Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Cost-effectiveness and budget impact analysis of viscosupplementation with hylan G-F 20 for knee and hip osteoarthritis
Authors Migliore A , Integlia D , Pompilio G , Di Giuseppe F, Aru C , Brown T
Received 22 November 2018
Accepted for publication 6 June 2019
Published 22 July 2019 Volume 2019:11 Pages 453—464
DOI https://doi.org/10.2147/CEOR.S194669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Alberto Migliore,1 Davide Integlia,2 Giuseppe Pompilio,2 Francesca Di Giuseppe,2 Cinzia Aru,2 Tray Brown3
1Unit of Rheumatology, San Pietro Fatebenefratelli Hospital, Rome, Italy; 2ISHEO Srl, Rome, Italy; 3Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK
Purpose: To estimate the cost-effectiveness and budget impact of viscosupplementation with one intra-articular (IA) injection of 6 mL hylan G-F 20 (Synvisc-One®,) and with three injections of 2 mL hylan G-F 20 (Synvisc®,3×2) in knee osteoarthritis (OA) patients compared with conventional support therapy (CST – eg, NSAIDs and acetaminophen) and the cost-effectiveness of one IA injection of 2 mL hylan G-F 20 (Synvisc®,1×2) in hip OA patients compared with CST from an Italian Health System perspective.
Methods: The model used was a Markov model with states for stages II–IV on the Kellgren–Lawrence scale and runs on 6-month cycles over a 5-year time horizon. A 3.5% discount was applied to both costs and utilities. Direct costs were determined from the perspective of the Italian National Health Service. A one-way and probabilistic sensitivity analysis was conducted for both comparisons.
Results: Hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL for knee OA were very likely to be cost-effective when compared to acetaminophen (ICER = €3,160.61 and €3,845.81 per QALY, respectively) and NSAIDs as both ICERs are below €25,000. The hip OA treatment by hylan G-F 20 1×2 mL was dominant compared to NSAIDs and very likely compared to acetaminophen. The results of the cost-effectiveness analysis were confirmed by one-way sensitivity analysis. The budget impact analysis for knee and hip OA showed a small increase in expenditure during 5 years.
Conclusions: Hylan G-F 20 1×6 mL/hylan G-F 20 is a cost-effectiveness treatment compared to NSAIDs and acetaminophen in the treatment of knee/hip OA in Italy. The treatment of hip and knee OA resulted in cost-saving with hylan G-F 20 1×2 mL and economically sustainable with hylan G-F 20 1×6 mL. However, Real Word Evidence studies should be conducted in order to estimate costs associated with both prosthetics and to understand the reduction of physiotherapy and medication due to hylan G-F 20.
Keywords: budget impact, cost-effectiveness, osteoarthritis, viscosupplementation, hylan G-F 20, total knee or hip replacement
Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease characterized by progressive damage of articular cartilage and underlying bone, pain and functional limitation.1 It is the most common form of arthritis and the sixth-leading cause of disability in the world,2 being comparable to that of asthma,3 according to WHO.
The hip and knee are the joints most frequently affected by OA, and they are associated with moderate to severe disability even in young adults.4 In European countries, OA estimated prevalence is 35% among people aged 50–59 years, and 55% for people over 70 years of age,5 while the lifetime risk is 45% and 25%,6,7 respectively, for knee and hip OA.
