Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Cost-consequence analysis for human recombinant growth hormone (r-hGH) treatment administered via different devices in children with growth hormone deficiency in Italy
Authors Foo J, Maghnie M, Colao A , Vlachaki I, Colombo G
Received 20 November 2018
Accepted for publication 6 April 2019
Published 22 August 2019 Volume 2019:11 Pages 525—537
DOI https://doi.org/10.2147/CEOR.S195265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Jason Foo,1 Mohamad Maghnie,2 Annamaria Colao,3 Ioanna Vlachaki,4 Giorgio Colombo5,6
1Global Health Economics, Mapi Group (An ICON plc Company), Houten, The Netherlands; 2Department of Pediatrics, IRCCS Istituto Giannina Gaslini Institute, University of Genova, Genova, Italy; 3Department of Clinical Medicine and Surgery, University Federico II of Naples, 80131 Napoli, Italy; 4Global Health Economics, ICON plc, London, UK; 5Department of Drug Science, Pavia University, Pavia, Italy; 6SAVE Studi – Health Economics & Outcomes Research, Milan, Italy
Correspondence: Jason Foo
Global Health Economics, Mapi Group (An ICON PLC Company), De Molen 84, Houten 3995 AX, The Netherlands
Email [email protected]
Background: The objective of this analysis was to evaluate the cost-consequence of recombinant human growth hormone (r-hGH) administered via the easypod auto-injector (Merck, Darmstadt, Germany) versus conventional devices in children with growth hormone deficiency in Italy.
Methods: A patient-level simulation, decision-analytical model was developed to estimate the average height gains and growth hormone treatment costs for a cohort of boys and girls until their bone maturation age. The calculations were performed using listed growth hormone drug prices (base case) and a scenario analysis was also conducted using published tender prices. Costs were discounted at 3%.
Results: Due to improved adherence and earlier identification of poor responders, patients receiving somatropin with easypod gained, on average, 3.2 cm more than patients receiving other r-hGH treatments. Somatropin with easypod had the second highest total cost including wastage (€96,710), but had the second lowest cost per cm gained (€7699/cm). In the scenario analysis, somatropin with easypod had the lowest cost per cm gained (€4708/cm) amongst all of the compared treatments.
Conclusion: Somatropin with easypod can be cost-saving versus all other r-hGH treatments except Omnitrope when listed drug prices are considered and can be cost-saving versus all other r-hGH treatments when tender drug prices are considered. The easypod device also facilitates cost savings in terms of reduced wastage.
Keywords: easypod, growth hormone treatment, growth hormone deficiency, cost-consequence analysis
Introduction
Recombinant human growth hormone (r-hGH) is used to treat growth hormone deficiency (GHD) in children and adults. Early intervention with long-term r-hGH treatment improves adult stature, with some patients reaching target final height.
However, there are a number of issues related to r-hGH treatment. Firstly, lack of adherence hampers growth potential: poor (or non-) adherence is associated with both individual and social treatment failures, such as less favorable clinical outcomes, lower quality of life and higher health care costs.2 Secondly, an equally important issue is poor response to r-hGH therapy (ie, not leading to significant catch-up growth). This can be measured in terms of change in height standard deviation score (HtSDS). Poor response can be prevented or corrected by adapting the treatment following the relevant guidelines. Thirdly, there is evidence that actual r-hGH consumption is much higher than anticipated, suggesting substantial wastage.3
Several devices for r-hGH administration have been developed that can be grouped into five broad categories including syringes with needle, injection pens, self-injection pens, needle-free devices and electronic devices. Easypod®, the electronic auto-injector device, is the only device that allows for adherence monitoring. It has a number of features, including pre-set dosing, adjustable injection settings and accurate monitoring of treatment adherence by an injection log that records injection history which can be accessed by patients or downloaded at their clinic to show which injections, if any, have been missed.4
Early identification of adherence levels to r-hGH therapy may improve the cost-effectiveness of the treatment5,6 through optimization of patients’ management and cost savings. Via easypod, the clinician has the necessary information to correctly identify patients with high adherence and poor response due to decreased sensitivity to growth hormone (GH) (GH “resistant”), and subsequently increase the GH dose in line with the approved dose range for GHD patients.
