Back to Journals » Research and Reports in Tropical Medicine » Volume 10
Culex quinquefasciatus Egg Membrane Alteration and Ovicidal Activity of Cipadessa baccifera (Roth) Plant Extracts Compared to Synthetic Insect Growth Regulators
Authors Ramkumar G, Karthi S, Shivakumar MS, Kweka EJ
Received 16 August 2019
Accepted for publication 14 November 2019
Published 29 November 2019 Volume 2019:10 Pages 145—151
DOI https://doi.org/10.2147/RRTM.S227590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Govindaraju Ramkumar, 1,* Sengodan Karthi, 1, 2,* Muthugounder S Shivakumar, 1 Eliningaya J Kweka 3, 4
1Molecular Entomology Laboratory, Department of Biotechnology, Periyar University, Salem 636 011, Tamil Nadu, India; 2Department of Biochemistry, Centre for Biological Sciences, K.S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode 637215, Tamil Nadu, India; 3Division of Livestock and Human Diseases Vector Control, Mosquito Section Tropical Pesticides Research Institute, Arusha, Tanzania; 4Department of Medical Parasitology and Entomology, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
*These authors contributed equally to this work
Correspondence: Eliningaya J Kweka
Division of Livestock and Human Diseases Vector Control, Mosquito Section Tropical Pesticides Research Institute, P.O. Box 3024, Arusha, Tanzania
Email [email protected]
Background: Insecticide resistance among mosquito vectors for synthetic insecticides still remains a major problem for control efforts. This study assessed the ovicidal potential of crude solvent extracts from the medicinal plant Cipadessa baccifera comparatively to standard registered synthetic insect growth regulators (IGR) on freshly laid eggs of Culex quinquefasciatus.
Method: Five plant extracts were prepared using different solvents. The batches of eggs were exposed to different concentrations of each solvent extract comparatively to synthetic IGR. The hatched eggs of Cx. quinquefasciatus were subjected to different concentrations. The first instars that emerged from the eggs were counted daily. The egg hatching inhibition was observed 24, 48 and 72 hrs post treatment. The desiccation median time (DT 50 and DT 90) was calculated.
Results: The percent egg hatching inhibition was inversely proportional to the concentration of extracts. The morphological damage to the eggs was observed. Among five solvent extracts, acetone extracts showed the highest ovicidal activity. The changes in eggshell morphology were observed. The maximum ovicidal activity was observed in acetone extracts with DT 50 value of 1.70 hrs (0.91– 2.22). The methanol plant extract using gas chromatography-mass spectrometry identified 14 compounds.
Conclusion: These results suggest that the acetone extracts of C. baccifera have the potential to be used as an ovicidal agent for controlling mosquito populations in aquatic stages. The biodegradability of the extracts has the advantage of being eco-friendly.
Keywords: mosquito, egg hatching, insect growth regulators, ovicidal activity plant extracts
Corrigendum for this paper has been published.
Introduction
Designing innovative vector control tools is of paramount importance due to the development of insecticide resistance among disease vectors.1 Chemical control is an effective strategy used extensively in vector control for decades. However, the evolution of insecticide resistance among mosquitoes to insecticides has increased in the last two decades.2
Culex quinquefasciatus (Say) serves as a vector for filariasis and arboviruses.3 Human filariasis is a major public health problem and remains a challenging problem socioeconomically in most tropical countries.4 Insect growth regulators have shown significant larvicidal efficacy against Aedes albopictus mosquito at low lethal doses as compared to microbial, organophosphates and synthetic pyrethroid insecticides.5 Some studies have disrupted hormonal balance inside the developing embryo.6 Su and Mulla reported the ovicidal activity of the neem products such as azadirachtin against Cx. quinquefasciatus.7
Insect growth regulators are comparatively safer to non-target organisms and have been recommended for mosquito control.8,9 Insect growth regulators (IGR) include chemicals with a unique mode of actions such as juvenile hormone analog, chitin synthesis inhibitor, ecdysone agonist.10–12 These IGRs have extended effects to the morphology and physiology of mosquito eggs.13,14 The surface morphology, physical structure and chemical composition of the eggs determine the ability of eggs to adapt and tolerate adverse conditions such as desiccation.15 Biopesticides provide an alternative to synthetic pesticides because of their generally low environmental pollution and low mammalian toxicity.16 Many herbal products have been used as natural insecticides before the discovery of synthetic organic insecticides.17
Cipadessa baccifera Miq. (Meliaceae) is a bushy shrub, distributed in Northern Circars. The active constituents isolated from the seeds of C. baccifera include cipadesin, 17a, 20R-dihydroxypregnan-3, 16-dione, 1, 4-epoxy-16- hydroxyheneicos-1, -3, -12, -14, -18.18
Therefore, the aim of this study was to evaluate the ovicidal effect and morphological changes in the eggs of Cipadessa baccifera extracts using acetone, ethyl acetate, methanol, chloroform and petroleum benzene solvent which was compared with two insect growth regulators (IGRs), against freshly laid eggs of Culex quinquefasciatus.