Limitations to job activity are relevant in people with OA if compared with a healthy age- and sex-matched population, leading to a reduction of working hours, difficulties in job applications, or early retirement due to the illness,8 and thus, causing relevant socioeconomic consequences and financial losses.9 Given the increasing incidence of OA with age, the extended life expectancy observed in Italy should result in a progressively higher number of people with this condition.10
Hip and knee OA are usually managed with systemic treatments such as analgesics and NSAIDs and, in the most advanced cases, with prosthesis. However, many patients cannot tolerate NSAID-induced side effects, predominantly gastrointestinal ulceration and bleeding.11 Furthermore, although surgical treatment of hip and knee OA is effective, it is not appropriate for all disease stages or for all patients, and it is also costly and not without risk.12
Viscosupplementation (VS) with intra-articular hyaluronic acid (IA-HA) is a well-established treatment option in knee OA and it is included in the professional guidelines for treatment of the disease in this joint, but it should be applied theoretically to all synovial joints in order to reduce pain, improve joint function and contrast joint damage.1,13,14 VS with hylan G-F 20 has been shown to be safe and effective treatment in patients suffering from both knee and hip OA.15–17
The use of treatments such as VS is a decision to be considered at the initial stage, since they involve more short-term costs, but represent savings in the medium and long term by delaying surgery and reducing NSAIDs and acetaminophen consumptions.17
The change from non-invasive (eg, NAIDS, etc.) to an invasive therapeutic solution could at first glance lead to a low adherence and acceptability by patients but unfortunately, there are few data available on this point. In particular, many of the conclusions regarding patient acceptability can be derived from clinical effectiveness in pain reduction. For example, the overall satisfaction rate assessed at 12 weeks in a study of two HA preparations was high, with over 80% of all patients being either “satisfied” or “very satisfied.”18
Briggs et al19 more recently on pretreatment patient expectations and posttreatment satisfaction reported that relief of pain and improved ability to walk were considered “very important” by 69% and 78% of the patients, respectively. So more data should be available in order to consider the economic consequences of adherence and acceptability of VS.
There are five injectable forms of HA approved by the United States Food and Drug Administration (FDA) including Hyalgan®, Supartz®, Orthovisc®, Synvisc®, and Euflexxa®. Each of these HA products differs in their origin, method of production, molecular weight, dosing instructions, biologic characteristics, and possibly clinical outcomes.15 Hylan G-F 20 is one of the VS products approved for marketing in Canada since 1992 and the United States since 1997 after public review of the data by a FDA advisory panel.20
The aim of this analysis was to determine the cost-effectiveness and the budget impact of hylan G-F 20 therapy compared with conventional supportive therapy (NSAIDs and acetaminophen) in the treatment of knee and hip OA in the Italian setting.
For knee OA, two different cost-effectiveness (CEA) and budget impact (BIA) analyses were performed: the first one with one IA injection of 6 mLhylan GF-20 (Synvisc-One® – hylan G-F 20 1×6 mL) and another with three injections of 2 mLhylan G-F 20 (Synvisc® – called hylan G-F 20 3×2 mL) per year. For hip OA, the CEA and BIA were performed considering one IA injection of 2 mL hylan G-F 20 every 6 months (Synvisc® – called hylan G-F 20 1×2 mL).
Methods
Model and transition probabilities
The model used was a Markov model with states for stages II–IV on the Kellgren–Lawrence (K-L) scale, and then states for either total knee replacement (TKR) or total hip replacement (THR), the after-replacement period, and death. In each of the K-L states, a decision tree was used to model treatment effectiveness, with different utility values applied for successful treatments depending on the intervention used and then the same utility value regardless of intervention for treatment failure. The model runs on 6-month cycles.
A 5-year horizon was chosen with a 3.5% discount applied to both costs and utilities. Patient progression through the K-L states was based on a rate of progression derived from Pavelka et al (2000) and Jordan JM et al (2011) studies, for knee and hip, respectively.21,22 TKR incidence was estimated by Weinstein et al (2013)23 and the incidence of revision knee prosthesis was estimated by Chawla et al (2017),24 while the incidences of THR and revision were estimated by Piscitelli et al (2013).
The annual probability of gastrointestinal and cardiovascular adverse events (AEs) was estimated by Katz et al (2016) and the annual incidence of pulmonary embolism by prosthesization was estimated by Memtsoudis et al (2009).25,26 The mortality rate is based on the Italian life table adjusted for the increase risk of death faced by people with OA.27
Efficacy
Effectiveness was defined as the number of patients having a meaningful reduction in knee OA symptoms and these values were derived from clinical trials.15,28–30 The same effectiveness values were used for hylan G-F 20 3×2 mL and hylan G-F 20 1×6 mL OA knee analyses, as they showed similar effectiveness.31
The same effectiveness values were used for both knee and hip analyses as there are few data from trials that were solely focused on hip OA as opposed to solely knee OA or a combination. This assumption is supported in the literature.17
Cost
Direct costs were determined from the perspective of Italy’s National Health Service and included direct costs of the drugs (NSAIDs and acetaminophen), the cost of administering hylan G-F 20 1×6 mL and hylan G-F 20, while the costs of gastrointestinal and cardiovascular AEs were estimates by Piscitelli et al (2012),10 Gerzeli et al (2005),32 and Stukerboom et al (2002)33 studies. The cost of hylan G-F 20 1×6 mL and hylan G-F 20 was communicated by the pharmaceutical company (Table 1).