Furthermore, the use of the easypod device, through its unique capacity for automatic dose adjustment and dosage optimization across cartridges, minimizes product wastage.
For these reasons, we describe in this study a model built to evaluate the cost-effectiveness of the easypod auto-injector versus conventional devices for r-hGH treatment in children with GHD in Italy.
A cost-consequence analysis is a variant of a cost-effectiveness analysis that presents health-related outcomes alongside costs and subsequently their relative value between alternatives. A patient-level simulation, decision-analytical model was developed to estimate the cost-consequence of r-hGH treatment with the combination of somatropin (Saizen®, Merck) administered via easypod, compared with other treatments for a hypothetical cohort of children with idiopathic growth hormone deficiency (IGHD) in Italy. The model structure captures the advantages of easypod over conventional devices in detecting earlier and more accurately low responders and non-adherent patients.
Methods
Design
This cost-consequence model consists of a decision tree (Figure 1) and a Markov model (Figure 2). The decision tree models how patients are evaluated in order to identify poor adherence or poor response to r-hGH. The Markov model represents the different levels of r-hGH use during the course of treatment. Average cumulative drug costs and height gained (in cm) per treatment arm were computed. The model was developed in Microsoft Excel using Visual Basic for Applications (VBA).
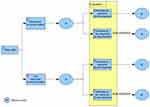 |
Figure 1 Decision tree. |
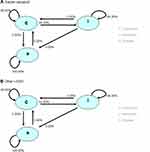 |
Figure 2 Model states. |
The analysis was performed on a cohort of 10,000 patients. The model followed the pre-defined number of patients through the Markov model states, from the treatment start age up to the full bone maturation age and recorded the final height and drug costs for each patient. The average height gained (in cm) at full bone maturation age, total drug costs, drug wastage costs as well as costs per cm gained for each treatment were estimated.
Baseline patient characteristics
In this model, 10,000 hypothetical patient profiles were generated by sampling values of certain patient characteristics: gender, age and height. The percentage of boys, the upper and lower starting age of treatment as well as the upper and lower HtSDS at the beginning of GH treatment were defined for the cohort (Table 1). Age and height samples were generated from uniform distributions: Uniform (lowest age, highest age), Uniform (lowest HtSDS, highest HtSDS). Using Italian reference heights per age and gender,7 (Supplementary materials, Table S1) the initial height of the patient was calculated as:

 |
Table 1 Patient baseline characteristics |
In addition, the full bone maturation age (BA) was defined for boys and girls as the age when adult height is reached, and was considered as the end of treatment for each individual in the cohort (18 years for girls and 19 years for boys).8 It was assumed that there are no further height gains after this age and the model calculations were stopped.
Poor responders and poorly adherent patients
During the treatment period, two reasons for growth failure were considered in the model: (a) poor adherence and (b) poor response to r-hGH (resistance). It is assumed that part of the cohort had lower r-hGH sensitivity and required a higher GH dose to achieve clinical benefits (poor responders) while another part of the population was poorly adherent and would not achieve the maximum clinical benefits, as these children missed injections of GH compared to the prescribed schedule of 6–7 injections per week.
Easypod can help physicians identify earlier and more accurately the cause of growth failure. Using easypod adherence recording files, the physician can detect these patients earlier and adapt the GH dose for poor responders if applicable or manage/motivate patients and caregivers to improve adherence in the case of poor adherence to GH treatment. Without a device being able to accurately record adherence, a percentage of the poorly adherent patients are falsely identified as poor responders (and could be subsequently overdosed, increasing costs and potential safety risks). On the other hand, a percentage of the poor responders are likely to be not definitively confirmed, with the subsequent consequence of being possibly under-dosed, not achieving maximal growth response and eventually decreasing the probability of adult height achievement.
