Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Cost-Effectiveness Analysis of Antithyroid Drug (Propylthiouracil) Compared to Radioactive Iodine for the Treatment of Graves’ Disease in Ethiopia
Authors Mengistu HS , Getahun KT, Alemayehu L, Gezahign S
Received 2 December 2021
Accepted for publication 1 April 2022
Published 11 April 2022 Volume 2022:14 Pages 221—229
DOI https://doi.org/10.2147/CEOR.S350984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Habtamu Solomon Mengistu,1 Kidus Tesfaye Getahun,2 Lake Alemayehu,3 Sifrash Gezahign3
1Department of Pharmaceutics and Social Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Aman, Ethiopia; 2St. Peter’s Specialized Hospital, Federal Ministry of Health, Addis Ababa, Ethiopia; 3Addis Ababa Health Bureau, Federal Ministry of Health, Addis Ababa, Ethiopia
Correspondence: Habtamu Solomon Mengistu, Tel +251 921 40 65 05, Email [email protected]
Background: Graves’ disease is an autoimmune disorder caused by stimulating antibodies. The peak incidence of Graves’ disease occurs among patients aged 30 to 60 years. Radioactive iodine (RAI) and antithyroid drug (ATD) have been well-established therapies for the treatment of Graves’ disease for several decades. However, there remain large variations in practice among physicians in the preferred modality and the method of administration.
Objective: To assess the cost-effectiveness of ATD (propylthiouracil) compared to RAI from a health care payer perspective in Ethiopia.
Methods: Markov model was constructed by using TreeAge software 2021 with different parameters, such as ATD, RAI, treatment failure, treatment success, hypothyroidism, and supplemental thyroxine to conduct a cost-effectiveness analysis. A hypothetical 40-year-old female patient with symptomatic Graves’ hyperthyroidism was simulated to estimate expected lifetime health outcomes, quality-adjusted life years (QALYs) and costs, discounted at 3%.
Results: RAI has lesser QALYs (14.19) and is less expensive (US$ 3583.22), while ATD has higher QALYs (16.54) and is more expensive (US$ 12531.68). The result showed that there was no dominant treatment option. The incremental cost-effectiveness ratio was US$ 3811.6 per QALY which was greater than one to three times the cost-effectiveness threshold of Ethiopia (US$ 783).
Conclusion: In this cost-effectiveness analysis, RAI was the preferred treatment strategy for Graves’ disease, since the cost needed to get one extra QALY through ATD was greater than one to three times the cost-effectiveness threshold of Ethiopia.
Keywords: antithyroid drugs, radioactive iodine, Graves’ diseases
Introduction
Background
Graves’ disease is an autoimmune disorder caused by stimulating antibodies such as thyroid-stimulating immunoglobulin (TSI), which bind to and activate thyrotropin receptors on thyroid cells, thus not only inducing the synthesis and secretion of thyroid hormone but also hypertrophy and hyperplasia of thyroid follicles. It is the most common cause of spontaneous hyperthyroidism in patients 30 to 60 years of age. It represents 50–80% of all cases of thyrotoxicosis and with 20 to 30 cases per 100,000 individuals each year. In 95% of the cases, thyrotoxicosis is due to Graves' disease, but few could be due to solitary toxic adenoma or multinodular goiter or associated with obstetric conditions such as gestational trophoblastic neoplasia.1,2
Thyroid disorders are common endocrine disorders encountered in the African continent. The amount of autoimmune thyroid disorders in the continent is uncertain due to underdiagnosis and underreporting, but the few published studies show a prevalence rate ranging from 1.2% to 9.9%, with Graves’ disease being the most frequent of these conditions. In Ethiopia, the prevalence (disease burden) of autoimmune thyroid disease is reported to be 1.2% and approximately 3% of women and 0.5% of men develop Graves’ disease during their lifetime.3–5
Hyperthyroidism can be readily diagnosed based on serum thyroid function tests in patients with typical signs or symptoms.2 Once thyrotoxicosis has been identified by laboratory values, the thyroid radioiodine uptake and scan may be used to help distinguish the underlying etiology.6 The typical characteristics of Graves’ disease are goiter and eye disease, and patients with long-standing untreated hyperthyroidism may develop atrial fibrillation (10% to 15% of patients) or heart failure (5.8% of patients).7 Radioactive iodine (RAI) and antithyroid drugs (ATDs) have been well-established therapies for the treatment of Graves’ disease for several decades. However, there remain large variations in practice among physicians in the preferred modality and the method of administration.8
