Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Cost-effectiveness of adding Endocuff® to standard colonoscopies for interval colorectal cancer screening
Authors Yu TM , Tradonsky A , Tang J, Arnold RJG
Received 12 January 2019
Accepted for publication 11 April 2019
Published 31 July 2019 Volume 2019:11 Pages 487—504
DOI https://doi.org/10.2147/CEOR.S201328
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Tiffany M Yu,1 Alison Tradonsky,1 Jun Tang,1 Renée JG Arnold1,2
1Department of Life Sciences, Navigant Consulting, Inc, San Francisco, CA, USA; 2Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Background and aims: Higher screening colonoscopy adenoma detection rates (ADRs) correlate with reduced risk of interval colorectal cancer (CRC). The Endocuff® device has been shown to improve ADRs compared to standard colonoscopy (SC). This cost-effectiveness analysis compared interval CRC screening using Endocuff®-assisted colonoscopy (EC) vs SC.
Methods: A decision-analytic Markov model followed patients through screening, CRC diagnosis, progression, remission, and death. ADRs, CRC progression, and utilities were from literature. CRC incidence, stage distribution, and mortality were from the Surveillance, Epidemiology, and End Results (SEER) and SEER-Medicare linked databases. Screening and annual patient costs were from public databases and literature. Endocuff® device average sales price was applied. Lifetime device and medical costs were evaluated separately for device purchaser, health plan, and accountable care organization (ACO) perspectives.
Results: Consistent use of EC instead of SC was expected to reduce lifetime risks of interval CRC and related death by 0.98% and 0.19%, respectively, preventing one case per 102 patients and one death per 526 patients. Survival and quality-of-life (QoL) improved by 0.025 life-years and 0.011 quality-adjusted life-years (QALYs) per patient on average. EC instead of SC led to incremental cost-effectiveness ratios to the device purchaser of $4,421 per life-year gained and $9,843 per QALY gained, and $199 or $87 average cost-savings per patient to the health plan or ACO, respectively.
Conclusion: Endocuff® for screening colonoscopies was expected to reduce interval CRC incidence and death, improve QoL, and be cost-effective to the device purchaser and cost-saving to a health plan or ACO.
Keywords: adenomatous polyps, colorectal neoplasm, colonic polyps, adenocarcinoma, interval cancer
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer among men and women, and the third leading cause of cancer-related death in the US.1 In 2018, 97,220 new cases of colon cancer and 43,030 new cases of rectal cancer were projected to be diagnosed.1 That year, CRC was projected to cause 50,630 deaths. Lifetime risk of CRC is 4.5% for men and 4.2% for women. Of incident CRC patients, 39% are diagnosed with localized disease, 35% with regional, and 21% with distant, according to data reported by the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.2 Five-year survival for these patients is 89.9%, 71.3%, and 13.9%, respectively.
The majority of CRCs derive from adenomatous polyps (adenomas), which take over a decade on average to become malignant.3,4 This provides a window of opportunity to prevent CRC through early adenoma identification and removal during regular CRC screening. Most adenomas are asymptomatic, so early detection depends on effective screening modalities.5 Optical colonoscopy is the most common modality for CRC screening. The adenoma detection rate (ADR), defined as the proportion of screening colonoscopy patients in which at least one adenoma is detected, is an evidence-based quality measure for colonoscopy used by gastroenterology specialty societies and the Centers for Medicare and Medicaid Services (CMS).6,7 Conventional adenomas are the precursors to, at minimum estimates, 70% of all CRCs, emphasizing the importance of adenoma detection in effective CRC prevention.8
Even among experienced endoscopists, the adenoma miss rate with colonoscopy was estimated to be 17%, and may be as high as 24%.9,10 Factors correlated with the miss rate include adequacy of colon preparation, adenoma location within the colon, and colonoscope withdrawal time.11 Modifications to the basic colonoscope have been suggested to improve adenoma detection, including changing the colonoscope (eg, using a wide-angled lens or numerous lenses) and/or adding accessory devices (eg, distal attachments).12,13
Data from an integrated healthcare delivery organization showed that a 1% increase in ADR was associated with a 3% decrease in the risk of interval CRC and a 5% decrease in the risk of interval CRC-related death.14 Endocuff® (Arc Medical Design Ltd, Leeds, UK; US distribution by Olympus Corporation of the Americas) is a mechanical device placed on the distal end of the colonoscope, and aids in the discovery of adenomas and polyps within the colon. The device has soft flexible arms that extend from its fixed base. The arms collapse backward during colonoscope insertion and advancement, and extend during examination and withdrawal, allowing flattening of the colon folds to reduce slippage and enhance visualization of the colon. A meta-analysis of published studies showed Endocuff®-assisted colonoscopy (EC) increased ADRs by 14.0% compared to standard optical colonoscopy (SC).15–17 This cost-effectiveness analysis evaluated potential CRC outcomes and costs over a lifetime with consistent interval CRC screening using EC compared to SC in the US.