 |
Table 1 Costs of drugs, AEs and total joint replacement used in CEA and BIA |
Regarding the choice of acetaminophen and NSAIDs drugs used in the analysis, the expert opinion of Prof. Alberto Migliore34 and the most widely used drugs in the Italian context were considered (Table S1). Moreover, in order to estimate the cost of each drug, the average cost between originator cost and generic drug costs was assumed (using CODIFA Database) (Table S2).
Knee and hip IA injection administration costs were estimated from outpatient service costs of Italian National Health Service35 and for hip VS was considered the ultrasound-guided IA injection technique because IA injections, may be dangerous to infiltrate the hip owing to the uncertainty of injecting into the joint cavity. The ultrasound guidance extends the same benefits as achieved by VS in OA of the knee to OA of the hip but it brings an additional cost.36
It was assumed that other related costs such as specialist visit costs would be similar regardless of treatment and have therefore been excluded. This decision is discussed further in the sensitivity analysis.
Indirect costs were also included based on productivity loss on treatment failures. This was derived using the average number of work days OA suffers miss multiplied by the average daily salary in Italy (OECD, 2016).37,38 Kingsbury et al study37 is the only one updated scientific publication that estimates working days lost for knee and hip OA in Italy, but it also considers finger OA. Therefore, indirect costs related to productivity loss might be overestimated just in the CEA and not in BIA because it takes into account only the direct cost. Furthermore to estimate accurately productivity loss, it is necessary to conduct Real Word Evidence studies by using regional or hospital databases.
These costs are included regardless of age based on the argument that the productivity of retired people should be included in for equity reasons.39
Budget impact analysis
The BIA has been generated for knee and hip. Hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL were used for knee and hylan G-F 20 1×2 mL for hip models, considering simulation with a time horizon of 5 years, for a cohort of 1,000 patients for states 2, 3 and 4 according to K-L scale and the other input parameters used in the cost-effectiveness model. The consumption of drugs at stages 2–4 was estimated by Thomas et al (2017)40 and expert opinion.
Two scenarios have been analyzed: treatment of knee/hip OA with and without hylan G-F 20 1×6 mL/hylan G-F 20 and include cost associated with drug assumption (NSAIDs, acetaminophen, PPI, COX2), AE incidence, TKR/THR and knee/hip revision. In the VS scenario for knee and hip joints, we used a Kaplan–Meier survival curve to estimate the delay in performing a knee and hip operation, as reported in Waddell et al (2007) and in Van Den Bekerom et al (2008), respectively.41,42 Considering the lack of Kaplan–Meier curve for hylan G-F 20 1×6 mL, Waddell's study was used for hylan G-F 20 1×6 mL too. Considering the time horizon of BIA, the hip and knee prosthetics reduces the economic burden of hip and knee OA due to joint replacement and prosthesis revision costs, and management of AEs too.
As in the CEA, the choice of PPI and COX2 drugs used in the analysis are based on expert opinion and most widely used drugs in Italy (Table S2). Drug costs were estimated based on the average between the cost of originator and the cost of generic drugs (using the CODIFA Database). The perspective used to perform this analysis is one of the public payer of the Italian National Health System.
Results – CEA
Base case analysis – knee
After running the model with the base case values, ICERs for the comparison of hylan G-F 20 1×6 mL, hylan G-F 20 3×2 mL versus both acetaminophen and NSAIDs were determined for the treatment of knee OA (Table 2, Table 3).
 |
Table 2 Base case ICERs – hylan G-F 20 1×6 mL |
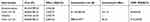 |
Table 3 Base case ICERs – hylan G-F 20 3×2 mL |
The results show that hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL for OA knee treatment are very likely to be cost-effective compared to acetaminophen and NSAIDs as the ICERs for both comparisons are well below €25,000, one of the suggested cost-effectiveness thresholds for Italy (Mencacci et al, 2013)43 (Tables 2 and 3).