The time until identification of the cause of growth failure was included in the model. Time has an impact on the adult height of low responding patients, as the model assumed that their r-hGH dose was not adjusted until the evaluation date. The link between adherence and years of therapy has been shown to correlate negatively with years of therapy.9 For patients using easypod, it was assumed that the evaluation of response and adherence allows detection of poor responders and poorly adherent patients after completion of the first 6 months (1 cycle). For patients using devices other than easypod, it was assumed that identification of the cause of growth failure did not occur until completion of 12 months of therapy (2 cycles) as data on adherence are not available and growth response is the main criteria used to define poor response and subsequent action (Table 2).
 |
Table 2 Parameters for low response and non-adherence identification |
When devices other than easypod (which do not allow for adherence monitoring) were used for the administration of the r-hGH, it was assumed that a proportion of poorly adherent patients is diagnosed by the physician as poor responders.10 To capture this part of the treatment process, the proportion of poorly adherent patients (24%)11 and of those falsely identified as poor responders (60%, assumption) were defined as input parameters in the model (Table 2). Subsequently, the percentage of patients who received an unnecessary dose increase was calculated as the percentage of poorly adherent patients multiplied by the percentage of those falsely identified as poor responders (Table 2). This subset of patients has an impact on the treatment costs, as the improper dose increase is not considered to have effects on patients’ adult height.
In case of poor adherence, the use of devices other than easypod could also lead to a misdiagnosis of poor responder incorrectly identified as poorly adherent patients. The model used as inputs the percentage of poor responding patients (resistance) and the percentage of patients that are poor responders but not identified as such (Table 2). Subsequently, the percentage of poor responding patients that were identified as such was equal to the percentage of poor responding patients (30%)10 minus the percentage of patients that were poor responders not identified as such (15%, assumption) (Table 2). For these patients, the model assumes that r-hGH dose was increased thus increasing costs and also adult height.
For poor responding patients not identified as such (incorrectly diagnosed), the model assumes that r-hGH dose was not adjusted, therefore without increasing drug costs but also no benefit for adult height. These patients remained indeed on the initial height percentile or maintained their HtSDS values until their bone maturation age, even if they were adherent.
Model states
Each individual patient could transit in the model through four states with a horizon equal to the treatment time (age at full bone maturation minus the treatment start age). The states were:
- Continuous (continuously taking r-hGH)
- Intermittent (missing a number of doses)
- Discontinued (stopped taking r-hGH)
- Achievement of bone age (skeletal maturity): model termination.
During the first cycle, all patients entered in the 1) continuous state. The model structure and the possible transitions are shown in Figure 2 alongside the transition probabilities. Adherence data from Lass et al12 showing that treatment adherence tended to be higher in children treated with easypod than with another device for r-hGH (easypod: 65% good, 35% medium/poor adherence; other devices: 48% good, 52% medium/poor adherence) was used to estimate the transition probabilities in the model. Based on expert opinion, it was assumed that no patients discontinued r-hGH treatment prior to their age of full bone maturation.
Efficacy
The main parameter to measure the efficacy of r-hGH treatment is height that represents the main goal of therapy, ie, normalization of linear growth and achievement of a “normal adult stature” with minimal risks and cost.13
HtSDS expresses height relative to standards for children of the same age and sex, allowing comparisons that are independent of age or gender. The normal population mean for height corresponds to zero HtSDS and a normal HtSDS is included between –2 and +2 standard deviations. Increase over time in HtSDS (upward percentile crossing) implies catch-up growth and a decrease implies growth failure. HtSDS was used to define height gain in the first year of r-hGH treatment (Table 3). We used weighted averages from Ranke et al14 to calculate the HtSDS gain for the first year (ages 2–12 years), with the assumption that 40% of the population had severe GHD and 60% of the population had less severe GHD. We then extrapolated the values for HtSDS gains from 13 to 19 years. After the first year and during the treatment period, the HtSDS gains are determined by patient’s age, level of adherence, presence of poor response (resistance) and total treatment time.
 |
Table 3 First year height SDS gain by age |
The relative reduction in HtSDS gains for each state is defined in Table 4. In case of continuous treatment, it was assumed that HtSDS gain declines by 25% compared to gain obtained during the first year (assumption), while for intermittent treatment (85.7% adherence),15 gain declines faster, ie, by 70% per year (assumption).
