Back to Journals » OncoTargets and Therapy » Volume 10
CT-guided 125I interstitial brachytherapy for pelvic recurrent cervical carcinoma after radiotherapy
Authors Tong L, Liu P, Huo B, Guo Z, Ni H
Received 13 April 2017
Accepted for publication 1 June 2017
Published 17 August 2017 Volume 2017:10 Pages 4081—4088
DOI https://doi.org/10.2147/OTT.S139571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chiung-Kuei Huang
Lina Tong,1 Ping Liu,1 Bin Huo,2 Zhi Guo,1 Hong Ni1
1Department of Interventional Therapy, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin, China; 2Department of Oncology, The Second Hospital of Tianjin Medical University, Tianjin, China
Objective: This retrospective study aimed to evaluate the feasibility, safety, and clinical efficacy of computed tomography (CT)-guided 125I seed interstitial brachytherapy for pelvic recurrent cervical cancer in patients with a history of pelvic radiotherapy.
Methods: From March 2011 to December 2015, 35 pelvic recurrent lesions (33 patients) were reirradiated using this type of salvage therapy. The medical history, dose–volume histogram parameters, complications, local control, overall survival (OS), and affected factors were analyzed.
Results: All patients were followed-up until expiration, and the median duration of follow-up was 16 months. The 1-, 3-, 6-, 12-, and 18-month local control rates were 84.5%, 74.2%, 60.0%, 55.5%, and 33.3%, respectively. The symptoms significantly improved after implantation. The median local tumor progression-free survival (LTPFS) and OS times were 7 months (range, 1–19 months) and 12 months (range, 2–42 months), respectively. The 1- and 2-year OS rates were 65.5% and 43.6%, respectively. In univariate analysis, a good performance status, a tumor diameter <4 cm, an interval time from last radiotherapy to seed implantation longer than 6 months and D90 (dose delivered to 90% of the target volume) ≥130 Gy were prognostic factors for LTPFS. Cox proportional hazards regression analysis revealed that tumor size and D90 were independent factors affecting LTPFS (P=0.033, hazard ratio [HR] =3.357, 95% CI =1.105, 10.212; P=0.035, HR =2.766, 95% CI =1.072, 10.212). Good performance status was identified as an independent factor affecting OS (P=0.001, HR =0.086, 95% CI =0.019, 0.387). Two patients showed grade 3–4 toxicity – 1 patient had rectovaginal fistula and 1 patient had incomplete intestinal obstruction – and 3 cases showed seed migration in our analysis. No grade 5 events occurred.
Conclusion: Reirradiation with CT-guided 125I seed interstitial brachytherapy is a safe, effective, and minimally invasive method to treat patients with recurrent cervical cancer after radiotherapy.
Keywords: 125I seed, brachytherapy, recurrent, cervical cancer
Introduction
Even in the era of radical hysterectomy with platinum-based chemoradiotherapy, most cervical cancer patients will eventually relapse, including 11%–22% of those in International Federation of Gynecology and Obstetrics (FIGO) stages Ib–IIa and 28%–64% of those in FIGO stages IIb–IVa.1 Recurrent cervical cancer leads to devastating consequences such as pelvic pain, physical obstruction, vaginal bleeding and a dismal 1-year survival rate between 15% and 20%.2
The treatment modality for recurrent cervical cancer is dependent on previous therapy, location and extent of the recurrence, tumor size, time of disease-free interval, and the patient’s performance status.3 Pelvic exenteration may represent a curative treatment option only in highly selected cases with a small lesion confined to the vagina; the postoperative morbidity rate is high and reported to be between 13% and 64%.4 Cisplatin-containing combination chemotherapy is administered with palliative intent to recurrences; however, the improvement in median survival is low. Despite the combination of chemotherapy and bevacizumab, the median survival time was increased by only 3.7 months.5,6 Since 70% of patients received pelvic radiotherapy, reirradiation is usually impossible because of potential damage to the intestine and bladder.7 Other modern radiotherapy techniques need to be found so that they can be effectively used for patients with recurrent cervical cancer.
Interstitial implantation of 125I seeds according to the computerized three-dimensional treatment planning system (TPS) is considered an advanced form of conformal radiotherapy, and it has shown efficacy in antitumor response and symptom relief for different tumors.8,9 The 125I seed can deliver a cumulative dose directly into the target tumor with a very sharp dose gradient outside the implanted volume, thus sparing nearby normal tissues and providing dose escalation.10 This is particularly important for the treatment of recurrent tumor with a history of irradiation.