The ICER is higher hylan G-F 20 3×2 mL compared to hylan G-F 20 1×6 mL analysis due to the higher cost of hylan G-F 20 3×2 mL due to the three injection administration. Another important aspect that should be considered is the reduction in NSAIDs-related AEs with hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL.
Serious AEs, defined as either cardiovascular events, including myocardial infarction, stroke, coronary heart disease, and congestive heart failure, or gastrointestinal events, including gastric or duodenal ulcers, gastrointestinal hemorrhage, and intestinal obstruction, and related mortality from NSAIDs use were modeled. Out of a cohort of 1,000 patients over the 5-year horizon, there were 55 and 36 serious AEs and 10 and 8 deaths simulated in the NSAIDs cohort for hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL, respectively.
Base case analysis – hip
The results for hylan G-F 20 1×2 mL for hip OA treatment indicate additional QALYs and cost compared to acetaminophens with the ICER well below €25,000, while hylan G-F 20 1×2 mL compared to NSAIDs was dominant, which means that hylan G-F 20 1×2 mL is the most efficacy and less expensive treatment option (Table 4).
 |
Table 4 Base case ICERs – hylan G-F 20 1×2 mL |
As discussed in the knee analysis, another aspect that should be considered is the reduction in NSAIDs-related AEs that hylan G-F 20 provides. Out of a cohort of 1,000 patients over the 5-year horizon, there were 26 serious AEs and five deaths simulated in the NSAIDs cohort. The lower numbers, when compared to the knee analysis, are due to the higher rate of hip replacements, thus fewer patients are taking NSAIDs.
One-way sensitivity analysis
A one-way sensitivity analysis was conducted for hylan G-F 20 1×6 mL, hylan G-F 20 3×2 mL and hylan G-F 20 1×2 mL for both comparisons. In all cases, the results of hylan G-F 20 1×6 ml and hylan G-F 20 3×2 mL remained robust ICER under €17,000 to any parameters within plausible ranges, except for the following three scenarios.
- Comparison with acetaminophen when the utility from an effective acetaminophen treatment exceeded the utility from an effective hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL treatment. This scenario is however very unlikely as a review of clinical trials has shown hylan G-F 20 to have a more positive impact on the health state of patients with OA than acetaminophen (Towheed et al, 2006).44
- Comparison with NSAIDs when the efficacy of hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL is less than NSAIDs. This scenario is however very unlikely as clinical trials have shown hylan G-F 20 to have a more positive effect on patients’ health states (Bellamy et al, 2006).45
- Comparison with NSAIDs where the utility assigned to treatment failure, at the upper value in the potential range increases the ICER to €22,000 for hylan G-F 20 1×6 mL and €25,000 for hylan G-F 20 3×2 mL. This scenario is, however, an unrealistic scenario (not plausible) as it suggests that the treatments analyzed actually worsen the patients’ condition.
The one-way sensitivity analysis results for hylan G-F 20 1×2 mL remained robust (maintaining the ICER under €7,000) for any parameters within plausible ranges. To further explore the uncertainty of the effectiveness of hylan G-F 20 for hip OA, a scenario analysis was run by giving each intervention the same likely range of effectiveness and running 1,000 iterations of the simulation (Monte Carlo Simulation) while drawing from distributions reflecting the likely range of the other parameters. Table 5 shows very similar results to the base case results with hylan G-F 20 1×2 mL dominating the other interventions.
 |
Table 5 Scenario analysis results |
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was conducted to address any uncertainty in the analyses for both comparisons. The results are presented as cost-effectiveness planes and a cost-effectiveness acceptability curves (CEAC). To demonstrate the spread of potential values, the incremental costs and QALYs from the 10,000 iterations are plotted in Figures 1A, 2A and 3A for the comparison with acetaminophen and Figures 1B, 2B and 3B for the comparison with NSAIDs, for hylan G-F 20 1×6 mL, hylan G-F 20 3×2 mL and hylan G-F 20 1×2 mL, respectively. For both comparisons of hylan G-F 20 1×6 mLand hylan G-F 20 3×2 mL, the majority of these points are in the top-right quarter, indicating in both cases that hylan G-F 20 1×6 mLand hylan G-F 20 3×2 mL are more effective and more expensive. This mirrors the results of the base case scenario.
