancers where numerous mechanisms have been proposed to account for the ability of curcumin to induce apoptosis in malignant cells.34 THAL is an immunomodulatory agent, it can be used as a potential compound for treatment of malignant and immunological disorders. THAL is known as a potent inhibitor of angiogenesis which used for the treatment of cancer.35 Hence, we examined the combination effect of CUR and THAL on AML cell lines including U937 and KG-1, to measure the level of STAT3 and BCL-XL gene expression after treatment with these compounds. Since STAT3 plays an important role in the up-regulation of BCL-XL, it is reasonable to assume that down-regulation of STAT3 and subsequently BCL-XL lead to appropriate percent of apoptosis in both cell lines. Our results indicated that CUR significantly inhibited cell proliferation and induced apoptosis in KG-1 and U937 cell lines. CUR in combination with THAL has more effects on proliferation and apoptosis. Cytotoxic effect of CUR on AML cell lines was demonstrated in previous studies.20,36 Rao et al reported curcumin inhibited proliferation and induced apoptosis and G1/S arrest in both DNR-insensitive KG1a, Kasumi-1 and DNR-sensitive U937 cells. We observed cell arrest at the subG1/G1 phase of the cell cycle. Curcumin-induced apoptosis was associated with reduced expression of both BCL-2 mRNA and protein.36 Giannopoulos et al reported an apoptotic effect of THAL on CLL cell lines.37 CUR promoted an appropriate effect of bortezomib and THAL on multiple myeloma (MM) and also demonstrated that CUR mediated this effect by down-regulation of several gene products such as BCL-XL.38 Zhuang et al reported that the activation of STAT3 (as a critical transcriptional activator of MCL-1 and BCL-XL) by Src in melanoma cells induces the upregulation of MCL-1 and BCL-XL.39 Furthermore, a previous study verified that increase BCL-XL expression related with increase of STAT3 expression.18 Ghosh et al showed that CUR can inhibit STAT3 activation34 also Mackenzie et al demonstrated that inhibition of STAT3 by CUR leads to decreased level of some antipoetic protein such as BCL-XL and BCL-2.40 Zhao et al indicated that downregulation of STAT3 by RNA interference leads to significant induction of apoptosis and also inhibition of proliferation in HL-60 AML cells.12 Han et al and Woo et al in two separate studies reported that CUR downregulated the expression of survival genes such as BCL-XL.41,42 In this study, Real-Time PCR analysis showed that the gene expression of STAT3 and BCL-XL significantly decreases in KG-1 cell line with single and combination doses of CUR and THAL, contrary to KG-1 we observed an increasing level of STAT3 and BCL-XL gene expression in U937 cell line. Moreover, our previous studies indicated that THAL and CUR had a significant effect on VEGFs (as a critical regulator of angiogenesis) and PI3K/AKT/mTOR pathway [24, 29].
Conclusion
In conclusion, we found that the combination of CUR and THAL significantly reduced the viability of U937 and KG-1 cells. The information derived from this study suggested that downregulation of STAT3 and subsequently BCL-XL lead to apoptosis in AML cell lines. We used CUR as a herbal compound that has lower side effects in comparison with chemotherapy agents, for enhancement of CUR effect’s we combined CUR with THAL. Our finding demonstrated that the combined effect of CUR/THAL may be a novel therapeutic strategy for AML cells.
Acknowledgment
The study was financially supported by the Hematology, Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences (HORCSCT).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Zhou H-S, Carter BZ, Andreeff M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol Med. 2016;13(2):248. doi:10.20892/j.issn.2095-3941.2016.0023
2. Zahedpanah M, Shaiegan M, Ghaffari SH, Nikbakht M, Nikugoftar M, Mohammadi S. Parthenolide induces apoptosis in committed progenitor AML cell line U937 via reduction in osteopontin. Rep Biochem Mol Biol. 2016;4(2):82–88.