Methods
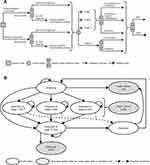
A decision-analytic Markov model was used to compare EC to SC for guideline-appropriate CRC screening for US patients over a lifetime (Figure 1A). CRC screening patients were tracked through health states representing screening (no CRC diagnosis), CRC diagnosis, metastasis, remission, and death (Figure 1B). Probabilities of transitioning between health states were applied annually. Patient outcomes included CRC incidence, CRC-related death, life-years, and quality-adjusted life-years (QALYs).
Three stakeholder perspectives were evaluated: the device purchaser, the health plan, and the fully integrated accountable care organization (ACO) responsible for both device and downstream payer-borne costs. Endocuff® device and medical costs were considered separately and together, depending on perspective. Lifetime Endocuff® device costs were considered for the device purchaser; lifetime medical costs were considered for the health plan. The fully integrated ACO was assumed to be responsible for device and medical costs.
Reductions in lifetime risks were evaluated using the number needed to treat (NNT) to avoid one CRC case or CRC-related death. Cost-effectiveness was evaluated using incremental cost-effectiveness ratios (ICERs): cost per life-year gained, and cost per QALY gained. Annual 3% discount rates were applied to costs and QALYs.18 This analysis was developed in accordance with guidelines for cost-effectiveness analyses.19–22
Modeled patient pathway
Screening
Patients entered the model with CRC screening initiation at age 50 based on the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines.23 At each screening over their lifetimes, patients in the SC arm of the model underwent standard optical colonoscopy, while patients in the EC arm used Endocuff® to augment their colonoscopies. In accordance with NCCN Guidelines, time intervals between screenings were 5 years for patients with high-risk characteristics and 10 years for patients without high-risk characteristics (average-risk patients). The proportions of high-risk and average-risk screening patients with SC screening were based on the distribution of high- and average-risk screening colonoscopies (Healthcare Common Procedure Coding System [HCPCS] codes G0105 and G0121) conducted in 2016 under CMS (Table 1).24 ADRs with SC and EC (25.8% and 39.8%, respectively) were from published colonoscopy studies comparing SC and EC, and were used to calculate CRC incidence rates in each model arm in later years.25–29 Increased adenoma detection with EC would also be expected to increase the proportion of patients considered high-risk due to identification of more sessile adenomas or more adenomas per patient.23 In the absence of data on the magnitude of that increase, it was assumed that the proportion of screening patients considered high risk with EC vs SC would increase by the same magnitude as ADR (14.0%).
 |
Table 1 Model inputs |
Colon cancer incidence rates
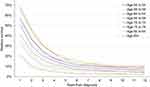
CRC incidence rates by age in the screened population (ie, interval cancer) were calculated based on 2014 overall US incidence rates from the SEER database (Figure 2, grey line).30 The model assumed that CRC prevention benefits of screening would not be realized in the first screening year, so incidence with SC in that year was set equal to the overall population. Improved visualization of the colon with EC may increase the number of CRCs detected in the short term. A systematic review and meta-analysis found that the sensitivity of SC for CRC detection was 94.7%, suggesting that 5.3% of CRCs may be undetected by SC, indicating that the maximum relative increase in CRC detection is 6%.31 To account for this possibility, the CRC incidence with EC in the first year was assumed to be 1.06 times the incidence with SC (Table 1).31 This conservative assumption was selected in order to maximize the cancer treatment-related costs associated with use of EC.
 |
Figure 2 Colorectal cancer incidence rates by age. Abbreviations: SC, standard colonoscopy; EC, Endocuff®-assisted colonoscopy. |
In subsequent years, interval CRC incidence by age with SC was calculated using overall US incidence rates,30 screening prevalence,5 and CRC hazard ratio with SC compared to no screening32 (Equation 1A). Interval CRC incidence rates with EC were calculated using the incidence rates with SC, the difference in ADR with EC vs SC, and the CRC hazard ratio for ADR improvements14 (Equation 1B).