Since 2010, 125I brachytherapy has been used for pelvic recurrent cervical cancer with a history of pelvic irradiation at our institution. The aim of this study was to review and update our experience with permanent 125I interstitial brachytherapy in patients with recurrent cervical cancer after radiotherapy.
Materials and methods
Ethical statement
This study was approved by the Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital and was carried out in compliance with the Helsinki Declaration. Patients who signed written informed consent for this study were included, and personal identification information was removed.
Patients
We identified all patients with a history of external beam radiotherapy or high-dose rate (HDR) brachytherapy to the pelvic cavity who underwent interstitial implantation of 125I seeds at our institution from March 2011 to December 2015. The eligibility criteria were as follows: histologically proven recurrent cervical cancer; Karnofsky performance status (KPS) score of 60 or higher; interviewed by surgeons who had considered them unsuitable for salvage surgery or patients had refused surgery; no severe dysfunction of the kidneys, liver, blood coagulation or bone marrow; and informed consent before undergoing seed implantation. In total, 33 patients were treated, and their therapy records were reviewed. This study was approved by the Institutional Review Board of our medical institution. The patient and tumor characteristics are listed in Table 1.
Seed implantation technique
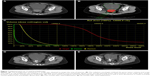
Three days prior to seed implantation, the patients underwent a detailed tumor volume study using CT scans with a thickness of 5 mm (Figure 1A). The images were transferred to a TPS (Beijing Aerospace and Aviation University, Beijing, China), which was used to reconstruct the three-dimensional images of the focus. The radiation oncologist outlined the gross tumor volume (GTV) and organs at risk for disease on each transverse image (Figure 1B). The planning target volume included the entire GTV with a 0.5 to 1.0 cm margin in each direction that was covered by the 90% isodose curve. The prescribed dose (PD) of the 125I seed implant was 90–150 Gy, which was adjusted according to the adjacent structures. The dose–volume histogram, isodose curves of different percentages, D90 (PD delivered to 90% of the target volume), total number and activity of 125I seeds were calculated (Figure 1C).
The patients were placed on a clear liquid diet 12 hours prior to the procedure. If the recurrent tumor was adjacent to the bladder or rectum, they required urethral catheterization and coloclysis with diluted contrast medium before interventional therapy. After adequate local anesthesia, 18-gauge needles were implanted into the mass and were spaced at a distance of 1.0 cm in a parallel array performed under CT guidance according to the plan. Precautions were taken to avoid puncture of large blood vessels, pelvic autonomic nerves or important organs. The 125I seeds (model 6711, 4.5 mm long and 0.8 mm in diameter; radioactivity, 11.1–37 MBq; average energy, 27–35 keV; half-life of 59.6 days; radiation range, 1.7 cm; initial dose rate, 7 cGy/h; China Institute of Atomic Energy, Beijing, China) were inserted through each needle, and the space between seeds (center-to-center) was kept at 0.5–1.0 cm. Postplacement CT was performed to document the distribution of the 125I seeds in the tumor. Additional seed implants would be performed immediately if a low-dose region was detected to optimize the treatment parameters. All patients received 4–6 cycles of platinum-based intra-arterial infusion chemotherapy and paclitaxel or docetaxel intravenous chemotherapy after implantation.
Evaluation of the effect and follow-up
Routine follow-up consisted of symptom improvement, physical examination and laboratory tests including measurements of the hematic convention, squamous cell cancer-Ag, chest radiography, and abdominopelvic ultrasonography. Pelvic CT was performed 1–2 months after the procedure and at 3-month intervals thereafter, or earlier if a new clinical sign or symptom appeared.
Local control (LC) was evaluated based on imaging findings in accordance with the Response Evaluation Criteria in Solid Tumors.11 The toxicities were assessed from the start date of 125I seed implantation. The severity of complications was graded according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer grading system.12
Statistical analysis
The local tumor progression-free survival (LTPFS) and overall survival (OS) curves were calculated using the Kaplan–Meier method. Univariate analysis was performed to define the predictive factors influencing LTPFS and OS using the log-rank test. The relative importance of the covariates in determining predictive factors was also assessed with a multivariate Cox proportional hazard model. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using IB Social Sciences (SPSS), version 19.