Materials and Methods
Mosquito Rearing
Culex quinquefasciatus mosquito eggs were collected from the National Centre for Disease Control (NCDC), Mettupalayam, Tamil Nadu, India. The eggs were kept in plastic trays containing dechlorinated tap water and maintained at 27±2°C and 75–85% relative humidity under 14:10 light and dark photoperiod. The hatched larvae were reared in dechlorinated tap water in plastic trays and provided with dog biscuits and yeast powder in the ratio of 3:1 as a larval food. Once emerged, the adults were transferred to mosquito rearing cages, holding 10% sugar solution, a food source for adults.
Insect Growth Regulators
The ovicidal efficacy of the two insect growth regulators, a chitin synthesis inhibitor (buprofezin: Buprolord, 25% SC, United Phosphorus Limited, Gujarat) and an ecdysone agonist (Azadirachtin: NeemAzal®, 1% EC, EID Parry India Ltd, India) was determined. The fresh 1% stock solution of each class of insect growth regulator was prepared in dechlorinated tap water. The eggs were exposed to different concentrations (0.1, 0.3, 0.5, 1 and 2 mg/mL).
Plant Materials
The leaves of Cipadessa baccifera were collected from Kolli Hills of the Eastern Ghats in the Namakkal district of the Southeast Tamil Nadu (10°12ʹ–11°7ʹ N, 76° - 77°56 ʹE and Altitude 1300 m above sea level). The voucher specimen was numbered and kept in a herbarium for reference.
Preparation of Plant Extracts
The leaves were dried under shade for 7–10 days. The dried leaves were powdered using commercial electrical stainless-steel blender. Three hundred grams of powdered leaves were extracted in five different solvents: chloroform (400mL), ethyl acetate (400mL), acetone (400mL) petroleum benzene and methanol (300mL) in a Soxhlet apparatus (boiling point range 50–80ºC) for 8 hrs. The extracts were concentrated under reduced pressure of 22–26mm Hg at 45ºC and the residues obtained were stored at room temperature.
Egg Exposure to Plant Extracts and Insect Growth Regulators
Freshly laid eggs of Cx. quinquefasciatus were incubated at 24 ± 1°C and 75–80% RH for 48 hrs to obtain embryonated eggs, which were treated with different concentrations (0.1, 0.3, 0.5, 1 and 2 mg/mL) of insect growth regulators. The embryonated eggs from the same batch unexposed to any chemicals served as control. The ovicidal effects of insect growth regulators were observed on freshly laid and embryonated eggs of Cx. quinquefasciatus (150–200 eggs/raft/replicate). Control experiments were conducted in dechlorinated tap water in three replicates. For ovicidal activity, a slightly modified method of Su and Mulla was performed.19 The leaf extracts were diluted in the ethanol to achieve various concentrations ranging from 0.1, 0.3, 0.5, 1 and 2 mg/mL. The hatching rates were calculated 48 hrs post treatment by following the formula:
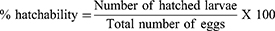
Egg Hatching Inhibition
The first instars that emerged from the embryonated eggs were counted daily. The unhatched freshly laid eggs were observed under a fluorescence microscope for any morphological changes and abnormalities that may have been caused from the exposure to insect growth regulators and plant extracts. The percentage of unhatched eggs in control experiments was adjusted with treatments by using Abbott’s formula.20 The chi-square test was used to determine the significant differences between the proportion of hatched and unhatched egg groups (p< 0.05).