In Ethiopia, there are limited data comparing treatment modalities for Graves’ disease. In other settings, most of the literature demonstrates RAI to be an effective treatment strategy for the treatment of hyperthyroidism due to Graves’ disease. RAI is given in a single dose in capsule or solution dosage form, which consolidates adherence level and reduces overall treatment cost and can be repeated with up to two additional doses in cases of treatment failure. RAI does not need any special equipment for administration and has a lesser probability of resulting relapse as compared to ATDs.9
Considering the lower prescription burden and treatment time course of RAI compared to ATDs, the study hypothesizes that RAI might represent a more cost-effective solution than the current standard of care with ATDs in Ethiopia. Given the limited comparative evidence of these treatment modalities in this setting, the study sought to model the treatment of Graves’ hyperthyroidism with each of these treatment decisions to determine their relative cost-effectiveness using a Markov model.3
Markov models are widely utilized in the medical literature, and they provide a framework for modeling the cost-effectiveness of medical decision-making, with potentially substantial applications in decision support systems and health economics analysis. They reflect relatively simple mathematical models that are simple enough for non-data scientists or non-statisticians to understand. The proof of a basic assumption, the Markov property, must be done with extreme care; otherwise, no further analysis may be performed.10
Methods
ATD and RAI were chosen because the two are the latest treatment modalities available for the treatment of Graves’ disease. Cost-effectiveness analysis was chosen since it can compare the cost and health outcomes of the two options. The literature review was carried out on November 2, 2021, using PubMed and Google scholar with various combinations of search terms. The transition probability of each variable and the cost of medications were assessed in the model.
The study used a health care payer perspective with a lifetime horizon after treatment with ATDs and RAI. The study considered a lifetime horizon because of the potential for relapse and retreatment with lifelong medications in Graves’ disease pattern. The target population included the adult population aged 30–60 because the disease is most prevalent around this age. The outcomes included Quality Adjusted Life Years (QALYs) and cost because the study measured the cost needed to avoid illnesses and gain life years. The health care payer perspective is used since all the costs are invested entirely by the patient and indirect costs are not considered. A discount rate of 3% for cost and outcomes was used after the first year. The overall study parameters used in this study are illustrated in Table 1.
 |
Table 1 Summary of the Study Design |
Health Economic Modelling
A 40-year-old female patient used to capture the impact of the diseases in a younger patient is considered as the base case. Markov model was constructed (Figures 1 and 2) with TreeAge software 2021 and includes the following parameters:
- Graves’ diseases treatment alternatives – RAI and ATD.
- Estimates – Euthyroidism, hypothyroidism, treatment failure, death, procedure success, procedure failure, and relapse.
- Costs of medications, procedures, and laboratory tests.
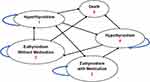 |
Figure 1 Schematic diagram of Markov model for ATD. |
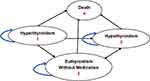 |
Figure 2 Schematic diagram of Markov model for RAI. |
The model has a cycle length of one year with a total of 37 cycles to capture all the outcomes of both treatment options. The current life expectancy in Ethiopia is 67.07 and the study assumed the disease to start at the age of 30. Therefore, the expected life year with the disease becomes 37. Deterministic sensitivity analyses were conducted on data with a wide variation or uncertainty of probability to examine the impact of the uncertainty of variables used in the analysis on the output of the model.