For acetaminophen comparison of hylan G-F 20 1×2 mL, the majority of these points appears to be to the right of the y-axis and evenly split by the x-axis, suggesting that hylan G-F 20 1×2 mL is more effective and about the same cost. For the NSAIDs comparison, the majority of the points are in the lower right-hand quadrant, suggesting that hylan G-F 20 is more effective and cheaper.
The CEAC shows the probability that hylan G-F 20 1×6 mL, hylan G-F 20 3×2 mL and hylan G-F 20 1×2 mL are cost-effective when compared to either acetaminophen or NSAIDs for a range of values for the societal willingness-to-pay for a QALY. The CEAC is generated by running 10,000 iterations of the simulation (Monte Carlo simulation) while drawing from distributions reflecting the likely range of parameters and calculating the proportion of these iterations wherein the ICER is below a certain threshold (Drummond et al, 2015).39
The hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL CEACs for both comparisons are shown in Figures 1C and 2C for acetaminophen and Figures 1D, and 2D for NSAIDs. These show that while there are some differences in two curves, both are likely to be cost-effective even at thresholds well below €25,000 for hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL. For example, the probability that hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL are the most cost-effective treatment is over 50% at a threshold of €4,000 and €5,000 when compared to acetaminophen, and €7,000 and €12,000 compared with NSAIDs, respectively. When a threshold of €25,000 was considered, there is a probability of 74.9% and 75.8%, respectively, for hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL, of being cost-effective compared to acetaminophen and of 56.2% and 53.8% when compared to NSAIDs.
The hylan G-F 20 1×2 mL CEACs for both comparisons are shown in Figure 3C for acetaminophen and Figure 3D for NSAIDs. Figure 3C shows that hylan G-F 20 1×2 mL has an approximate 80% probability of being cost-effective at any threshold above €2,000 when compared with acetaminophen. The CEAC comparing hylan G-F 20 with NSAIDs demonstrates that hylan G-F 20 does have a probability of approximately 60% of being cost-effective up to a threshold of €50,000.
Results – BIA
Considering the model structure, literature review to measure transition probabilities from each state starting from state 2 and expert opinion, the budget increase per patient per year within a 5-year time horizon is €99.99 and €122.49 for hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL, respectively. The higher result for hylan G-F 20 3×2 mL is due to the higher administering cost for three injections versus hylan G-F 20 1×6 mL (one injection).
However, the results show that using hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL for treatment of knee OA lead to a very marginal impact on health-care expenditures for the Italian National Health Service (Table 6 and Table 7).
 |
Table 6 Budget impact for hylan G-F 1×6 mL (knee) |
 |
Table 7 Budget impact for hylan G-F 20 3×2 mL (knee) |
For OA hip treatment with hylan G-F 20 1×2 mL, the budget impact showed an additional net cost during 5 years, approximately €151.06 per patient per year, compared to the scenario without hylan G-F 20 1×2 mL (Table 8).
 |
Table 8 Budget impact for hylan G-F 20 1×2 mL (hip) |
Discussion
Considering Markov model simulations and ICER per QALY results, we found that both hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL compared to the best supportive care were very likely to be cost-effective when compared to acetaminophen (ICER = €3,160.61 and €3,845.81 per QALY, respectively) and NSAIDs (ICER = €7,440.07 and €10,229.83 per QALY, respectively) as both ICERs are below €25,000.
The hip OA treatment by hylan G-F 20 1×2 mL was dominant compared to NSAIDs and very likely compared to acetaminophen (ICER = €937.10 per QALY). The one-way analysis demonstrated that results remained robust for any parameters within plausible ranges, keeping the ICER under €17,000, €17,000 and €7,000 for hylan G-F 20 1×6 mL, hylan G-F 20 3×2 mL and hylan G-F 20 1×2 mL, respectively. The CEACs for knee showed a probability of 74.9% and 75.8% of being cost-effective compared to acetaminophen and of 56.2% and 53.8% when compared to NSAIDs, respectively, for hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL, considering a threshold of €25,000.
