Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Cranial versus Caudal Direction Technique of Native Percutaneous Kidney Biopsy: A Randomized Controlled Trial
Authors Jaturapisanukul S , Chavanisakun C, Benjakul N , Ngamvichchukorn T, Laungchuaychok P, Kurathong S, Pongsittisak W
Received 28 December 2022
Accepted for publication 18 March 2023
Published 28 March 2023 Volume 2023:16 Pages 93—101
DOI https://doi.org/10.2147/IJNRD.S400639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Solos Jaturapisanukul,1,2 Chutima Chavanisakun,3 Nontawat Benjakul,3 Tanun Ngamvichchukorn,1 Punnawit Laungchuaychok,1 Sathit Kurathong,1,2 Wanjak Pongsittisak1,2
1Division of Nephrology and Renal Replacement Therapy, Department of Internal Medicine, Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand; 2Vajira Renal-Rheumatology-Autoimmune Disease Research Group, Bangkok, Thailand; 3Department of Anatomical Pathology, Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand
Correspondence: Wanjak Pongsittisak, Tel +66818345228, Email [email protected]
Background: Percutaneous kidney biopsy (PKB) is the gold standard for diagnosing various kidney diseases, but it can result in potential complications. This study aimed to compare kidney tissue adequacy and safety between the two biopsy techniques, including cranial direction (CN) and caudal direction (CD), of needle biopsy under real-time ultrasonogram guidance.
Methods: This single-center, prospective, single-blinded, randomized trial included patients undergoing native PKB from July 5, 2017, to June 30, 2019. Patients were randomized to the CN and CD groups. Adequacy and complications between the two groups were analyzed. All PKBs were performed under real-time ultrasonogram guidance with a 16-gauge kidney biopsy needle.
Results: A total of 107 participants were enrolled (53 in the CD group and 54 in the CN group). The CD group has more glomeruli than the CN group but with no statistical significance (16 versus 11, p = 0.0865). The CD group obtained more adequate kidney tissue samples than the CN group (69.8% versus 59.3%, p = 0.348). The number of inadequate glomeruli tissue sampling is similar in both groups (14 versus 15, respectively). Furthermore, the CN group had more adverse events, including Hb decline ≥ 10% after kidney biopsy, perinephric hematoma size ≥ 1 cm, hematuria, and the need for blood transfusion, than the CD group.
Conclusion: The CD technique of the percutaneous kidney biopsy in the native kidney has fewer complications and was possibly more effective than the CN technique.
Keywords: kidney biopsy, needle trajectory, post-biopsy complication
Introduction
Percutaneous kidney biopsy (PKB) is the gold standard to obtain information for diagnosing various kidney diseases.1,2 Adequate tissue samples are necessary for diagnosis. Ideally, the biopsy tissue should contain a predominate kidney cortical area because this is the location of the glomerulus.2 A sufficient number of glomeruli improves the accuracy of pathological diagnosis of focal kidney lesions. However, biopsy complications, such as hematuria, hematoma, and perinephric hemorrhage, which are associated with multiple procedures, should be a concern.
The real-time ultrasonogram-guided PKB with the automated kidney biopsy needle shows more benefits in biopsy yield and decreases post-kidney biopsy complications.3,4 This modality has become a widespread technique in this era. The cortex of the inferior pole of the kidney is usually a target for performing a native kidney biopsy, and biopsy needle direction in clinical practices has two techniques: cranial direction (CN) and caudal direction (CD). CD obtained more cortex and glomeruli in ex vivo porcine kidneys.5 Other retrospective and prospective observational studies revealed that the CD biopsy technique is safe and effective.6,7 However, this was not confirmed by human randomized controlled trials (RCTs). Thus, this study aims to compare the efficacy and complications of CN versus CD biopsy techniques.
Materials and Methods
Design
This randomized, single-blinded, single-center study compares the efficacy and complications of CD versus CN in patients undergoing native PKB from July 5, 2017, to June 30, 2019, in a university hospital in Bangkok, Thailand.
Participants
Patients who underwent PKB during the study were eligible participants. The inclusion criteria were ages of 18–65 years and indications of performing a PKB decided by a consultant nephrologist. All screened participants have a complete blood count (CBC), prothrombin time/international normalized ratio (PT/INR), activated partial thromboplastin time (aPTT), and serum creatinine for any bleeding risk. Any medications that increase bleeding risk, including anticoagulants, antiplatelet agents, and nonsteroidal anti-inflammatory drugs, were noted and discontinued before PKB, and informed consent was obtained.