GC-MS Analysis and Identification of Compounds
The plant extract of C. baccifera was analysed by gas–liquid chromatography (Polaris Q Ion Trap GC/FID) and mass spectrometry (PerkinElmer Q-700 equipment). The methodology developed by Cheng et al was used.21
Statistical Analysis
Data were subjected to Probit analysis in SPSS version 25 (IBM Corp., Armonk, NY, USA). Desiccation median time DT50 (defined as the duration at which 50% of eggs were desiccated) and median desiccation time DT90 (defined as the duration at which 90% of eggs were desiccated) of the eggs were calculated. Comparisons between proportions of DT50 and DT90 were computed using the chi-square test.
Results
Effects on Embryonic Development
The acetone extract of C. baccifera produces morphological changes in the eggshell, resulting in damage to the egg membrane and decreased egg hatchability (Figure 1). No significant relationship between the rates of egg hatching inhibition and abnormalities of egg morphology was observed in treatments using IGR.
 |
Figure 1 (A)Control Operculum open and egg hatching. (B and C) Abnormal egg hatching and operculum open from side wall damaged after exposure to Cipadessa baccifera acetone plant extract. |
Egg Hatchability in Insect Growth Regulators
The hatchability of freshly laid eggs of Cx. quinquefasciatus in insect growth regulators was also found to be dosage dependent. The egg hatching inhibition of Cx. quinquefasciatus was higher in eggs exposed to azadirachtin (75%) than in buprofezin (55%) at 0.1mg/mL concentration, and the trend was also observed in other higher concentrations.
Egg Hatchability in Plant Extracts
Among the plant extracts tested for egg hatchability inhibition, the acetone extract of C. baccifera exerted 98% egg hatching inhibition at a concentration of 0.1 mg/mL, whereas higher concentration resulted in 100% eggs hatching inhibition. In the control arm, egg hatchability was 100%.
Effects on Egg Hatching Patterns
Egg hatching pattern in azadirachtin and buprofezin was similar to that of control, while that of freshly laid eggs of C. baccifera extracts was dosage dependent. The median desiccation time (DT50) of Cx. quinquefasciatus eggs was observed to be higher for acetone extract (1.70 hrs). There was no significant difference in hatching inhibition of freshly laid eggs at any concentrations when exposed to other solvent extracts of C. baccifera (P > 0.05) (Table 1).
 |
Table 1 Estimation of Desiccation Time of Culex quinquefasciatus Eggs |
GC-MS Analysis
The methanol plant extract using gas chromatography-mass spectrometry identified 14 compounds in the C. baccifera plant chemical constitutions. The major peaks were 4H-1-benzopyran-4-one,3,5,7-trimethoxy-2-(3,4,5-trimethoxyphenyl) (41.6%), palmitic acid vinyl ester (5.7%), octadecanoic acid,1-[[(1-oxohexadecyl)oxy]methyl]-1,2-ethanediyl ester (8.4%), octadecanoic acid,1-[[(1-oxohexadecyl)oxy]methyl]-1,2-ethanediyl ester (5.6%), cyclopropane carboxylic acid, and 2-methyl-, 2,6-di-t-butyl-4-methylphenyl ester (5.4%) (Table 2).
 |
Table 2 Chemical Composition of Methanol Leaf Extract from C. baccifera |
Discussion
The findings of the current study have shown that plant extracts have the potential to contribute in alternative pesticides for disease vector control. The environment is an incomparable reservoir of natural products that exhibit structural features, which have not been found in terrestrial natural products.22 Several studies have demonstrated that plants are an excellent source of components with biological activity such as antifungal, antiviral, phytotoxic and larvicidal activities.23–28 The screening of medicinal plants for mosquito larvicidal activity may eventually lead to their use in natural product-based mosquito abatement practices.24–32
Plants may serve as a suitable alternative source of insecticides to complement the toolbox of available synthetic insecticides for the future. Due to their low mammalian toxicity, the crude extracts have many active compounds with different acting mechanisms, most inexpensive and are readily available in many areas of the world.33 Different parts of plants contain complex chemical composition with unique biological activity which is thought to be due to toxins from different secondary metabolites, which may act as mosquitocidal agents.33 The plant crude extracts seemed to be more effective than single isolated compound which might be attributed to the natural synergistic effect that acts in many different ways to inhibit the development of an embryo in eggs. The results of this study showed that the acetone extract of C. baccifera showed 98% egg hatching inhibition (2% hatchability) was recorded at 24, 48 and 72 hrs at 0.1, 0.3, 0.5, 1 and 2 mg/mL, respectively. It was also found that the acetone extracts of C. baccifera damage the eggshell and subsequently increases hatching inhibition.