Face Validity
Physicians and other health care professionals of Black Lion Specialized Hospital were consulted about the actual pattern of Grave’s disease and the standard of care. Feedback was taken during the structuring of the Markov model and constructed accordingly. Expert opinions (assumptions) were included for the values of variables that were not available in the literature. The follow-up durations, necessary laboratory tests, and dose tapering concepts of ATD were taken from physician recommendations.
Results
Cost-Effectiveness Analysis
The overall lifetime cost and effectiveness of ATD are higher than RAI, but it was not a cost-effective option in the willingness to pay (WTP) US$ 783. RAI is a cost-effective option at this threshold as depicted in Figure 3.
 |
Figure 3 Cost-effectiveness analysis of RAI and ATD. |
Base Case Results
The two treatment strategies were undominated (Table 2) with each QALY costing US$ 252 for RAI and US$ 757 for ATD.
 |
Table 2 Base Case Results for ATD versus RAI Undiscounted and Discounted (3%) |
Cost Data
All cost data (ATD, RAI, laboratory, and personnel costs) were gathered from Black Lion Specialized Hospital and the latest medication price lists of Ethiopian Pharmaceutical Supply Agency (EPSA) with the recent monetary value of Ethiopian Birr (ETB) were considered for one year. Laboratory-related costs include thyroid function tests, liver function tests (LFT), complete blood count (CBC), blood group, and hematocrit (HCT) testing costs. Cost data of ATD (PTU) and RAI were converted with a current (November 19, 2021) exchange rate of 1 ETB for 0.021 US Dollar. The overall treatment costs for the two alternatives were calculated based on the treatment protocols followed by Black Lion Specialized Hospital which is the main referral center in Ethiopia. Therefore, ATD’s overall treatment cost (US$ 12,531.68) is around three and a half times higher than RAI’s overall treatment cost (US$ 3583.22). This may be due to the duration and frequency of the treatment (ie, RAI is given a single dose for a maximum of three doses, whereas ATD is given for a prolonged period with multiple frequencies per day).
On the other hand, the QALYs of ATD (16.54) is 1.165 times higher than that of RAI (14.19). The incremental cost-effectiveness ratio was 3811.6 which means it costs this much to get one extra QALY through ATD over RAI to choose as a cost-effective option. However, the incremental cost-effectiveness ratio (ICER) result was greater than one up to three times the WTP of Ethiopia, and ATD was not considered cost-effective over RAI. The model key assumptions (Table 3), transition probabilities (Table 4), and utility values (Table 5) are illustrated in the tables.
 |
Table 3 Economic Model Assumptions |
 |
Table 4 Transition Probabilities |
 |
Table 5 Health-Related Quality of Life (HRQoL) Weights |
Deterministic Sensitivity Analysis
To ascertain the uncertainty attached to the effectiveness of the treatment options, one-way and two-way sensitivity analyses were conducted by changing the probability, utilities, and cost of ATD and RAI.
One-Way Sensitivity Analysis
In one-way sensitivity analysis (SA), altering the value of any transition probability and utilities across the range of ± 20% of the values of variables did not alter the preferred cost-effective option of the base case results. The ICER was influenced by the utility of hyperthyroidism for RAI. As the utility of hyperthyroidism increases from a lower limit (0.744) to a higher limit (1), the ICER increases for ATD and remains the same for RAI.
Two-Way Sensitivity Analysis
In the two-way sensitivity analysis, altering the value of any variable across the range of ± 20%19 did not alter the preferred cost-effective option. RAI remains a cost-effective strategy when the utility of hypothyroidism for RAI and the utility of hyperthyroidism for RAI increases within the specified range.