Equation 1. Interval CRC incidence rates in the screened population
A. Standard colonoscopy
(1A)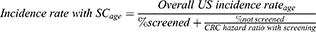
B. Endocuff®-assisted colonoscopy
(1B)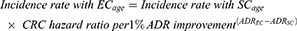
Stage at interval cancer diagnosis
CRC stages were defined using the American Joint Committee on Cancer (AJCC) 7th edition staging system. Stage distribution at interval CRC diagnosis was analyzed using the SEER-Medicare linked database. Approval for this analysis was obtained from the New England Independent Review Board. Patients diagnosed with CRC as their first primary cancer in 2012–2013 were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes (Appendix 1: Table S1). For patients with Medicare claims data (available from 2006 on), claims preceding their diagnosis date were examined for screening colonoscopies using an approach similar to published Medicare claims analyses (Appendix 1: Figure S1).33,34
Screening colonoscopies were identified using HCPCS and Current Procedural Terminology (CPT) codes (Appendix 1: Tables S2 and S3). Colonoscopies were not considered screening if they were conducted within 1 month following a diagnosis that may have necessitated a colonoscopy (Appendix 1: Table S4), or within 6 months preceding CRC diagnosis as those may have been diagnostic. Patients were considered high risk if their most recent screening colonoscopy included biopsy or tumor/polyp/lesion removal, or the claim included ICD-9-CM code V16.0 (family history of CRC) (Appendix 1: Table S5). Patients had interval CRC if the time between their most recent screening colonoscopy and their CRC diagnosis was within the appropriate interval for their risk group.
Disease progression
Patients diagnosed in stages I-III were assumed to undergo surgical resection in accordance with clinical guidelines.23 The annual rate of distant recurrence among patients diagnosed with stage I CRC was 1.5% based on the 7.1% 5-year recurrence rate from a multi-center retrospective database analysis.35 Annual rates of distant recurrence or death among patients diagnosed with stage II or III CRC (5.3% and 11.1%, respectively) were from 3-year progression-free survival rates in the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-08 randomized clinical trial (85.4% and 71.7%, respectively).36
Remission
CRC survivors whose disease did not progress for 5 years were assumed to enter remission, based on the expectation that CRC-related risks (ie, distant recurrence and cancer-specific mortality), quality-of-life (QoL) decrements, and costs would decrease over time among surviving patients (Figure 1B). This assumption is supported by the relative survival among stage IV CRC patients in later years (Figure 3) and QoL among CRC survivors (Table 1). The same risks, QoL, and costs were applied for the remission and screening health states.
 |
Figure 3 Relative survival with Stage IV CRC compared to no cancer. Analyses were conducted using SEER*Stat.30 Data were used to inform CRC-related death. 95% CIs are illustrated in Appendix 2. Abbreviation: CRC, colorectal cancer. |
Mortality
Cancer-related and non-cancer-related mortalities were included. Non-cancer-related mortality by age was based on vital statistics published by the Centers for Disease Control and Prevention, and applied to all patients.37 CRC-related mortality was only applied for patients with stage IV CRC, assuming that cancer-related death would only occur after metastasis.
Cancer-related mortality was assessed in the SEER database using period analyses of relative survival with stage IV CRC compared to the non-cancer population (Figure 3; Appendix 2: Figure S2).30 Relative survival was analyzed by age at stage IV diagnosis and years since diagnosis. The base year was 2013 and relative survival at each year after diagnosis was determined using data from cohorts of patients diagnosed across three calendar years. For example, 10-year relative survival was based on patients diagnosed in 2001–2003, 9-year relative survival was based on patients diagnosed in 2002–2004, 8-year relative survival was based on patients diagnosed in 2003–2005, and so on.
Quality-of-life
QoL of each health state was measured with a utility ranging from zero (death) to one (perfect health). The utility of no cancer, applied for the screening and remission health states, was from a large survey of the US adult population.38 Utilities for CRC by stage were from surveys of colorectal adenoma patients and CRC survivors.39,40
Costs
Mean lifetime direct medical costs were considered from the perspective of the device purchaser (device costs), the health plan (medical costs), and the fully integrated ACO (responsible for both device and medical costs). Costs are reported in 2017 US Dollars.
CRC screening
CRC screening costs were applied in the first year of the model, and again at guideline-recommended intervals.