The key exclusion criteria were (1) patients who underwent a kidney transplant, (2) serum creatinine of >3.5 mg/dL for 3 months, (3) small-size kidneys (length of <8 cm, width of <3.5 cm), (4) coagulopathy and/or thrombocytopenia, (5) uncontrolled hypertension (>160/100 mmHg), (6) obesity (body mass index [BMI] of >35 kg/m2), (7) single kidney, and (8) refusal to sign the inform consent. The full exclusion list was announced at the Thai Clinical Trial Registry (TCTR20170706001: https://www.thaiclinicaltrials.org/show/TCTR20170706001). We generated participants’ randomization groups by a web-based block of four after study enrollment.
Intervention
All PKBs are performed by 4 nephrologists who were well-trained in both techniques with kidney biopsy experience at least 20 times per year. Prior this study started, all of the nephrologists who included to perform a kidney biopsy in this study have experience in CD and CN technique at least 10 times. The biopsy needle was a 16-gauge, automated, spring-loading gun kidney biopsy needle (Biox®). All participants underwent kidney biopsy in the prone position with local anesthesia. All eligible patients were randomly assigned to the CN or CD group. The lower pole of a kidney was selected as the biopsy target under real-time ultrasonogram guidance.
Kidney biopsies were planned with two attempts for each individual; however, adding more attempts to collect adequate tissue depended on the operator’s decision. A pathologist blinded from the procedure promptly examined the specimens under light microscopy to obtain sufficient renal tissue for light, immunofluorescence, and electron microscopy studies.
CD Technique
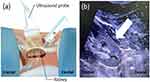
The kidney biopsy needle tip is pointed to the legs and 45–60 degrees with the participant’s back. The needle was directed to the cortex of the inferior pole of the kidney. The needle tip was advanced to the kidney capsule, and then the biopsy needle was triggered to collect the kidney tissue (Figure 1).
CN Technique
The kidney biopsy needle tip is pointed to the head and 45–60 degrees with the participant’s back, then proceeded the same as the CD technique (Figure 2).
Post-Kidney Biopsy Monitoring
All participants were admitted for post-kidney biopsy care and monitoring for 24 h. We prescribe bed rest in a supine position for 8 h and monitored vital signs every 15 min for 2 h, every 30 min for 4 h, and then hourly for the remaining observation period. Post-biopsy kidney ultrasonogram by blinded radiologist (1 person) and CBC were checked after 24 h to detect complications. Moreover, the participant receives the standard of care as an attending physician if a clinically suspected complication is observed.
Outcomes
The primary outcome was the average number of glomeruli, which is calculated from the total number of glomeruli divided by the number of core tissue. The secondary outcomes were an adequate sampling of tissue and the number of undiagnostic tissues by a pathologist. The adequate sampling tissue was defined as tissue comprising the cortex and medulla part, >20 glomeruli, and at least one vessel. The undiagnostic tissue was the tissue with an insufficient structure for pathological diagnosis by a pathologist. The outcomes were reported by a pathologist who was blinded for the randomization. Factors associated with obtaining adequate sampling tissue were also analyzed.
Adverse Events
Specific adverse events include Hb decline ≥10% after kidney biopsy, perinephric hematoma size ≥1 cm, hematuria, blood transfusion, embolization, nephrectomy, and post-kidney biopsy complication-related mortality. Serious adverse events and other adverse events were observed.
Statistical Analysis
Chunduri et al reported an average number of glomeruli of 21 ± 12.8 A sample size of 52 participants per group was necessary to detect a 10-glomeruli efficacy difference between the two techniques with a two-sided 5% significance level and a power of 85%.