Results
125I seed interstitial brachytherapy
Brachytherapy was performed successfully for 33 patients with 35 lesions under CT guidance. All patients underwent postoperative dose verification using the TPS. The median number of 125I seeds used was 50 (range, 20–95). Of 33 patients, 3 (8.5%) patients did not reach the TPS criteria after the first procedure and subsequently received additional implantations. Postplanning evaluation showed that the actual D90 ranged from 115.1 to 160.7 Gy (mean, 133.9 Gy). The median maximum dose to the bladder and rectum were 34.6 (range, 5.1–80.1 Gy) and 35.9 Gy (range 7.0–54.3 Gy), respectively.
LC and LTPFS
As shown in Table 2, the 1-, 3-, 6-, 12- and 18-month LC rates were 84.5%, 74.2%, 60.0%, 55.5% and 33.3%, respectively. The tumor volume was significantly reduced (Figure 1D and E). Kaplan–Meier curves for LTPFS are presented in Figure 2A, with a median LTPFS time of 6 months (range, 1–19 months). According to univariate analysis, KPS ≥80, tumor diameter <4 cm, interval time from last radiotherapy to seed implantation ≥6 months and D90≥130 Gy showed a better LTPFS (Table 3). As shown in Table 4, tumor size (Figure 2B) and D90 (Figure 2C) were also identified as independent factors affecting the LTPFS.
Overall Survival
The mean OS of this study group was 12 months (range, 2–42 months). The 1- and 2-year OS rates were 65.5% and 43.6%, respectively (Figure 2D). Better OS was found in patients who had no distant metastasis when the 125I brachytherapy was received and who had a good performance status (Table 3). Cox proportional hazards regression analysis revealed that a good performance status was the only independent factor affecting OS (Table 4, Figure 2E).
Relief of clinical symptoms
In this study, the major clinical symptoms were pain (66.7%), fatigue (75.6%), abnormal defecation (33.3%), abdominal distension (30.3%), urinary irritation (36.3%), vaginal discharge (39.3%), vaginal bleeding (30%), tenesmus (48.5%) and leg edema (9.1%). The remission rates of clinical symptoms after 125I interstitial brachytherapy are shown in Table 5.
Complications
A total of 2 patients (6.1%) had grade 3–4 toxicity including 1 patient with rectovaginal fistula and 1 patient who had incomplete intestinal obstruction, after treatment for 4 and 2 months, respectively. Seven patients had grade 1–2 toxicity including mild hematuria (3.0%) lasting for 1–3 days, a small amount of colporrhagia (12.1%), and vaginal secretion (9.1%) that disappeared within 2–3 weeks. There were 2 cases of seed migration in our analysis. No grade 5 events occurred.
Discussion
Treatment of recurrent cervical cancer is still a current challenge, especially for those with a history of radiotherapy. Most of the recurrent tumors could not be resected because of local extension, postradiation fibrosis, regional and distant metastasis, patient state, or their resection would cause unacceptable functional disability.13 Although chemotherapy combined with targeted therapy has been widely used in clinical practice, the improvement in median survival is low. Reirradiation of the previously irradiated area is dangerous as the complications may increase significantly, up to 30%–56%.14
125I seeds belong to the low-dose rate brachytherapy which has advantages of high precision, strong adaptability and minimal peripheral organ damage. In addition, 125I seeds slowly emit continuous, low-dose X-rays and γ-rays, for a delivery dose of 160–180 Gy within the local tissue during the half-life of 125I, which can efficiently inhibit the proliferation and promote the apoptosis of tumor cells.15 In contrast, the surrounding normal tissue receives <25% of the dose delivered to the tumor cells.16 This emission of radiation from the 125I seeds also allows the normal tissue, which receives sublethal or potentially lethal doses of radiation, to have sufficient time for repair and recovery.17 125I seeds can provide additionally radiation because the therapeutic benefit is theoretically boosted by natural increases in the local dose after radiation-induced tumor shrinkage brings the radioactive source closer to the target. Together, these characteristics support 125I seeds as an optimal treatment for recurrent cervical cancer, especially for patients who previously received radiotherapy.