Average SC or EC cost included the colonoscopy procedure, pathology, and serious adverse events (AEs). Colonoscopy procedure cost for SC was the average 2017 CMS fee schedule cost for types of colonoscopies used for screening (Table S2),41–43 weighted by distributions of procedures and settings-of-care reported in the 2016 CMS Physician/Supplier Procedure Summary File.24 The additional cost of surgical pathology examination (CPT code 88305) was weighted by the proportion of colonoscopy procedures with polyp removal. For EC, the distribution of colonoscopy procedure types was shifted toward those including polyp removal by the difference in ADR from SC to EC (+14.0%). AEs cost was equal for EC and SC screening and was from an analysis of hospitalizations occurring within 14 days of screening or surveillance colonoscopy.44
Endocuff® device cost was $30, based on the distributor’s average sales price. Each EC colonoscopy required one device.
Annual costs
Annual cost for patients in the screening (no CRC diagnosis) health state was the average healthcare expenditure per Medicare beneficiary reported by CMS (Table 1).45 Annual costs for CRC patients by stage at diagnosis and phase of disease were from retrospective claims analyses.46–48 The initial phase was the year post-diagnosis or distant recurrence, the terminal phase was the last year of life, and the continuing phase was the intervening years. Patients who experienced distant recurrence after diagnosis with stage I-III CRC incurred costs associated with stage IV CRC following distant recurrence. Patients in remission incurred costs equal to those in the high-risk screening population.
Sensitivity analyses
One-way sensitivity analyses evaluated the potential influence of input uncertainty on the ICER per QALY. Inputs were varied across individual ranges (Table 1). The probability of being considered high-risk may be higher with EC than SC so this was varied from equal to SC up to +25% over base case. The ADRs with EC and SC were varied simultaneously to opposite ends of their 95% CIs. CRC utility values in the year after diagnosis and in subsequent years were varied together for each CRC stage. Endocuff® device cost was varied from 75% of average sales price up to list price ($50). All other inputs were varied over 95% CIs where reported or ±25% otherwise.
Results
Consistent use of Endocuff® is expected to decrease incidence of interval CRC in the screened population (Figure 2, black line “SC screening”, and blue line “EC screening”). Lifetime risk of CRC in the screened population decreased 0.98% with EC vs SC and risk of CRC death decreased 0.19%. The NNT to avoid one case of CRC with EC vs SC was 102 patients and the NNT to avoid one CRC-related death was 526 patients. Survival and QoL were expected to improve with EC compared to SC by 0.0254 life-years and 0.0114 QALYs per patient on average due to the decreased probability of developing CRC.
Total per-patient lifetime cost from the device purchaser perspective was $112.27 with consistent EC screening compared to SC screening (Table 2). This translated to ICERs of $4,421 per life-year gained, $9,843 per QALY gained, $11,505 per avoided CRC, and $59,035 per avoided death due to CRC. From the health plan perspective, lifetime costs per patient were expected to decrease $199.22. From the ACO perspective encompassing both the device costs and medical costs, CRC screening using EC compared to SC resulted in overall cost-savings of $86.95 per patient over a lifetime.
 |
Table 2 Results |
Savings were largely due to avoidance of CRC-related costs. Mean per-patient CRC costs over a lifetime decreased $1,283.99 with EC compared to SC. Non-device screening costs increased by $377.76 due to projected increases in screening frequency and need for polypectomies and pathology evaluations with increased ADR with EC vs SC. Costs associated with survival without CRC diagnosis increased $707.02 as patients were less likely to be diagnosed with CRC and spent more time in the cancer-free health state with EC vs SC.
In one-way sensitivity analyses, the top-ranked most influential inputs on the ICER per QALY from the device purchaser’s perspective were 1) ADRs with EC and SC, 2) cost of the Endocuff® device, and 3) annual time preference discount rate (Figure 4A). The only scenario under the device purchaser’s perspective that showed an ICER above $20,000 per QALY gained was when the ADRs with SC and EC were varied across their 95% CIs such that the improvement in ADR was only 2.6%, resulting in an ICER of $44,029 per QALY gained. One-way sensitivity analyses showed a $50,000 ICER per QALY gained when ADR with EC was 28.1%, representing a 2.3% improvement over base case ADR with SC. When the Endocuff® device cost was set equal to list price, the ICER was $16,405 per QALY gained.