Tornado Diagram
To test the validity of the findings, sensitivity analysis was conducted in the form of a Tornado diagram with a net monetary benefit of US$ 783. Primarily, three variables (ie p_die_othercause; u_hyperthyroidism RAI and u_hyperthyroidism RAI) influence the values of the findings to a significant extent, but a sensitivity analysis was conducted for the two variables as shown in Figure 4. Generally, in all sensitivity analyses, the cost-effectiveness result of RAI was consistent with the base case result, and the findings were robust.
 |
Figure 4 Tornado diagram with net monetary benefits (NMB). |
Discussion
Graves’ disease prevalence was found to be 41.1% in Ethiopia, which was comparatively lower than in other African countries.4 In the base case analysis, RAI has lesser QALYs (14.19) and is less expensive (US$ 3583.22), while ATD has higher QALYs (16.54) and is more expensive (US$ 12,531.68). It is evident that RAI is less effective than ATD through QALYs analysis of the Markov model using TreeAge software. Moreover, the values of QALYs of the two alternatives were found to be significantly different for the adjusted life year of 37 cycles. The result also showed that there was no dominant treatment option.
The incremental cost-effectiveness ratio was 3811.6. ATD treatment strategy needs US$ 3811.6 to get one extra QALY to be cost-effective over RAI, but this value was greater than the cost-effectiveness threshold of Ethiopia (US$ 783), and ATD was not considered a cost-effective strategy for the treatment of Graves’ diseases. The cost-effectiveness ratio of RAI (US$ 252.47/QALY) was lower than ATD (US$ 757.64/QALY) which makes RAI the preferred treatment option in the Ethiopian context. Almost all results were stable to changes in many key parameters and structural uncertainty tested in sensitivity analyses.
A study from England supported this result which assessed the cost per cure from hyperthyroidism (not just Graves’ disease), which captured all medical costs for two-year post-diagnosis. The study demonstrated that RAI was substantially cheaper over a short-term horizon than ATDs per cure. However, the result will not be changed if assessed for long-term costs or quality of life.12
Research conducted in Japan assessed the cost-effectiveness of RAI, ATDs, and subtotal thyroidectomy for the treatment of Graves’ disease. The lifelong utility of the high-fixed-dose RAI alone strategy with a 5% rate of discounting was highest (lifelong utility for 30 years; 15.2/patient), and the utility of ATD plus RAI strategy with 1% side effects of the ATD was lowest (14.1/patient). A low-fixed-dose RAI alone strategy had the lowest cost-effectiveness ratio and was widely used in Japan.22 This study also suggested that the cost-effectiveness ratio of RAI was lower than ATD, which makes RAI to be preferred.
The study on cost-utility analysis modeling lifetime cost and effectiveness of the treatment of graves’ disease in England and Australia states that RAI is the most effective and least expensive strategy dominating ATD. This study used a governmental perspective and the results were different from the base case analysis of this study. This may be due to the difference in the health care system, and RAI was not used as a primary treatment option in Ethiopia to estimate lifetime cost.20
However, the first limitation associated with this study was that the model does not consider a particular therapy that may not be valid as a first-line choice under certain conditions, such as using RAI in pregnant women 4–6 months before pregnancy. The second limitation was the use of PTU for analysis of this study, which is the major ATD used in Ethiopia, but it carries a risk of serious liver disease; however, methimazole is the main ATD used in large part due to its lower risk of liver problems. The third limitation was that the baseline data were gathered from studies done abroad, which are way different from Ethiopian health care settings. This was due to the lack of relevant data in Ethiopia.
Conclusion
In this cost-effectiveness analysis, RAI had less cost-effectiveness ratio than ATD for the treatment of Graves’ disease. The cost needed to get one extra QALY through ATD was greater than one to three times the cost-effectiveness threshold of Ethiopia.
Therefore, RAI was the preferred treatment strategy over ATD. The results were robust to substantial sensitivity analysis, adding to the validity of the results. Policymakers in Ethiopia should include RAI as one treatment option for Graves’ disease. However, conducting further research on cost-effectiveness analysis for treatment options of Graves’ disease by using validated weights from accepted methodologies is still very important.
Ethical Approval
Ethical approval was obtained from Addis Ababa University, School of Pharmacy.
Acknowledgments
We would like to acknowledge immensely the health care professionals at Black Lion Specialized Hospital for their unlimited and kind help throughout the data collection procedure.
Author Contributions
All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article was submitted, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Funding
No funding was available.
Disclosure
The authors reported no conflicts of interest in this work.