Chitinous egg development was affected in buprofezin-treated embryos, and probably, pharate larvae used the body pressure for egg hatching. Our results showed less toxicity to egg hatching and embryo development when exposed to buprofezin and azadirachtin. In contrast, buprofezin and azadirachtin substantiate their role in altering hormonal actions during embryonic development of the Culex eggs rather than altering egg hatching process.34,35 Suman et al have suggested that less toxicity to larvicidal and ovicidal efficacies are governed by the type and concentration of different classes of insect growth regulators.14
In the present study, Cx. quinquefasciatus eggs were most susceptible to acetone plant extract of C. baccifera as compared to the two IGRs. Previous studies reported that diflubenzuron, pyriproxyfen and azadirachtin were less toxic to Cx. quinquefasciatus eggs at a WHO-recommended concentrations.7,8,36 In order to manage mosquito populations at the egg stage, the eggs need to be exposed at higher concentrations of insect growth regulators for shorter rather than long durations. Freshly laid eggs are more vulnerable to the toxicity of insect growth regulators than embryonated eggs. Similar observations using insect growth regulators were made by Miura et al.36,37 Miura and colleagues suggested hat, freshly laid and 2–14-hr-old eggs were more vulnerable to diflubenzuron exposure.37 Meanwhile, Vasuki demonstrated species-specific variation on ovicidal action of chitin synthesis inhibitor and juvenile hormone analog against eggs of An. stephensi (Liston), Cx. quinquefasciatus and Ae. aegypti.36 These differences may be attributed to the inability of insect growth regulators to disrupt hormone actions during egg development and the loss of shell permeability due to endochorion tanning and wax layer formation.38 The interspecific variations in Ae. aegypti eggs from different habitats have shown differences in the desiccation of survival time.39 Moreover, this study could not find any relationship between eggs and ovicidal activity of insect growth regulators based on egg desiccation time, and this could be one of the reasons for differential ovicidal efficacy of insect growth regulators.
The isolation of compounds from leaf extracts of C. baccifera could lead to the development of natural mosquitocidal products to complement the synthetic insecticides. The use of natural plant-based products by individuals and communities would generate local employment, reduce dependence on expensive imported synthetic products and stimulate local efforts to enhance public health.29 For example, the essential oil extracted from Citronella and Eucalyptus provides the active ingredients of some commercial repellents. Such substances are sold under several brand names.40
Limitation of the Study
This study had no resources to study other mosquito families’ response to these extracts.
Conclusion
This study has shown that the acetone extract of Cipadessa baccifer, possesses ovicidal activity on Cx. quinquefasciatus eggs at very low concentrations, but no significant activity, eggshell morphology and ovicidal efficacy were observed in insect growth regulators. These results open the possibility for further investigations of the efficacy of growth inhibition properties of plant-based extracts.
Ethics Approval
This study was approved by the Indian Council of Medical Research and Periyar University ethics committees.
Availability of Data and Materials
The data related to the conclusion of this study have been included in this manuscript.
Acknowledgments
The authors would like to thank the Indian Council of Medical Research for the financial support they provided. We also thank the Department of Biotechnology, Periyar University, Salem, for providing the infrastructural facility for carrying out this research work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chang X, Zhong D, Lo E, et al. Landscape genetic structure and evolutionary genetics of insecticide resistance gene mutations in Anopheles sinensis. Parasit Vectors. 2016;9(1):228. doi:10.1186/s13071-016-1513-6
2. Amer A, Mehlhorn H. Persistency of larvicidal effects of plant oil extracts under different storage conditions. Parasitol Res. 2006;99(4):473–477. doi:10.1007/s00436-006-0183-2
3. Aditya G, Bhattacharyya S, Kundu N, Saha G, Raut S. Predatory efficiency of the water bug Sphaerodema annulatum on mosquito larvae (Culex quinquefasciatus) and its effect on the adult emergence. Bioresour Technol. 2004;95(2):169–172. doi:10.1016/j.biortech.2004.02.007
4. Rajasekariah G, Parab P, Chandrashekar R, Deshpande L, Subrahmanyam D. Pattern of Wuchereria bancrofti microfilaraemia in young and adolescent school children in Bassein, India, an endemic area for lymphatic filariasis. Ann Trop Med Parasitol. 1991;85(6):663–665. doi:10.1080/00034983.1991.11812623
5. Ali A, Nayar J, Xue R-D. Comparative toxicity of selected larvicides and insect growth regulators to a Florida laboratory population of Aedes albopictus. J Am Mosq Control Assoc. 1995;11(1):72–76.