Continuous variables were tested for normal distribution by histogram and the Kolmogorov–Smirnov test. Normal distribution variables were analyzed for mean and standard deviation (SD) and both groups were compared by the Student’s t-test. We performed the non-parametric analysis by the median, interquartile range, and comparison with the Mann–Whitney U-test for the non-normal distribution variable. The categorical variables were presented by number and percentage. The Chi-Square or Fisher’s exact test was performed to compare statistical differences between the groups for categorical variables. We analyzed the univariable and multivariable logistic regression model from the univariable model to find the factors associated with adequate sampling tissue. Variables with a p-value of <0.3 were selected to perform the multivariable model. A p-value of <0.05 was used as a threshold for statistical significance. R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Result
Baseline Characteristic
This study included 208 patients who met the inclusion criteria from the 343 patients referred for PKB and screened from July 5, 2017, to June 30, 2019. Of the 208 patients, 91 were excluded and 10 refused to participate. Thus, 107 participants were randomized at an approximate 1:1 ratio into the CD and CN groups with 53 and 54 participants, respectively (Figure 3). The baseline characteristics were similar between both groups (Table 1). The overall mean (SD) age was 41.6 (13.3) years. Female was predominant in the study. Waist circumference was not different in both groups (p = 0.205). However, some BMI in the CD group is lower than in the CN group (CD, 23.5 ± 3.9 versus CN, 25.2 ± 5.7; p = 0.075). The top three most common comorbid disease was hypertension (35.5%), systemic lupus erythematosus (27.1%), and type 2 diabetes mellitus (15.9%). The biopsy indication was comparable in both groups—the common indications included nephrotic syndrome, acute glomerulonephritis, and undiagnosed proteinuria. Additionally, laboratory parameters regarding creatinine, hemoglobin, platelet, and coagulation were not statistically different.
 |
Table 1 Baseline Characteristic |
The left-side kidney (93.5%) was preferable for performing PKB. The kidney dimension was not statistically different in both groups, as shown in Table 1. The median number of a needle biopsy pass was 2 (interquartile range [IQR], 1) times, which was not different between both groups (p = 0.180).
Outcome
The number of glomeruli from the CD group (median [IQR]: 16 [11] glomeruli) was more than the CN group (median [IQR]: 11 [12] glomeruli) but not statistically significant, as shown in Table 2 (p = 0.865). Regarding kidney tissue adequacy, the CD group obtained more adequacy sampling tissue than the CN group (69.8% versus 59.3%, respectively). Reasons for inadequate tissue sampling from the CN technique include an inadequate number of vessels (77.2%), an inadequate number of glomeruli (68.2%), no obtained medulla (31.8%), and no obtained cortex (4.5%), whereas the CD technique was the inadequate number of vessels (87.5%), an inadequate number of glomeruli (87.5%), and no obtained medulla obtained (50.0%). The number of inadequate glomeruli tissue sampling is similar in both groups (14 versus 15, respectively).
 |
Table 2 Outcomes |
Adverse Events
The CN group had more complications than the CD group, ie, perinephric hematoma size ≥1 cm, hematuria, and required blood transfusion (Table 3). However, no serious adverse events, including biopsy-related death or hematoma requiring embolization or nephrectomy, occurred in either group.
 |
Table 3 Adverse Event |
Factors Associated with Adequate Kidney Tissue
Younger age, higher waist circumference, higher length of the kidney, lower serum creatinine, and CD techniques were associated with adequate sampling tissue (p < 0.3) by the univariable logistic regression model (Table 4). However, the remaining factor was waist circumference after the multivariable model analysis (p = 0.0283).
 |
Table 4 Logistic Regression for Factors Associated with Adequate Tissue |
Discussion
PKB plays an essential role in obtaining information about diagnoses, treatment options, and prognoses of various kidney diseases. The pathological kidney disease diagnosis depends on specimen quality.1,2,9 Inadequate kidney tissue may lead to misdiagnosis; for example, an insufficient number of glomeruli cannot provide an exclusion diagnosis of focal segmental glomerulosclerosis (FSGS) from minimal change disease.10,11 In contrast, kidney biopsy remained an invasive procedure with potential risks. Several post-kidney biopsy complications, such as pain, hematuria, perinephric hematoma, or serious adverse events, including massive perinephric hematoma requiring blood transfusion, embolization, or nephrectomy needed for significant bleeding, may occur.1,2,9
Several PKB research studies about various PKB techniques for efficacy and safety issues, such as real-time ultrasonogram guidance,3,4,12,13 size of gauge needles,8,14,15 type of kidney biopsy needle,16,17 and prophylactic medication before kidney biopsy.18–20 However, the comparison of cranial and caudal directions of the kidney biopsy needle is rarely discussed.
Ideally, the biopsy core should be predominately contained cortical tissue because this is the location of glomeruli.2 However, the juxtamedullary junction and medulla portion remained necessary to diagnose some kidney diseases such as myeloma cast kidney,21 FSGS,22 or polyoma nephropathy.23 Additionally, the needle was fired transverse to the kidney cortex and medulla directly in the CD PKB technique (Figure 2). A needle tip may direct to the renal sinus, resulting in renal vessel injury and significant hematoma. The kidney cortex, where the needle was fired parallel, may have lower risk of reaching the renal sinus and decreased risk of renal vessel injury compared to the CD of kidney biopsy (Figure 1). Then, the CN technique angulation is more likely to advance the needle biopsy tip injury toward nearby organs such as the spleen, liver, or diaphragm.