From the health plan perspective, the most influential variables were the 1) non-device cost of each EC screening, 2) cost of each SC screening, or 3) proportion of patients considered high-risk with EC screening (Figure 4B). Cost-additive results were only observed from the health plan perspective in three tested scenarios: i) when the non-device cost of each EC screening was high ($1,150 [base case $920]), ii) when the cost of each SC screening was low ($664 [base case $886]), and iii) when the proportion of patients considered high-risk with EC screening was high (80.1% [base case 64.1%]) (Figure 4). The ICERs per QALY gained in these three scenarios were $58,012, $49,830, and $7,635, respectively.
Discussion
This analysis found that consistent CRC screening with EC compared to SC was expected to improve patient survival and QoL and reduce risks of interval CRC and related death. Average survival and QoL per patient improved by 0.0254 life-years and 0.0114 QALYs with EC instead of SC, while lifetime risks of interval CRC or CRC-related death were expected to decrease by 0.98% and 0.19%, respectively. Lifetime cost to the device purchaser was expected to be $122 per patient screened consistently with EC instead of SC. The costs per life-year or QALY gained to the device purchaser were $4,421 and $9,843, respectively, well under the $50,000 willingness-to-pay (WTP) per life-year or per QALY gained threshold commonly discussed in the US, suggesting that the Endocuff® device for CRC screening and prevention would be cost-effective. One-way sensitivity analyses showed that cost-effectiveness was expected across all reasonable input ranges.
Due to reduced CRC risks and associated costs, EC was expected to be cost-saving to the health plan or fully integrated ACO. Expected average savings per patient screened consistently with EC instead of SC was $199 to a health plan not including the Endocuff® device costs, and $86 to a fully integrated ACO that pays for the Endocuff® device. To a fully integrated ACO stakeholder responsible for both the Endocuff® device cost and medical costs for CRC screening and treatment, consistent screening with EC was expected to be dominant over SC by reducing costs and improving patient outcomes.
While WTP thresholds per avoided CRC case, or avoided death due to CRC, are less commonly discussed and have not been established in the US, it appears that $11,505 per avoided CRC case and $59,035 per avoided CRC death may be considered cost-effective, especially in the context of the high per-patient costs of oncology treatments used to reduce the risk of cancer mortality. The reduction in lifetime risk of interval CRC by 0.98% with EC compared to SC corresponds to an NNT to avoid one CRC case of 102 patients. Lifetime risk of CRC in the general US population has been estimated at 4.3%,2 suggesting that this projected absolute reduction in interval CRC risk with EC compared to SC may represent a substantial relative risk reduction for the screened population.
With reduced interval CRC incidence, consistent screening with EC compared to SC was also expected to reduce risk of CRC death in the screened population by 0.19%. This translates to an NNT to avoid one CRC death of 526 patients, which is lower than the NNT reported for other recommended cancer preventative services. For example, biennial screening mammography as recommended by the US Preventative Services Task Force (USPSTF) was estimated in a meta-analysis of clinical trials to avoid 8 breast cancer deaths per 10,000 screened women 50–59 years of age.49 This translates to an NNT to avoid one breast cancer death with screening mammography of approximately 1,250 women, suggesting that the NNT reported in the current study to avoid one CRC death with EC instead of SC screening is within an acceptable range for cancer screening in the US.
Limitations
This analysis used Medicare claims data to determine the risk-group distribution in the SC screened population, the distribution of procedures used for screening colonoscopies, and to identify previously screened CRC patients to determine the incident stage distribution of interval CRC. Using claims data to identify screening colonoscopies was limited by the colonoscopy CPT procedure codes, which do not differentiate between colonoscopies performed for screening vs other purposes, or between high- and low-risk patients. Risk group distribution with SC screening was determined using HCPCS codes, which are screening-specific and differentiate high- vs low-risk patients, but these codes are only used when the screening colonoscopy was negative. Therefore, the proportion of patients who are high-risk may be underestimated. To evaluate the stage distribution of interval CRC, this study assessed older patients in the SEER-Medicare linked database using methodologies similar to previously published analyses of CRC screening colonoscopies.33,34 More research to characterize the CRC screening patient population and treatment patterns would be useful in evaluations of the progress and success of CRC screening.