6. Berger EM, Dubrovsky EB. Juvenile hormone molecular actions and interactions during development of Drosophila melanogaster. Vitam Horm. 2005;73:175–215.
7. Su T, Mulla M. Ovicidal activity of neem products (azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). J Am Mosq Control Assoc. 1998;14(2):204–209.
8. WHO. Pesticides and Their Application: For the Control of Vectors and Pests of Public Health Importance. Geneva: WHO; 2006.
9. Mulla M. The future of insect growth regulators in vector control. J Am Mosq Control Assoc News. 1995;11(2):269–273.
10. Mordue A, Morgan E, Nisbet A. Azadirachtin, a natural product in insect control. Compr Mol Insect Sci Biochem Mol Biol. 2005;2010:4.
11. Msangi S, Lyatuu E, Kweka EJ. Field and laboratory evaluation of bioefficacy of an insect growth regulator (Dimilin) as a larvicide against mosquito and housefly larvae. J Trop Med. 2011;2011.
12. Soin T, Swevers L, Kotzia G, et al. Comparison of the activity of non‐steroidal ecdysone agonists between dipteran and lepidopteran insects, using cell‐based EcR reporter assays. Pest Manag Sci. 2010;66(11):1215–1229. doi:10.1002/ps.1998
13. Suman D, Shrivastava A, Parashar B, Pant S, Agrawal O, Prakash S. Scanning electron microscopic studies on egg surface morphology and morphometrics of Culex tritaeniorhynchus and culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 2008;104(1):173–176. doi:10.1007/s00436-008-1162-6
14. Suman DS, Shrivastava AR, Pant S, Parashar BD. Differentiation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) with egg surface morphology and morphometrics using scanning electron microscopy. Arthropod Struct Dev. 2011;40(5):479–483. doi:10.1016/j.asd.2011.04.003
15. Jagadeeshan S, Singh RS. Rapid evolution of outer egg membrane proteins in the Drosophila melanogaster subgroup: a case of ecologically driven evolution of female reproductive traits. Mol Biol Evol. 2007;24(4):929–938. doi:10.1093/molbev/msm009
16. Liu S, Shi J, Cao H, Jia F, Liu X, Shi G. Survey of Pesticidal Component in Plant. Paper Presented At: Entomology in China in 21st Century. Proceedings of Conference of Chinese Entomological Society. Beijing, China: Science and Technique Press; 2000.
17. Mittal P, Subbarao S. Prospects of using herbal products in the control of mosquito vectors. ICMR Bull. 2003;33(1):1–10.
18. Luo X-D, Wu S-H, Ma Y-B, Wu D-G. Components of Cipadessa baccifera. Phytochemistry. 2000;55(8):867–872. doi:10.1016/S0031-9422(00)00247-8
19. SU M. Activity and biological effects of neem products against arthropods of medical and veterinary importance. J Am Mosq Control Assoc. 1999;15(2):133–152.
20. Finney DJ. Probit Analysis.
21. Cheng Z, Zhou X, Hu B, et al. Tissue distribution of capilliposide B, capilliposide C and their bioactive metabolite in mice using liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2016;
22. Sukumar K, Perich MJ, Boobar L. Botanical derivatives in mosquito control: a review. J Am Mosq Control Assoc. 1991;7(2):210–237.