Previous studies by Karam et al revealed that caudal needle direction biopsy to ex vivo porcine kidney obtained more yield than cephalic needle direction.5 Other retrospective and prospective observational studies revealed the caudal angulation needle approach under real-time ultrasonogram guidance in obtaining cortical tissue from the lower pole of the native kidney as an effective technique with very few complications.6,7 Hence, our study is the first human RCT that compared CD to CN in native kidney biopsy.
Our result demonstrates more glomeruli obtained in the CD group than in the CN group, with the same kidney tissue adequacy outcome but not statistically significant. The average glomerular obtained by real-time ultrasonogram guidance from previous studies ranged from 11 to 21 glomeruli, similar to our study. Interestingly, the CD group revealed a lower risk of complication, probably because more cortex and less medulla tissue were obtained. This hypothesis was confirmed by Sawicka et al24 who reported that the needle biopsy angle results in less medulla tissue associated with adequate sampling specimen and lesser complication.
However, the CD technique usually requires patients to hold their breath to access the lower pole of the kidney. Patients with volume overload or who are not cooperative will have difficulty holding their breath. The CD technique is difficult in these populations, while the lower pole of the kidney in the CN technique is easy to access and does not require patients to hold their breath during the procedure.
Interestingly, in multivariate analysis, waist circumference is the only factor associated with adequate kidney tissue biopsy. It is possibly due to performance bias. The nephrologist tends to insert a kidney biopsy needle deeply into the kidney cortex and possibly more carefully when performing a kidney biopsy if the patient is overweight and has increased weight circumference. However, it is less likely to have a clinical significance (OR 1.05).
As its strength, our study is the first RCT that revealed the CD technique’s efficacy and safety. However, our study has some limitations. First, the angle of the needle biopsy was not recorded. We allowed the operators to perform kidney biopsy as an assigned technique by varying the needle angle (45–60 degrees). Second, CN still has more experience than CD, although our nephrologists who performed PKB are well-trained in both techniques. Third, the CN group tends to have more body weight and waist circumference compared to the CD group, although it is not statistically significant. Lastly, standardized biopsy adequacy is not available.1,2 We use this definition based on the ability to diagnose focal kidney disease although almost kidney diseases in non-transplantation patients usually do not require a medulla portion for diagnosis.
In conclusion, the CD technique of the percutaneous kidney biopsy in native kidney has fewer complications and was possibly more effective than the CN technique.
Data Availibiity
Data are available on request to the corresponding author due to privacy/ethical restrictions.
Ethical Consideration
This study was approved by the institutional review board of Navamindradhiraj University (COA 70/2560) and was registered with the Thailand Clinical Trial Registry (TCTR20170706001: https://www.thaiclinicaltrials.org/show/TCTR20170706001). This study compiles with the Declaration of Helsinki.
Acknowledgment
The authors thank all nurses, nephrologist and pathologist staff in the Department of Medicine and Department of pathology, Faculty of Medicine, Navamindradhiraj University and Vajira Hospital.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Navamindradhiraj University Research Fund.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Najafian B, Lusco MA, Alpers CE, Fogo AB. Approach to kidney biopsy: core curriculum 2022. Am J Kidney Dis. 2022;80(1):119–131. doi:10.1053/j.ajkd.2021.08.024
2. Luciano RL, Moeckel GW. Update on the native kidney biopsy: core curriculum 2019. Am J Kidney Dis. 2019;73(3):404–415. doi:10.1053/j.ajkd.2018.10.011
3. Prasad N, Kumar S, Manjunath R, et al. Real-time ultrasonogram-guided percutaneous renal biopsy with needle guide by nephrologists decreases post-biopsy complications. Clin Kidney J. 2015;8(2):151–156. doi:10.1093/ckj/sfv012
4. Pongsittisak W, Wutilertcharoenwong N, Ngamvichchukorn T, et al. The efficacy of blind versus real-time ultrasonogram-guided percutaneous renal biopsy in developing country. SAGE Open Med. 2019;7:2050312119849770. doi:10.1177/2050312119849770
5. Karam AR, Vijayaraghavan G, Khan A, Ustun B, Hussain S. Renal biopsy: comparative yield of cranial versus caudal needle trajectory. An ex vivo Analysis Nephrol. 2013;18(4):304–306.