3. Roboz GJ, Guzman M. Acute myeloid leukemia stem cells: seek and destroy. Expert Rev Hematol. 2009;2(6):663–672. doi:10.1586/ehm.09.53
4. Walter RB, Appelbaum FR, Tallman MS, Weiss NS, Larson RA, Estey EH. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as paradigm. Blood. 2010;116(14):2420–2428. doi:10.1182/blood-2010-05-285387
5. Mohammadi S, Ghaffari SH, Shaiegan M, et al. Acquired expression of osteopontin selectively promotes enrichment of leukemia stem cells through AKT/mTOR/PTEN/β-catenin pathways in AML cells. Life Sci. 2016;152:190–198. doi:10.1016/j.lfs.2016.04.003
6. Haghi A, Mohammadi S, Heshmati M, Ghavamzadeh A, Nikbakht M. Anti-vascular endothelial growth factor effects of sorafenib and arsenic trioxide in acute myeloid leukemia cell lines. Asian Pac J Cancer Prev. 2017;18:1655–1661. doi:10.22034/APJCP.2017.18.6.1655
7. Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers. 2014;6(2):926–957. doi:10.3390/cancers6020926
8. Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11(6):595–596. doi:10.1038/nm0605-595
9. Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10(1):48–54. doi:10.1038/nm976
10. Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19(56):6613–6626. doi:10.1038/sj.onc.1204086
11. Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8(5):409–422. doi:10.1517/14728222.8.5.409
12. Zhao J, Xu Y, Zong Y, et al. Inhibition of Stat3 expression induces apoptosis and suppresses proliferation in human leukemia HL-60 cells. Hematology. 2011;16(4):232–235. doi:10.1179/102453311X13025568941925
13. Benekli M, Baumann H, Wetzler M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J Clin Oncol. 2009;27(26):4422–4432. doi:10.1200/JCO.2008.21.3264
14. Epling-Burnette PK, Liu JH, Catlett-Falcone R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107(3):351–362. doi:10.1172/JCI9940
15. Bi L, Yu Z, Wu J, et al. Honokiol inhibits constitutive and inducible STAT3 signaling via PU. 1-induced SHP1 expression in acute myeloid leukemia cells. Tohoku J Exp Med. 2015;237(3):163–172. doi:10.1620/tjem.237.163
16. Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10(1):105–115. doi:10.1016/S1074-7613(00)80011-4
17. Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. cell. 1993;74(4):609–619. doi:10.1016/0092-8674(93)90509-O
18. Lee JH, Schütte D, Wulf G, et al. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 2006;15(2):201–211. doi:10.1093/hmg/ddi430
19. Mohammadi S, Zahedpanah M, Nikbakht M, et al. Parthenolide reduces gene transcription of prosurvival mediators in U937 cells. Exp Oncol. 2017;39(1):30–35. doi:10.31768/2312-8852.2017.39(1):30-35
20. Mohammadi S, Ghaffari SH, Shaiegan M, et al. Curcumin veto the effects of osteopontin (OPN) specific inhibitor on leukemic stem cell colony forming potential via promotion of OPN overexpression. Int J Hematol Oncol Stem Cell Res. 2016;10(3):120.
21. Jha AK, Nikbakht M, Parashar G, Shrivastava A, Capalash N, Kaur J. Reversal of hypermethylation and reactivation of the RARbeta2 gene by natural compounds in cervical cancer cell lines. Folia Biol (Krakow). 2010;56(5):195–200.