23. Bauer A, Kirby W, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4_ts):493. doi:10.1093/ajcp/45.4_ts.493
24. Kweka EJ, Nyindo M, Mosha F, Silva AG. Insecticidal activity of the essential oil from fruits and seeds of schinus terebinthifolia Raddi against African malaria vectors. Parasit Vectors. 2011;4(1):129. doi:10.1186/1756-3305-4-129
25. Kweka EJ, Senthilkumar A, Venkatesalu V. Toxicity of essential oil from Indian borage on the larvae of the African malaria vector mosquito, Anopheles gambiae. Parasit Vectors. 2012;5(1):277. doi:10.1186/1756-3305-5-277
26. Mdoe FP, Cheng -S-S, Lyaruu L, Nkwengulila G, Chang S-T, Kweka EJ. Larvicidal efficacy of cryptomeria japonica leaf essential oils against Anopheles gambiae. Parasit Vectors. 2014;7(1):426. doi:10.1186/1756-3305-7-426
27. Mdoe FP, Cheng -S-S, Msangi S, Nkwengulila G, Chang S-T, Kweka EJ. Activity of cinnamomum osmophloeum leaf essential oil against Anopheles gambiae s.s. Parasit Vectors. 2014;7(1):209. doi:10.1186/1756-3305-7-209
28. Thomas A, Mazigo HD, Manjurano A, Morona D, Kweka EJ. Evaluation of active ingredients and larvicidal activity of clove and cinnamon essential oils against Anopheles gambiae (sensu lato). Parasit Vectors. 2017;10(1):411.
29. Bowers WS, Sener B, Evans PH, Bingol F, Erdogan I. Activity of Turkish medicinal plants against mosquitoes Aedes aegypti and Anopheles gambiae. Int J Trop Insect Sci. 1995;16(3–4):339–341. doi:10.1017/S1742758400017379
30. Kweka EJ, Mosha F, Lowassa A, et al. Ethnobotanical study of some of mosquito repellent plants in North-Eastern Tanzania. Malar J. 2008;7(1):152. doi:10.1186/1475-2875-7-152
31. Kweka EJ, Mosha FW, Lowassa A, et al. Longitudinal evaluation of Ocimum and other plants effects on the feeding behavioral response of mosquitoes (Diptera: culicidae) in the field in Tanzania. Parasit Vectors. 2008;1(1):42. doi:10.1186/1756-3305-1-42
32. Kweka EJ, Munga S, Mahande AM, et al. Protective efficacy of menthol propylene glycol carbonate compared to N, N-diethyl-methylbenzamide against mosquito bites in Northern Tanzania. Parasit Vectors. 2012;5(1):189. doi:10.1186/1756-3305-5-189
33. Ramkumar G, Karthi S, Muthusamy R, Natarajan D, Shivakumar MS. Adulticidal and smoke toxicity of Cipadessa baccifera (Roth) plant extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus. Parasitol Res. 2015;114(1):167–173. doi:10.1007/s00436-014-4173-5
34. Canales M, Naranjo V, Almazán C, et al. Conservation and immunogenicity of the mosquito ortholog of the tick-protective antigen, subolesin. Parasitol Res. 2009;105(1):97–111. doi:10.1007/s00436-009-1368-2
35. Suman DS, Wang Y, Bilgrami AL, Gaugler R. Ovicidal activity of three insect growth regulators against Aedes and Culex mosquitoes. Acta Trop. 2013;128(1):103–109. doi:10.1016/j.actatropica.2013.06.025
36. Vasuki V. Effect of insect growth regulators on hatching of eggs of three vector mosquito species. J Anim Sci. 1990;99(6):477–482.
37. Miura T, Schaefer CH, Takahashi RM, Mulligan FS. Effects of the insect growth inhibitor, Dimilin,® on hatching of mosquito eggs. J Econ Entomol. 1976;69(5):655–658. doi:10.1093/jee/69.5.655
38. Clements A. The biology of mosquitoes, Vol. I; Development, nutrition and reproduction. Parasitol Today. 1993;9(4):147. doi:10.1016/0169-4758(93)90183-G
39. Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90(3):353–358. doi:10.1007/BF00317691
40. Trongtokit Y, Rongsriyam Y, Komalamisra N, Apiwathnasorn C. Comparative repellency of 38 essential oils against mosquito bites. Phytother Res. 2005;19(4):303–309. doi:10.1002/(ISSN)1099-1573
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