6. Khan D, Ahmed I, Yaqoob J, Hilal K, Sayani R. Ultrasonogram guided native renal biopsy: using a caudal angulated needle path to yield adequate cortical sample. Pak J Radiol. 2014;24(4):123–127.
7. Prasad N, Shukla R, Behera M, et al. Comparison of yield and complications of craniocaudal versus caudocranial needle trajectory for kidney biopsy. J Vasc Access. 2020;21(1):73–78. doi:10.1177/1129729819854009
8. Chunduri S, Whittier WL, Korbet SM. Adequacy and complication rates with 14- vs. 16-gauge automated needles in percutaneous renal biopsy of native kidneys. Semin Dial. 2015;28(2):E11–4. doi:10.1111/sdi.12332
9. Hogan JJ, Mocanu M, Berns JS. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11(2):354–362. doi:10.2215/CJN.05750515
10. Nissen CJ, Moreno V, Davis VG, Walker PD. Increasing incidence of inadequate kidney biopsy samples over time: a 16-year retrospective analysis from a large national renal biopsy laboratory. Kidney Int Rep. 2022;7(2):251–258. doi:10.1016/j.ekir.2021.11.026
11. Fogo A. Minimal change disease. Am J Kidney Dis. 1999;33(3):E1.
12. Chung S, Koh ES, Kim SJ, et al. Safety and tissue yield for percutaneous native kidney biopsy according to practitioner and ultrasonogram technique. BMC Nephrol. 2014;15(1):96. doi:10.1186/1471-2369-15-96
13. Rao NS, Chandra A. Needle guides enhance tissue adequacy and safety of ultrasonogram-guided renal biopsies. Kidney Res Clin Pract. 2018;37(1):41–48. doi:10.23876/j.krcp.2018.37.1.41
14. Corapi KM, Chen JL, Balk EM, Gordon CE. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60(1):62–73. doi:10.1053/j.ajkd.2012.02.330
15. Xie W, Xu J, Xie Y, et al. Adequacy and complication rates of percutaneous renal biopsy with 18- vs. 16-gauge needles in native kidneys in Chinese individuals. BMC Nephrol. 2020;21(1):337. doi:10.1186/s12882-020-01987-3
16. Kim D, Kim H, Shin G, et al. A randomized, prospective, comparative study of manual and automated renal biopsies. Am J Kidney Dis. 1998;32(3):426–431. doi:10.1053/ajkd.1998.v32.pm9740159
17. Donnelly S, Goodyer P, Mauer M. Comparing the automated versus manual method of needle biopsy for renal histology artefacts. Nephrol Dial Transplan. 2008;23(6):2098–2100. doi:10.1093/ndt/gfn061
18. Athavale A, Kulkarni H, Arslan CD, Hart P. Desmopressin and bleeding risk after percutaneous kidney biopsy. BMC Nephrol. 2019;20(1):413. doi:10.1186/s12882-019-1595-4
19. Leclerc S, Nadeau-Fredette AC, Elftouh N, Lafrance JP, Pichette V, Laurin LP. Use of desmopressin prior to kidney biopsy in patients with high bleeding risk. Kidney Int Rep. 2020;5(8):1180–1187. doi:10.1016/j.ekir.2020.05.006
20. Izawa J, Matsuzaki K, Raita Y, et al. Intravenous tranexamic acid in percutaneous kidney biopsy: a randomized controlled trial. Nephron;2022. 1–8. doi:10.1159/000526325
21. Menè P, Stoppacciaro A, Lai S, Festuccia F. Light Chain cast nephropathy in multiple myeloma: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis. 2022;15:173–183. doi:10.2147/IJNRD.S280179
22. Shabaka A, Tato Ribera A, Fernández-Juárez G. Focal segmental glomerulosclerosis: state-of-the-art and clinical perspective. Nephron. 2020;144(9):413–427. doi:10.1159/000508099
23. Nankivell BJ, Renthawa J, Shingde M, Khan A. The importance of kidney medullary tissue for the accurate diagnosis of Bk virus allograft nephropathy. Clin J Am Soc Nephrol. 2020;15(7):1015–1023. doi:10.2215/CJN.13611119
24. Sawicka K, Hassan N, Dumaine C, et al. Direction of the biopsy needle in ultrasonogram-guided renal biopsy impacts specimen adequacy and risk of bleeding. Can Assoc Radiol J. 2019;70(4):361–366. doi:10.1016/j.carj.2018.11.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.