The CEACs for hip showed that hylan G-F 20 1×2 mLhas an approximately an 80% probability of being cost-effective compared with acetaminophen at any threshold above €2,000, and nearly 60% of the probability if compared with NSAIDs up to a threshold of €50,000.
In addition to the cost-effectiveness of hylan G-F 20 1×6 mL and hylan G-F 20 3×2 mL, the BIA for knee OA showed a small increase in expenditure during a 5-year timeframe, resulting in an increased budget impact per patient per year of €99.99 for hylan G-F 20 1×6 mL and €122.49 for hylan G-F 20 3×2 mL. An additional net cost during 5 years approximately €151.06 per patient per year was shown in the BIA for hip OA treatment with hylan G-F 20 1×2 mL.
One of the strengths of the cost-effectiveness model is that it includes the most relevant grades of OA (Kellgren & Lawrence grades 1–4) and is stratified by age.
One of the main limitations of the study is that some variables were taken from the literature based on non-Italian populations where disease management and progression can be different. This could change the results of budget impact and cost-effectiveness models.
Conclusion
Knee and hip OA is one of the leading cause of chronic pain, disability and reduced quality of life. Furthermore, the clinical and economic burden of knee and hip OA is significant, and is increasing due to demographic changes, longer life expectancy, higher levels of obesity, and reduced levels of physical fitness.
In the analysis, VS with hylan G-F 20 1×6 mL/hylan G-F 20 showed a decrease in medication consumption and drug-related AEs, and a delay of prosthesization, leading to a reduction of the economic burden due to OA on the Italian National Health Service. Thus, it is important to consider that current OA treatment showed several side effects, such as cardiovascular and gastrointestinal events caused by anti-inflammatory drugs and pulmonary embolism due to prosthesization. Moreover, total prosthesization probably involves higher costs due to hospital and home physiotherapy.
Hylan G-F 20 can also be used in the treatment of OA patients not eligible for prosthetic treatment (mainly elderly patients) and in patients where the intake of anti-inflammatory drugs presents an unfavorable benefit-risk ratio.
Delaying prosthesis means that some patients will no longer be eligible for prosthetic treatment because they are likely to develop comorbidities. VS is an important treatment option for these patients to maintain a good quality of life and reducing the intake of painkillers.
The important issue, in addition to the necessity of identifying the characteristics of the eligible patient, is to understand the potential of hylan G-F 20 from a broader social perspective because OA is a widespread and has a considerable societal impact. Real Word Evidence studies, utilizing regional or hospital databases, are currently lacking but could provide more precise costs estimates for both prosthetics (costs of physiotherapy in hospital/domiciliary regimen and drug consumption) and hylan G-F 20/hylan G-F 20 1×6 mL (in particular, the reduction of physiotherapy and medications related to hylan G-F 20 intake).
Acknowledgments
This study received unconditional funding from Sanofi Italia.
Disclosure
AM receives grants as consultant from Pfizer, UCBPharma, Merck, Roche, Brystol-MyersSquibb, IBSA, Sanofi-Aventis, and Fidia Pharma. AM also reports personal fees from FIDIA, IBSA, ABIOBEN, ROCHE, GUNA, Novartis, and Sanofi, outside the submitted work. DI is the CEO of ISHEO Srl and has received grants from Abbvie, Merck Serono, Bristol Myers Squibb, Pierre Fabre, Eli Lylli, Boehringer Ingelheim, Angelini, and Fidia Pharma. The authors report no other conflicts of interest in this work.
References
1. Migliore A, Procopio S. Effectiveness and utility of hyaluronic acid in osteoarthritis. Mini Rev Clin Cases Mineral Bone Metab. 2015;12(1):31–33.
2. Symmons D, Mathers C, Pfleger B. Global Burden of Osteoarthritis in the Year 2000. Geneva: World Health Organization; 2003. Available from: http://www.who.int/healthinfo/statistics/bod_osteoarthritis.pdf.
3. Mathers CD, Vos ET, Stevenson CE, Begg SJ. The Australian burden of disease study: measuring the loss of health from diseases, injuries and risk factors. Med J Aust. 2000;172:592–596.