 |
Table 4 Efficacy |
Resource use and costs
Details of drug usage, including r-hGH dose per kg (0.025 mg per kg, based on the approved label),16 does increase in case of poor responder identified patients (also based on the approved label) and adherence in case of intermittent r-hGH use15 are shown in Table 5.
 |
Table 5 Drug usage |
Although it is not a direct input in the model, body mass index (BMI) was used for the r-hGH dose calculations. For each patient, the required r-hGH dose per age was calculated using (sampled) height and BMI. Given that BMI of patients with IGHD is similar to the healthy population (difference of mean BMI SDS −0.1 up to −0.2),17 the Italian BMI tables (Supplementary materials, Table S2) were used.7
Based on BMI and height, the daily dose was calculated using the following formula:

For the drug costs calculations, 30.25 days per month were assumed. The dose per kg and the percent increase of the dose have an impact on costs but not on adult patients’ height.
The prices of the products used in this analysis, their cartridge size and the percentage of use in the Italian market for each size are presented in Table 6. In the case-base analysis, the listed drug prices were used from the Italian official journal.18 A scenario analysis was also conducted where the published tender prices were used (Table 7).19
 |
Table 6 Drug costs – base case |
 |
Table 7 Drug costs – scenario analysis |
Costs were discounted at a rate of 3% per year.20
Wastage
Easypod can be used in two ways: a) manual mode, enabling the patient to self-administer partial doses when each cartridge is nearly finished, thereby avoiding wastage of the remaining contents or b) automatic mode avoiding two injections on the days when a new cartridge is inserted in the device.21
Since the administration of partial doses in order to finish each cartridge means that the patient has to undergo two injections on the same day, in automatic mode the daily dose is adjusted slightly (by +10% or +25% according to the choice of the user or the health care professional), so that the entire contents of each cartridge can be administered as complete doses. If the dose administered has been adjusted to below the prescribed dose during the use of a cartridge, when the next cartridge is inserted, easypod will adjust the dose administered to exceed the prescribed dose. In view of this compensation and the magnitude of the dose adjustments, it may be concluded that the patient will not be under- or over-treated. This way, the contents of each cartridge are used optimally, while avoiding the duplicate injections resulting from the administration of partial doses when easypod is used in the manual mode. In the model, we have assumed that the automatic mode is enabled and the extent of adjustment is 10% (based on the most commonly used setting in Italy).
The calculation of wastage cost was implemented as part of the model. During the treatment period, for each cycle, the individually required r-hGH dose was calculated on patients’ actual height and BMI. For each product, given its cartridge size, the wastage (mg/day) was calculated as follows:
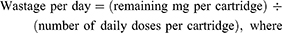
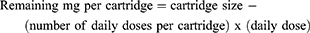

The cost of wastage was calculated as:
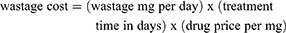
A threshold for the meaningful wastage (as a percentage of the daily dose) was defined as 75%.16 This means that if the remaining amount of drug, before a new cartridge was inserted was lower than this threshold, then the remaining was considered as wastage. In any other case, it was assumed that the patient used the entire contents of the cartridge.
Sensitivity analyses
In order to assess the impact of changes in key model parameters on the model results, the following model parameters were varied in one way sensitivity analyses; discount rate (0%, 5%), percent of non-adherent patients (falsely) identified as low responders (without easypod) (±30%), percent of patients that are low responders and not identified as such (without easypod) (±30%), transition probabilities for somatropin with easypod, transition probabilities for other r-hGH (±30%), per cent reduction in HtSDS gains per year for intermittent r-hGH treatment (±30%) and per cent reduction in HtSDS gains per year for continuous r-hGH treatment (±30%). The model tested ±30% of the mean to understand the impact this parameter has on results.