Increased ADR with EC screening was expected to increase the need for polyp removals and increase the proportion of patients in the high-risk screening group, thus increasing screening costs. In the absence of robust data describing the relationship between ADR and risk designation, this analysis assumed that the increases in polypectomy use and in the proportion of patients considered high-risk were equal to the increase in ADR. The definition of a patient’s risk group is multifactorial, depending on the number, size, and type of adenomas detected. For this reason, the change in the screening risk-group distribution cannot be determined with certainty based on ADR alone, despite the metric’s established clinical significance. In particular, if EC improves detection of intermediate-to-high risk polyps, this may lead to higher proportions of patients being considered high risk and needing more frequent screening. The proportion of patients considered high-risk with EC is the third-most influential variable from the health plan perspective, suggesting that additional research on the impact of Endocuff® on CRC screening treatment patterns would be useful. Even so, Endocuff® was expected to be at least cost-effective in all risk-group distribution scenarios tested in the sensitivity analyses.
Potential detection of CRC at earlier stages with EC compared to SC was not included in this model. This analysis applied the same incident CRC stage distribution to both the SC and EC arms of the model. However, if EC screening was to shift the distribution of incident CRCs toward earlier stages, then this would be expected to result in larger improvements in survival and reductions in cancer-associated treatment costs. The potential for diagnosis at an earlier stage due to improved screening with EC is unclear. Larger lesions are less likely to be missed by endoscopists during SC. Previous studies demonstrating improved adenoma detection with EC suggest that detection gains are often in smaller lesions.50 As such, these potential benefits were not included, and this analysis may present conservative estimates of the improvements in patient outcomes and cancer-related savings with Endocuff®.
The model hinges on the relationship between improved ADR during colonoscopies and decreased CRC incidence, as demonstrated in published studies of the benefit of CRC screening. This assumes that the additional adenomas identified by EC are clinically meaningful – ie, that they would have developed into interval CRCs before being detected at later screenings. The mean ADRs observed in studies comparing SC and EC (25.8% and 39.8%, respectively) were similar to those in published studies that have demonstrated decreased CRC incidence with increased physician ADRs (median 25.7% for the 3rd physician quintile and 38.9% for the 5th quintile), suggesting that the CRC prevention benefit applies in the ADR ranges relevant to SC and EC.14
Endocuff®-augmented colonoscopy screening was projected to improve patient outcomes compared to SC screening. Mean survival and QoL were expected to increase, and risks of interval CRC and CRC-related death in the screened population were expected to decrease. Adding Endocuff® to screening colonoscopies was expected to be cost-effective to the device purchaser in the US, and decrease lifetime costs per patient to a health plan or fully integrated ACO.
Abbreviations
ACO, accountable care organization; ADR, adenoma detection rate; AE, adverse event; AJCC, American Joint Committee on Cancer; CMS, Centers for Medicare and Medicaid Services; CPT, Current Procedural Terminology; CRC, colorectal cancer; EC, Endocuff®-assisted colonoscopy; HCPCS, Healthcare Common Procedure Coding System; ICD-9-CM, International Classification of Diseases, 9th revision, Clinical Modification; ICER, incremental cost-effectiveness ratio; NCCN, National Comprehensive Cancer Network; NNT, number needed to treat; NSABP, National Surgical Adjuvant Breast and Bowel Project; QALY, quality-adjusted life-year; QoL, quality of life; SC, standard colonoscopy; SEER, Surveillance, Epidemiology, and End Results; USPSTF, US Preventative Services Task Force; WTP, willingness-to-pay.
Acknowledgments
The authors thank Andrew Layton for his assistance with SEER-Medicare data. Funding for this research was provided by Olympus Corporation of the Americas. An abstract of this paper was presented at Digestive Disease Week (DDW) in Washington, D.C. in June 2018 as a poster presentation. The poster’s abstract was published in an online supplement to Gastroenterology (https://doi.org/10.1016/S0016-5085(18)31771-2).
Disclosure
Tiffany M Yu, Alison Tradonsky, Jun Tang, and Renée JG Arnold were employees of Navigant Consulting at the time of this research. Navigant Consulting received funding for this research from Olympus Corporation of the Americas. The authors report no other conflicts of interest in this work.
References
1. American Cancer Society. Key statistics for colorectal cancer. 2018. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html.
2. National Cancer Institute. SEER cancer stat facts: colorectal cancer. Available from: http://seer.cancer.gov/statfacts/html/colorect.html.
3. Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93(5):1009–1013.
4. Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12(1):1–9, v.
5. American Cancer Society. Colorectal cancer facts & figures 2017–2019. 2017. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf.