22. Nakmareong S, Kukongviriyapan U, Pakdeechote P, et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn-Schmiedeberg’s Arch Pharmacol. 2011;383(5):519–529. doi:10.1007/s00210-011-0624-z
23. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
24. Salemi M, Mohammadi S, Ghavamzadeh A, Nikbakht M. Anti-vascular endothelial growth factor targeting by curcumin and thalidomide in acute myeloid leukemia cells. Asian Pac J Cancer Prev. 2017;18(11):3055. doi:10.22034/APJCP.2017.18.1.23
25. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39(1):56–68. doi:10.1002/biof.1068
26. Zorat F, Shetty V, Dutt D, et al. The clinical and biological effects of thalidomide in patients with myelodysplastic syndromes. Br J Haematol. 2001;115(4):881–894. doi:10.1046/j.1365-2141.2001.03204.x
27. Invernizzi R, Travaglino E, De Amici M, et al. Thalidomide treatment reduces apoptosis levels in bone marrow cells from patients with myelodysplastic syndromes. Leuk Res. 2005;29(6):641–647. doi:10.1016/j.leukres.2004.11.008
28. Adès L, Fenaux P. Immunomodulating drugs in myelodysplastic syndromes. ASH Educ Program Book. 2011;2011(1):556–560.
29. Mohammadi Kian M, Mohammadi S, Tavallaei M, et al. Inhibitory effects of arsenic trioxide and thalidomide on angiogenesis and vascular endothelial growth factor expression in leukemia cells. Asian Pac J Cancer Prev. 2018;19(4):1127–1134. doi:10.22034/APJCP.2018.19.4.1127
30. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi:10.1056/NEJMra1406184
31. Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Retracted: notch‐1 down‐regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106(11):2503–2513. doi:10.1002/cncr.v106:11
32. Yu S, Shen G, Khor TO, Kim J-H, Kong A-NT. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008;7(9):2609–2620. doi:10.1158/1535-7163.MCT-07-2400
33. Hossain D, Bhattacharyya S, Das T, Sa G. Curcumin: the multi-targeted therapy for cancer regression. Front Biosci (Schol Ed). 2011;4:335–355.
34. Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. Curcumin inhibits pro-survival pathways in CLL B-cells and has the potential to overcome stromal protection of CLL B-cells in combination with EGCG. Clin Cancer Res. 2009;15(4):1250. doi:10.1158/1078-0432.CCR-08-1511
35. Komorowski J, Jerczyńska H, Siejka A, et al. Effect of thalidomide affecting VEGF secretion, cell migration, adhesion and capillary tube formation of human endothelial EA. hy 926 cells. Life Sci. 2006;78(22):2558–2563. doi:10.1016/j.lfs.2005.10.016
36. Rao J, Xu D-R, Zheng F-M, et al. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J Transl Med. 2011;9(1):71. doi:10.1186/1479-5876-9-71
37. Giannopoulos K, Dmoszynska A, Kowal M, et al. Thalidomide exerts distinct molecular antileukemic effects and combined thalidomide/fludarabine therapy is clinically effective in high-risk chronic lymphocytic leukemia. Leukemia. 2009;23(10):1771–1778. doi:10.1038/leu.2009.98
38. Sung B, Kunnumakkara AB, Sethi G, Anand P, Guha S, Aggarwal BB. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol Cancer Ther. 2009;8(4):959–970. doi:10.1158/1535-7163.MCT-08-0905
39. Zhuang L, Lee CS, Scolyer RA, et al. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol. 2007;20(4):416–426. doi:10.1038/modpathol.3800750
40. Mackenzie GG, Queisser N, Wolfson ML, Fraga CG, Adamo AM, Oteiza PI. Curcumin induces cell‐arrest and apoptosis in association with the inhibition of constitutively active NF‐κB and STAT3 pathways in Hodgkin’s lymphoma cells. Int J Cancer. 2008;123(1):56–65. doi:10.1002/(ISSN)1097-0215
41. Han -S-S, Chung S-T, Robertson DA, Ranjan D, Bondada S. Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-X L, NF-κB, and p53. Clin Immunol. 1999;93(2):152–161. doi:10.1006/clim.1999.4769
42. Woo J-H, Kim Y-H, Choi Y-J, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24(7):1199–1208. doi:10.1093/carcin/bgg082
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.