A study by Sharma et al18 demonstrated the efficacy and safety of the interstitial implantation of 125I seeds in the treatment of recurrent gynecologic malignancies in 40 patients with previous radiotherapy; in this study, the tumor control rate reached 67%, and 33% of patients had a disease-free survival period longer than 2 years. In our study, the LC rates after 1, 3, 6, 12 and 18 months were 84.5%, 74.2%, 60.0%, 55.5% and 33.3%, respectively. At the time of this analysis, the median LTPFS time was 7.3 months, the median survival time was 14.0 months and the 1- and 2-year OS rates were 65.5% and 43.6%, respectively. These results suggest that 125I brachytherapy can be successfully used to improve LC in patients with cervical cancer after radiotherapy. We also found compare with the recurrent tumors outside pelvic cavity that accepted 125I seed brachytherapy, the LC for this study was poorer and the complications were more serious.8,19 The main consideration is that with 125I brachytherapy, the sharp fall-off in the radiation dose emphasizes the need to accurately define the clinical target volume, as any area left unimplanted will receive very little irradiation. This is especially difficult in the postirradiated pelvis, which is affected by the pelvic bone structure, vessels, nerves and motion of cavitation organs, such as the bowel and the bladder. It is also difficult to differentiate areas of radiation-related fibrosis from tumor tissue that may not be sensitive to the radiation emitted from 125I seeds.
As recent studies have indicated that local disease control is an important independent prognostic factor for the OS of patients, seeking how to improve the LTPFS is very important. Univariate analysis found that KPS, tumor size, interval time from last radiotherapy to seed implantation and D90 had an influence on LTPFS. Multivariate analysis suggested that tumor size was an independent factor related to LTPFS. These results were similar to those of Martinez-Monge et al, who assessed the treatment of recurrent rectal cancer with radioactive seed implantation.24 Because of tumor shrinkage, the dose distribution for large-volume tumors will become nonuniform, which can form radiation cold areas prone to relapse. Another independent factor for LTPFS is D90. According to the American Association of Physicists in Medicine,20 we set the PD of the 125I implant at 90–150 Gy, which was adjusted according to the adjacent structures. We found that with an actual D90>130 Gy, a better LTPFS rate was obtained.
Another finding in our study was that 125I brachytherapy showed a huge advantage concerning the improvement of clinical symptoms, especially pain, with a relief rate of 81.3%. Other symptoms also showed varying degrees of remission, which significantly improved the quality of life of the patients. Thus, this minimally invasive technique can give patients a relatively long period of time to recover because most of our patients were at the late stage of disease. After undergoing many other different types of therapies, the general status of the patients was poor, and they had no other effective treatment option.
Mabuchi et al21 reported the toxicity of using HDR interstitial brachytherapy for locally recurrent cervical cancer, and 13 (25%) of the 52 patients experienced grade 3 or worse complications. In our study, 2 patients (7.4%) had grades 3–4 toxicity including 1 patient with rectovaginal fistula and 1 patient with incomplete intestinal obstruction. Compared to the complications related to other types of radiotherapies, 125I brachytherapy appears more tolerable. Fistula formation may be due to the local higher dose area caused by tumor shrinkage that brings the radioactive source closer to the target. Ling et al22 suggested that the dose received in the target area should be <120% of the prescription dose; otherwise, it would be an “excess” dose. This analysis showed that the dose nonuniformity of the target area could be accepted after implantation for the treatment of prostate cancer. However, the unique characteristics of pelvic tumors warrant further clinical studies for confirmation.
With postoperative follow-up and review, 1 patient presented with seeds falling into the abdominal cavity and 1 patient presented with seeds shedding from the vagina. These findings may be related to the movement of the visceral organs after implantation, tumor necrosis or the implantation technique. No side effects on the digestive tract were observed. Radiotherapy with the traditional dose distribution will be affected by internal organ movement and setup error, while the dose distribution of the radioactive seed is not affected by patients or their internal organ motion; moreover, the position error of a single particle is not as sensitive, and the seed displacement and migration have little effect on the target dose distribution. The relevant research of Beaulieu et al23 showed that 125I shift and migration were more likely to affect the dose distribution of the target than to cause adverse reactions limiting tumor control and normal tissue. The goal of such therapy is to have a good treatment plan, skilled operation technology and strict quality assurance and quality control.
Conclusion
In patients with recurrent disease, decisions regarding additional therapy must consider both the likelihood of disease control and potential toxicities. Our study suggests that 125I brachytherapy is effective and feasible for treatment of recurrent cervical cancer that develops in a previously irradiated area. This study was limited by its retrospective nature and the relatively small sample size from a single institution. In addition, >60% of the patients had been treated within the previous 2 years, and the follow-up period was relatively short. Thus, further studies with a larger number of patients and long-term monitoring of this promising procedure are needed to reach a definite conclusion.
Disclosure
The authors report no conflicts of interest in this work.