Results
Base case
In the base case analysis, patients using somatropin with easypod achieved a height gain of 12.6 cm (estimated adult height according to the model: 164.9 cm) whilst patients receiving other r-hGH treatments achieved a height gain of 9.4 cm (estimated adult height: 161.7 cm) (Table 8). On average, patients receiving somatropin with easypod gained 3.2 cm more than patients receiving other r-hGH treatments (Table 8).
 |
Table 8 Average height at full bone maturation – base case |
Table 9 presents detailed results of total costs, wastage costs and cost per cm gained for each treatment. The average total cost, including wastage, was calculated for each drug. Somatropin with easypod was less expensive than Zomacton and was more expensive than Nutropin, Humatrope, Genotropin, Omnitrope and Norditropin (Table 9). The cost of the wasted drug was lowest for somatropin with easypod (€845), whilst for other r-hGH treatments, it ranged from €1275 (Omnitrope) to €6102 (Zomacton) (Table 9).
 |
Table 9 Average costs – base case |
While somatropin with Easypod had the second highest total cost including wastage (€96,710), it had the second lowest cost per cm gained (€7699/cm).
Scenario analyses
In the scenario analysis where published tender prices were used,19 similar height gains were seen for both arms as in the base case (Table 10). Total costs, including wastage, were reduced substantially compared with the total costs reported in the base case scenario (Table 11). Somatropin with easypod showed the biggest reduction in total costs (including wastage), with a reduction of €37,495 (€59,215 vs €96,710) when compared with the base case. The total costs (including wastage) for all other comparators were also lower in the scenario analysis, with reductions ranging from €8624 (Omnitrope) to €33,810 (Genotropin). As expected, the wastage costs also decreased in line with the total costs.
 |
Table 10 Average height at full bone maturation – scenario analysis |
 |
Table 11 Average costs – scenario analysis |
Similar to the base case, somatropin with easypod had the lowest wastage costs (€499) amongst all r-hGH treatments.
Finally, this scenario analysis resulted in lower costs per cm gained for all treatments, with somatropin with easypod reporting the lowest cost per cm gained (€4708/cm) amongst all treatments.
Sensitivity analyses
Five parameters impacted the overall conclusions of the base case analysis and resulted in somatropin with easypod having the third or fourth lowest cost per centimeter gained; reducing the per cent of patients that are low responders and not identified as such (without easypod) by 30%, increasing the per cent reduction in HtSDS gains per year for continuous r-hGH treatment by 30%, decreasing the per cent reduction in HtSDS gains per year for intermittent r-hGH treatment by 30%, decreasing the transition probability for the continuous state for Somatropin with easypod by 30%, increasing the transition probability for the continuous state for other r-hGH by 30%.
Discussion
This study is the first cost-consequence model to capture the cost-effectiveness of somatropin with easypod relative to the other licensed GH treatments in Italy. Many of the key model input data were derived from the literature and validated by a local clinical expert. As much as possible, Italian data sources were used (eg, for reference heights per age and gender, BMI tables, drug costs) and this was particularly important for scenario analysis where we used published tender prices for GH to better reflect real-world costs.
The costs of competing r-hGH treatment options with conventional devices without functions such as adherence tracking and automatic dose adjustment play an important factor in understanding the value of somatropin with easypod. This value of easypod is driven by the functions enabling physicians to earlier identify true poor responders and manage adherence in real-time. This is further supported by improved adherence seen in clinical trials and from real-world data.
The findings from the base case analysis using list prices showed that the use of somatropin with easypod, due to improved adherence and earlier identification of poor responders, translated into an average incremental height gain of 3.2 cm compared with other r-hGH treatments. Whilst Somatropin with easypod had the second highest total cost (€96,710), after Zomacton, it had the second lowest cost per cm gained. In the scenario analysis using tender prices, as to be expected, the incremental height gains were comparable with the base case; however, the findings for the total treatment costs, wastage costs and subsequently the cost per cm gained were lower than in the base case scenario.
The scenario analysis, which uses published tender prices, better reflects the real-world costs of GH treatments. The reductions in total costs (including wastage) are driven solely by the lower prices achieved through the regional tendering process in Italy. Somatropin with easypod had the third lowest total drug cost (including wastage) and the lowest cost per cm gained (€ 4708/cm) in the scenario analysis using tender prices. It is important to emphasize that in both the base case and scenario analyses, wastage costs, as a percentage of the total drug costs, were less than 1% for somatropin with easypod and ranged from 1.8% to 6.2% with other hGH treatments. Apart from changes to the drug costs (scenario analysis), the five model parameters with the biggest impact on the base case results were the percent of patients that are low responders and not identified as such (without easypod), percent reduction in HtSDS gains per year for continuous r-hGH treatment, percent reduction in HtSDS gains per year for intermittent r-hGH treatment, the transition probability for the continuous state for Somatropin with easypod and the transition probability for the continuous state for other r-hGH.