6. Centers for Medicare and Medicaid Services. Consensus core set: gastroenterology measures version 1.0. 2015. Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/Downloads/Gastroenterology-Measures.pdf.
7. Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101(4):873–885.
8. Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840 149 screening colonoscopies. Gut. 2007;56(11):1585.
9. Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The miss rate for colorectal adenoma determined by quality-adjusted, back-to-back colonoscopies. Gut Liver. 2012;6(1):64–70.
10. Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112(1):24–28.
11. Lee TJ, Rees CJ, Blanks RG, et al. Colonoscopic factors associated with adenoma detection in a national colorectal cancer screening program. Endoscopy. 2014;46(3):203–211. doi:10.1055/s-0033-1358831
12. Patel N, Darzi A, Teare J. The endoscopy evolution: ‘the superscope era‘. Frontline Gastroenterol. 2015;6(2):101–107. doi:10.1136/flgastro-2014-100448
13. ASGE Technology Committee, Konda V, Chauhan SS, et al. Endoscopes and devices to improve colon polyp detection. Gastrointest Endosc. 2015;81(5):1122–1129. doi:10.1016/j.gie.2014.10.006
14. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(14):1298–1306. doi:10.1056/NEJMoa1309086
15. Chin M, Karnes W, Jamal MM, et al. Use of the Endocuff® during routine colonoscopy examination improves adenoma detection: ameta-analysis. World J Gastroenterol. 2016;22(43):9642–9649. doi:10.3748/wjg.v22.i43.9642
16. Patil R, Ona MA, Ofori E, Reddy M. Endocuff®-assisted colonoscopy-A novel accessory in improving adenoma detection rate: a review of the literature. Clin Endosc. 2016;49(6):533–538. doi:10.5946/ce.2016.032
17. Facciorusso A, Del Prete V, Buccino RV, et al. Comparative efficacy of colonoscope distal attachment devices in increasing rates of adenoma detection: a network meta-analysis. Clin Gastroenterol Hepatol. 2017; 16(8):1209–1219.
18. Ramsey S, Willke R, Briggs A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8(5):521–533. doi:10.1111/j.1524-4733.2005.00045.x
19. Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices–overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–1. Value Health. 2012;15(6):796–803. doi:10.1016/j.jval.2012.06.012
20. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–7. Value Health. 2012;15(6):843–850.
21. Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–2. Value Health. 2012;15(6):804–811. doi:10.1016/j.jval.2012.06.016
22. Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–3. Value Health. 2012;15(6):812–820. doi:10.1016/j.jval.2012.06.014
23. National Comprehensive Cancer Network®. NCCN clinical practice guidelines in oncology (NCCN guidelines) colorectal cancer screening version 1.2017. 2017. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
24. Centers for Medicare and Medicaid Services. Physician/supplier procedure summary master file. 2016. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/NonIdentifiableDataFiles/PhysicianSupplierProcedureSummaryMasterFile.html.
25. Biecker E, Floer M, Heinecke A, et al. Novel endocuff®-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol. 2015;49(5):413–418. doi:10.1097/MCG.0000000000000166
26. Floer M, Biecker E, Fitzlaff R, et al. Higher adenoma detection rates with endocuff®-assisted colonoscopy – a randomized controlled multicenter trial. PLoS One. 2014;9(12):e114267. doi:10.1371/journal.pone.0114267
27. Lenze F, Beyna T, Lenz P, Heinzow HS, Hengst K, Ullerich H. Endocuff®-assisted colonoscopy: a new accessory to improve adenoma detection rate? Technical aspects and first clinical experiences. Endoscopy. 2014;46(7):610–614. doi:10.1055/s-0034-1365446
28. Sawatzki M, Meyenberger C, Marbet UA, Haarer J, Frei R. Prospective Swiss pilot study of Endocuff®-assisted colonoscopy in a screening population. Endosc Int Open. 2015;3(3):E236–239. doi:10.1055/s-0034-1391418
29. Marsano J, Tzimas D, Razavi F, et al. Endocuff® assisted colonoscopy increases adenoma detection rates: a multi-center study. Gastrointest Endosc. 2014;79(5 Suppl):AB550. doi:10.1016/j.gie.2014.02.911
30. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: incidence – SEER 9 Regs Research Data, Nov 2016 Sub (1973-2014) <Katrina/Rita Population Adjustment> – Linked To County Attributes – Total U.S., 1969-2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission. Available from: www.seer.cancer.gov.
31. Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection–systematic review and meta-analysis. Radiology. 2011;259(2):393–405. doi:10.1148/radiol.11101887
32. Wang YR, Cangemi JR, Loftus EV
33. Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100(2):418–424. doi:10.1002/cncr.20014
34. Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296(23):2815–2822. doi:10.1001/jama.296.23.2815
35. Teloken PE, Ransom D, Faragher I, Jones I, Gibbs P, Platell C. Recurrence in patients with stage I colorectal cancer. ANZ J Surg. 2015; 86(1-2):49–53.
36. Allegra CJ, Yothers G, O‘Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol. 2013;31(3):359–364. doi:10.1200/JCO.2012.44.4711
37. Centers for Disease Control and Prevention. National vital statistics report: United States life tables, 2014. 2017. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_04.pdf. Accessed August 22, 2017.
38. Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43(11):1078–1086.
39. Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94(6):1650–1657. doi:10.1111/j.1572-0241.1999.01157.x
40. Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88(6):1294–1303.
41. Centers for Medicare and Medicaid Services. Physician fee schedule. 2017. Available from: https://www.cms.gov/apps/physician-fee-schedule/.
42. Centers for Medicare and Medicaid Services. Ambulatory Surgical Center (ASC) payment – July 2017 ASC approved HCPCS code and payment rates. 2017. Avaialble from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/ascpayment/11_addenda_updates.html.
43. Centers for Medicare and Medicaid Services. Hospital outpatient PPS – July 2017 addendum B update. 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates-Items/2017-July-Addendum-B.html.
44. Leffler DA, Kheraj R, Garud S, et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Arch Intern Med. 2010;170(19):1752–1757. doi:10.1001/archinternmed.2010.373
45. Centers for Medicare and Medicaid Services. Health expenditures by state of residence, 1991–2014. 2017. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsStateHealthAccountsResidence.html.
46. Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JD, Kuipers EJ. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst. 2009;101(20):1412–1422. doi:10.1093/jnci/djp319
47. Lairson DR, Parikh RC, Cormier JN, Chan W, Du XL. Cost-utility analysis of chemotherapy regimens in elderly patients with stage III colon cancer. Pharmaco Econ. 2014;32(10):1005–1013. doi:10.1007/s40273-014-0180-8
48. Song X, Zhao Z, Barber B, Gregory C, Schutt D, Gao S. Characterizing medical care by disease phase in metastatic colorectal cancer. J Oncol Pract. 2011;7(3 Suppl):25s–30s.
49. Siu AL; Force USPST. Screening for breast cancer: U.S. Preventive Services task force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
50. Rex DK, Repici A, Gross SA, et al. High-definition colonoscopy versus Endocuff® versus EndoRings versus full-spectrum endoscopy for adenoma detection at colonoscopy: a multicenter randomized trial. Gastrointest Endosc. 2018;88(2):335–344.e332.
SEER-Medicare Analysis of Interval CRC Incident Stage Distribution
The Surveillance, Epidemiology, and End Results Program (SEER)-Medicare Linked Database was used to evaluate the incident stage distribution of interval colorectal cancer (CRC) diagnosed between guideline-appropriate colonoscopy screenings. Data were provided by the National Cancer Institute. Institutional review board exemption approval for this protocol was granted by the New England Independent Review Board.
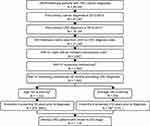 |
Figure S1 Patient selection flow chart.*ICD-9-CM codes in Table S1.†Procedure codes in Table S2.‡Screening colonoscopy is defined as a colonoscopy without (i) any excluded colonoscopy codes on the claim (Table S3), and (ii) without the excluded ICD-9 codes on the claim or in the month prior to the colonoscopy (Table S4).§CPT and ICD-9 codes in Table S5. Abbreviations: SEER, surveillance, epidemiology, and end results; CRC, colorectal cancer; AJCC, American Joint Committee on Cancer; ICD-9-CM, International Classification of Diseases – Ninth Edition – Clinical Modification; CPT, Current Procedural Terminology. |
 |
Table S1 ICD-9-CM codes used to identify CRC diagnosis |
 |
Table S2 Included procedure codes for routine colonoscopy screening |
 |
Table S3 Excluded procedure codes for routine colonoscopy screening |
 |
Table S4 Excluded diagnoses indicating potential non-screening colonoscopy |
 |
Table S5 Indicators of high-risk status after screening colonoscopy |
Seer Analyses of Relative Survival
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.