References
1. Food, Medicine and Health Care Administration and Control Authority. Standard Treatment Guidelines General Hospitals.
2. De Leo S, Lee YS. Braverman LE Hyperthyroidism. HHS Public Access. 2016;388(10047):906–918.
3. Ethiopia DHS. Final Report Central Statistical Agency Addis Ababa. Ethiopia DHS; 2011.
4. Ogbera AO, Kuku SF. Epidemiology of thyroid diseases in Africa. Indian J Endocrinol Metab. 2011;15(Suppl2):582.
5. Nyström HF, Jansson S, Berg G. Incidence rate and clinical features of hyperthyroidism in a long‐term iodine sufficient area of Sweden (Gothenburg) 2003–2005. Clin Endocrinol (Oxf). 2013;78(5):768–776.
6. Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ. 2006;332(7554):1369.
7. Kravets I. Hyperthyroidism: diagnosis and treatment. Am Fam Physician. 2016;93(5):363–370.
8. Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29.
9. Rajput R, Goel V. Indefinite antithyroid drug therapy in toxic Graves’ disease: what are the cons. Indian J Endocrinol Metab. 2013;17(Suppl1):588.
10. Komorowski M, Raffa J. Markov models and cost-effectiveness analysis: applications in medical research. Secondary Analysis Electronic Health Rec. 2016;2:351–367.
11. In H, Pearce EN, Wong AK, Burgess JF, McAneny DB, Rosen JE. Treatment options for Graves’ disease: a cost-effectiveness analysis. J Am Coll Surg. 2009;209(2):170–179.
12. Patel NN, Abraham P, Buscombe J, Vanderpump MPJ. The Cost-Effectiveness of Treatment Modalities for Thyrotoxicosis in a U.K. Center. Eur J Endocrinol. 2006;16:6.
13. Onimode YA, Ejeh JE, Orunmuyi AT. Adverse reactions to radioiodine 131I therapy of goiter in West African tertiary hospital. Mol Imaging Radionucl Ther. 2016;25(3):128.
14. Sundaresh V, Brito JP, Wang Z, et al. Comparative effectiveness of therapies for Graves’ hyperthyroidism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2013;98(9):3671–3677.
15. Hernández-Jiménez S, Pachón-Burgos Á, Aguilar-Salinas CA, et al. Radioiodine treatment in autoimmune hyperthyroidism: analysis of outcomes in relation to dosage. Arch Med Res. 2007;38(2):185–189.
16. Azizi F, Ataie L, Hedayati M, Mehrabi Y, Sheikholeslami F. Effect of long-term continuous methimazole treatment of hyperthyroidism: comparison with radioiodine. Eur J Endocrinol. 2005;152(5):695–701.
17. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21(6):593–646.
18. Abraham-Nordling M, Törring O, Hamberger B, et al. Graves’ disease: a long-term quality-of-life follow-up of patients randomized to treatment with antithyroid drugs, radioiodine, or surgery. Thyroid. 2005;15(11):1279–1286.
19. Metso S, Jaatinen P, Huhtala H, Luukkaala T, Oksala H, Salmi J. Long-term follow-up study of radioiodine treatment of hyperthyroidism. Clin Endocrinol (Oxf). 2004;61:641–648.
20. Donovan PJ, McLeod DS, Little R, Gordon L. Cost-utility analysis comparing radioactive iodine, anti-thyroid drugs and total thyroidectomy for primary treatment of Graves’ disease. Eur J Endocrinol. 2016;175(6):595–603.
21. Elberling TV, Rasmussen AK, Feldt-Rasmussen U, Hording M, Perrild H, Waldemar G. Impaired health-related quality of life in Graves’ disease. A prospective study. Eur J Endocrinol. 2004;151(5):549–555.
22. Yano F, Watanabe S, Hayashi K, et al. Cost-Effectiveness Analysis of Antithyroid Drug Therapy, 131I Therapy and Subtotal Thyroidectomy for Graves’ Disease. Eur J Endocrinol. 2007;56:65–76).
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