References
Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S43–S103. | ||
Long HR. Management of metastatic cervical cancer: review of the literature. J Clin Oncol. 2007;25(20):2966–2974. | ||
Kim YJ, Munsell MF, Park JC, et al. Retrospective review of symptoms and palliative care interventions in women with advanced cervical cancer. Gynecol Oncol. 2015;139(3):553–558. | ||
Sardain H, Lavoue V, Redpath M, Bertheuil N, Foucher F, Leveque J. Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review. Eur J Surg Oncol. 2015;41(8):975–985. | ||
Long HR, Bundy BN, Grendys EJ, et al; Gynecologic Oncology Group Study. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a gynecologic oncology group study. J Clin Oncol. 2005;23(21):4626–4633. | ||
Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2009;27(7):1069–1074. | ||
Barney BM, Petersen IA, Dowdy SC, Bakkum-Gamez JN, Klein KA, Haddock MG. Intraoperative Electron Beam Radiotherapy (IOERT) in the management of locally advanced or recurrent cervical cancer. Radiat Oncol. 2013;8:80. | ||
Wang J, Yuan H, Ma Q, et al. Interstitial 125I seeds implantation to treat spinal metastatic and primary paraspinal malignancies. Med Oncol. 2010;27(2):319–326. | ||
Hinnen KA, Battermann JJ, van Roermund JG, et al. Long-term biochemical and survival outcome of 921 patients treated with I-125 permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76(5):1433–1438. | ||
Lin L, Wang J, Jiang Y, et al. Interstitial 125I seed implantation for cervical lymph node recurrence after multimodal treatment of thoracic esophageal squamous cell carcinoma. Technol Cancer Res Treat. 2015;14(2):201–207. | ||
Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho. 2009;36(13):2495–2501. | ||
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. | ||
Hockel M, Dornhofer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. Lancet Oncol. 2006;7(10):837–847. | ||
Elst P, Ahankour F, Tjalma W. Management of recurrent cervical cancer. Review of the literature and case report. Eur J Gynaecol Oncol. 2007;28(6):435–441. | ||
Monk BJ, Tewari KS, Puthawala AA, Syed AM, Haugen JA, Burger RA. Treatment of recurrent gynecologic malignancies with iodine-125 permanent interstitial irradiation. Int J Radiat Oncol Biol Phys. 2002;52(3):806–815. | ||
DeWeese TL, Shipman JM, Dillehay LE, Nelson WG. Sensitivity of human prostatic carcinoma cell lines to low dose rate radiation exposure. J Urol. 1998;159(2):591–598. | ||
Sgouros G, Knox SJ, Joiner MC, Morgan WF, Kassis AI. MIRD continuing education: Bystander and low dose-rate effects: are these relevant to radionuclide therapy? J Nucl Med. 2007;48(10):1683–1691. | ||
Sharma SK, Forgione H, Isaacs JH. Iodine-125 interstitial implants as salvage therapy for recurrent gynecologic malignancies. Cancer. 1991;67(10):2467–2471. | ||
Suchorska B, Hamisch C, Treuer H, et al. Stereotactic brachytherapy using iodine 125 seeds for the treatment of primary and recurrent anaplastic glioma WHO degrees III. J Neurooncol. 2016;130(1):123–131. | ||
Nath R, Anderson LL, Luxton G, Weaver KA, Williamson JF, Meigooni AS. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys. 1995;22(2):209–234. | ||
Mabuchi S, Takahashi R, Isohashi F, et al. Reirradiation using high-dose-rate interstitial brachytherapy for locally recurrent cervical cancer: a single institutional experience. Int J Gynecol Cancer. 2014;24(1):141–148. | ||
Ling CC, Roy J, Sahoo N, Wallner K, Anderson L. Quantifying the effect of dose inhomogeneity in brachytherapy: application to permanent prostatic implant with 125I seeds. Int J Radiat Oncol Biol Phys. 1994;28(4):971–978. | ||
Beaulieu L, Archambault L, Aubin S, Oral E, Taschereau R, Pouliot J. The robustness of dose distributions to displacement and migration of 125I permanent seed implants over a wide range of seed number, activity, and designs. Int J Radiat Oncol Biol Phys. 2004;58(4):1298–1308. | ||
Martínez-Monge R, Nag S, Martin EW. 125Iodine brachytherapy for colorectal adenocarcinoma recurrent in the pelvis and paraortics. Int J Radiat Oncol Biol Phys.1998;42(3):545–550. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.