Two prior cost-effectiveness analyses have been published exploring the impact of easypod in patients with GHD.
Elashmawy et al 18 used a model very similar to ours with inputs adapted for Egyptian children born with GHD. They demonstrated that somatotropin delivered by easypod was cost-saving compared with somatropin delivered via prefilled syringe and cost-effective compared with somatropin delivered via regular subcutaneous syringe. Whilst the results of this study are generally in line with our results, the focus of Elashmawy et al was on the comparison of easypod with either prefilled syringes or regular subcutaneous syringe (irrespective of the brand of growth hormone).
Vitova et al19 performed a cost-utility analysis to compare somatotropin delivered via easypod to standard non-monitored somatotropin delivery. Interim results from the Easypod Connect Observational Study were used to populate a deterministic cohort model. Due to increased adherence of monitored patients, the authors reported a cost-effective incremental cost-effectiveness ratio (ICER) of 157,000 Czech Koruna. Whilst the type of cost-effectiveness analysis (cost-consequence vs cost-utility) differed from our analysis, their model also demonstrated improvements in efficacy of GH treatment as a result of increased adherence through the use of easypod to monitor treatment.
A limitation of our study is that there is a lack of comparative studies for the alternative r-hGH options and devices. The results of our analysis highlight the potential height gains and cost savings that can be achieved through the use of somatropin with electronic health solutions such as easypod.
Conclusion
Treatment with somatropin with easypod results in improved height gains compared with other r-hGH treatments through better adherence and earlier identification of poor responders. The easypod device also facilitates cost savings in terms of reduced wastage. Somatropin with easypod can be cost-saving versus all other r-hGH treatments except Omnitrope when listed drug prices are considered and can be cost-saving versus all other r-hGH treatments when tender drug prices are considered.
Abbreviations
BA, full bone maturation age; BMI, body mass index; GH, growth hormone; GHD, Growth hormone deficiency; HtSDS, height standard deviation score; IGHD, idiopathic growth hormone deficiency; r-hGH, recombinant human growth hormone; VBA, visual basic for applications.
Acknowledgments
The authors would like to thank Ekaterina Koledova for the model scientific review and constant scientific support. The analysis in this paper was presented at the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) Conference in 2018 as a poster presentation reporting interim findings. The poster’s abstract was published in Supplement 3 in Value in Health as well as being available through the ISPOR Scientific Database (available from https://tools.ispor.org/ScientificPresentationsDatabase/Presentation/87522).
Disclosure
Mr Jason Foo served as a consultant for Merck KGaA during the conduct of the study. Professor Giorgio Colombo reports grants from Merck Serono during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Farber RS, Kerrigan JR The multiple indications for growth hormone treatment of pediatric patients. Pediatr Ann 2006;35(12):926–932. [published Online First: 2007/01/24].
2. Haverkamp F, Johansson L, Dumas H, et al. Observations of nonadherence to recombinant human growth hormone therapy in clinical practice. Clin Ther 2008;30(2):307–316. [published Online First: 2008/03/18]. doi: 10.1016/j.clinthera.2008.02.017
3. Spandonaro F, Cappa M, Castello R, Chiarelli F, Ghigo E, Mancusi L The impact of real practice inappropriateness and devices’ inefficiency to variability in growth hormone consumption. J Endocrinol Invest 2014;37(10):979–990. [published Online First: 2014/08/12]. doi: 10.1007/s40618-014-0138-x
4. Dahlgren J Easypod: a new electronic injection device for growth hormone. Expert Rev Med Devices 2008;5(3):297–304. [published Online First: 2008/05/03]. doi: 10.1586/17434440.5.3.297
5. Koledova K, Stoyanov G, Yao M, et al. Analysis of results from the global, 5-year Easypod™ Connect Observational Study (ECOS) study in children with growth disorders.