4. D’Ambrosia RD. Epidemiology of osteoarthritis. Orthopedics. 2005;28 Suppl:S201–5.
5. Hunter DJ, Neogi T, Hochberg MC. Quality of osteoarthritis management and the need for reform in the US. Arthritis Care Res (Hoboken). 2011;63(1):31–38. doi:10.1002/acr.20278
6. Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–1213. doi:10.1002/art.24021
7. Murphy LB, Helmick CG, Schwartz TA, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18(11):1372–1379. doi:10.1016/j.joca.2010.08.005
8. Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC. Pain exacerbation as a major source of lost productive time in US workers with arthritis. Arthritis Rheum. 2005;53:673–681. doi:10.1002/art.21453
9. Woolf AD. The bone and joint decade 2000–2010. Ann Rheum Dis. 2000;59:81–82. doi:10.1136/ard.59.2.81
10. Piscitelli P, Iolascon G, Di Tanna G, et al. Socioeconomic burden of total joints arthroplasty for symptomatic hip and knee osteoarthritis inthe Italian population: a 5-year analysis based on hospitalization records. Arthritis Care Res. 2012;64:1320–1327. doi:10.1002/acr.21706
11. Migliore A, Tormenta S, Martin LS, et al. The symptomatic effects of intra-articular administration of hylan GF 20 on osteoarthritis of the hip: clinical data of 6 months follow-up. Clin Rheumatol. 2006;25:389–393. doi:10.1007/s10067-005-0052-x
12. John R, Watterson JR, Esdaile JM. Viscosupplementation: therapeutic mechanisms andclinical potential in osteoarthritis of the knee. J Am Acad Orthop Surg. 2000;8:277–284.
13. American College of Rheumatology Subcommittee on osteoarthritis guidelines recommendations for the medical management of osteoarthritis of the hip and the knee: 2000 update. Arthritis Rheum. 2000;43:1905–1915. doi:10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P
14. Pendleton A, Arden N, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2000;59:936–944. doi:10.1136/ard.59.12.936
15. Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113–119. originally published online March 19, 2009. doi:10.1136/ard.2008.094623
16. Sarvajeet Pal, Sreedhar Thuppal, Reddy KJ, et al. Long-term (1-year) safety and efficacy of a single 6-mL injection of Hylan G-F 20 in Indian patients with symptomatic knee osteoarthritis. Open Rheumatol J. 2014;8:54–68. doi:10.2174/1874312901408010054
17. Migliore A, Giovannangeli F, Granata M, et al. Hylan G-F 20: review of its safety and efficacy in the management of joint pain in osteoarthritis, clinical medicine insights: arthritis and musculoskeletal disorders. 2010;3:55–68.
18. Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(2):154–162. doi:10.1016/j.joca.2005.09.003
19. Briggs KK, Matheny LM, Steadman JR. Can hylan g-f 20 with corticosteroid meet the expectations of osteoarthritis patients? Am J Orthop (Belle Mead NJ). 2012;41(7):311–315.
20. Berkowitz DF.
21. Pavelka K, Gatterova J, Altman RD. Radiographic progression of knee osteoarthritis in a Czech cohort. Clin Exp Rheumatol. 2000;18:473–477.
22. Jordan JM, Sowers MF, Messier SP, et al. Methodologic issues in clinical trials for prevention or risk reduction in osteoarthritis. Osteoarthritis Cartilage. 2011;19:500–508. doi:10.1016/j.joca.2010.10.031
23. Weinstein AM, Benjamin N. Rome estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385–392. doi:10.2106/JBJS.L.00206
24. Chawla H, van der List JP, Christ AB, et al. Annual revision rates of partial versus total knee arthroplasty: a comparative meta-analysis. Knee. 2017;24(2):179–190. doi:10.1016/j.knee.2016.11.006
25. Katz JN, Smith SR, Collins JE, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis Cartilage. 2016;24(3):409–418. doi:10.1016/j.joca.2015.10.006
26. Memtsoudis SG, Besculides MC, Gaber L, Liu S, González Della VA. Risk factors for pulmonary embolism after hip and knee arthroplasty: a population-based study. Int Orthop. 2009;33(6):1739–1745. doi:10.1007/s00264-008-0659-z
27. Nuesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Juni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. doi:10.1136/bmj.d1165
28. Cannon GW, Cladwell JR, Holt P, et al. Rofecoxib, a specific inhibitor of cyclooxygenase 2, with clinical efficacy comparable with that of diclofenac sodium: results of a one-year, randomized, clinical trial in patients with osteoarthritis of the knee and hip. Arthritis Rheum. 2000;43(5):978–987. doi:10.1002/1529-0131(200005)43:5<978::AID-ANR4>3.0.CO;2-0
29. James WH, Ward JR, Egger MJ, et al. Comparison of naproxen and acetaminophen in a two-year study of treatment of osteoarthritis of the knee. Arthritis Rheum. 1993;36(9):1196–1206. doi:10.1002/art.1780360904
30. Kamath CC, Kremers HM, Vanness DJ, O’Fallon WM, Cabanela RL, Gabriel SE. The cost-effectiveness of acetaminophen, NSAIDs, and selective COX-2 inhibitors in the treatment of symptomatic knee osteoarthritis. Value Health. 2003;6(2):144–157. doi:10.1046/j.1524-4733.2003.00215.x
31. Conrozier T, Jerosch J, Beks P, et al. Prospective, multi-centre, randomised evaluation of the safety and efficacy of five dosing regimens of viscosupplementation with hylan G-F 20 in patients with symptomatic tibio-femoral osteoarthritis: a pilot study. Arch Orthop Trauma Surg. 2009;129(3):417–423. doi:10.1007/s00402-008-0601-2
32. Gerzeli S, Tarricone R, Zolo P, Colangelo I, Busca MR, Gandolfo C. The economic burden of stroke in Italy. The EcLIPSE study: economic longitudinal incidence-based project for stroke evaluation. Neurol Sci. 2005;26(2):72–80. doi:10.1007/s10072-005-0439-0
33. Sturkenboom MCJM, Romano F, Simon G, et al. The iatrogenic costs of NSAID therapy: a population study. Arthritis Care Res (Hoboken). 2002;47(2):132–140. doi:10.1002/art.10268
34. Migliore A
35. Nomenclatore prestazioni ambulatoriali 2013. Italian Ministry of Health. Available from: http://www.salute.gov.it/portale/temi/p2_6.jsp?id=3662&area=programmazioneSanitariaLea&menu=vuoto.
36. Migliore A, Martini LSM, Alimonti A, Valente C, Tormenta S. Efficacy and safety of viscosupplementation by ultrasound-guided intra-articular injection in osteoarthritis of the hip. Osteo Arthritis Cartilage. 2003;11:305–306. doi:10.1016/S1063-4584(03)00008-6
37. Kingsbury SR, Gross HJ, Isherwood G, Conaghan PG. Osteoarthritis in Europe: impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology (Oxford). 2014;53(5):937–947. doi:10.1093/rheumatology/ket463
38. OECD. (2016). Average annual wages. Available from: https://stats.oecd.org/Index.aspx?DataSetCode=AV_AN_WAGE.
39. Drummond M, Sculpher M, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes.
40. Thomas T, Amouroux F, Vincent P. Intra articular hyaluronic acid in the management of knee osteoarthritis: pharmaco-economic study from the perspective of the national health insurance system. PLoS One. 2017;12(3):e0173683. doi:10.1371/journal.pone.0173683
41. Waddell DD, Bricker DC. Total knee replacement delayed with Hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113–121.
42. Van Den Bekerom MPJ, Rys B, Mulier M. Viscosupplementation in the hip: evaluation of hyaluronic acid formulations. Arch Orthop Trauma Surg. 2008;128(3):275–280. doi:10.1007/s00402-007-0374-z
43. Mencacci C, Di Sciascio G, Katz P, Ripellino C. Cost-effectiveness evaluation of escitalopram in major depressive disorder in Italy. Clinicoecon Outcomes Res. 2013;5:87–99. doi:10.2147/ceor.s39492
44. Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257. doi:10.1002/14651858.CD004257.pub2
45. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321. doi:10.1002/14651858.CD005321.pub2
Supplementary materials
 |
Table S1 Drugs and cost used in cost-effectiveness and budget impact |
 |
Table S2 COX2 and proton pump inhibitor (PPI) costs used in budget impact |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