6. Koledova E, Stoyanov G, Ovbude L, Davies PSW Adherence and long-term growth outcomes: results from the Easypod™ connect observational study (ECOS) in paediatric patients with growth disorders. Endocr Connect 2018;7(8):914–923. [published Online First: 2018/10/10]. doi: 10.1530/EC-18-0172
7. Cacciari E, Milani S, Balsamo A, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest 2006;29(7):581–593. [published Online First: 2006/09/08]. doi: 10.1007/bf03344156
8. Greulich W, Pyle S Radiographic Atlas of Skeletal Development of the Hand and Wrist.
9. Maggio MC, Vergara B, Porcelli P, et al. Improvement of treatment adherence with growth hormone by easypod device: experience of an Italian centre. Ital J Pediatr 2018;44(1):113. [published Online First: 2018/09/29]. doi: 10.1186/s13052-018-0548-z
10. Bang P, SF A, Argente J, et al. Identification and management of poor response to growth-promoting therapy in children with short stature. Clinical Endocrinology 2012;77(2):169–181. [published Online First: 2012/05/01]. doi: 10.1111/j.1365-2265.2012.04420.x
11. Bagnasco F, Di Iorgi N, Roveda A, Gallizia A, Haupt R, Maghnie M Prevalence and correlates of adherence in children and adolescents treated with growth hormone: a multicenter Italian study. Endocr Pract 2017;23(8):929–941. [published Online First: 2017/06/15]. doi: 10.4158/ep171786.or
12. Lass N, Reinehr T Low treatment adherence in pubertal children treated with thyroxin or growth hormone. Horm Res Paediatr 2015;84(4):240–247. [published Online First: 2015/08/19]. doi: 10.1159/000437305
13. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH research society. GH research society. J Clin Endocrinol Metab 2000;85(11):3990–3993. [published Online First: 2000/11/30]. doi: 10.1210/jcem.85.11.6984
14. Ranke MB, Lindberg A Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab 2010;95(3):1229–1237. [published Online First: 2010/01/26]. doi: 10.1210/jc.2009-1471
15. Cutfield WS, Derraik JG, Gunn AJ, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One 2011;6(1):e16223. [published Online First: 2011/02/10]. doi: 10.1371/journal.pone.0016223
16. Saz-Parkinson Z, Granados MS, Amate Blanco JM Study of Adherence to Recombinant Growth Hormone Treatment of Children with a GH Deficiency: Contributions to Treatment Control and Economic Impact. Madrid: Agencia de Evaluación de Tecnologías Sanitarias (AETS) Instituto de Salud Carlos III; 2013.
17. Pitukcheewanont P, Desrosiers P, Steelman J, et al. Issues and trends in pediatric growth hormone therapy – an update from the GHMonitor observational registry. Pediatr Endocrinol Rev 2008;5 Suppl 2:702–707. [published Online First: 2008/05/28].
18. Elashmawy AA, Anwar GM, Elsedfy H, et al. Cost-effectiveness of EasypodTM device versus other somatotropin delivery techniques in Egypt in treatment of growth hormone deficiency. Value Health 2017;20(9):A586. doi: 10.1016/j.jval.2017.08.1062
19. Vitova V, Tichopad A Cost-effectiveness of somatropin administration with increased adherence due to monitoring compared to non-monitored administration in patients with growth hormone deficiency. Value Health 2013;16(7):A622–A623. doi: 10.1016/j.jval.2013.08.1825
20. Capri S, Ceci A, Terranova L, Merlo F, Mantovani L Guidelines for economic evaluations in Italy: recommendations from the Italian group of pharmacoeconomic studies. Drug Inf J 2001;35(1):189–201. doi:10.1177/009286150103500122
21. Loche S, Salerno M, Garofalo P, et al. Adherence in children with growth hormone deficiency treated with r-hGH and the easypod device. J Endocrinol Invest 2016;39(12):1419–1424. [published Online First: 2016/07/14]. doi: 10.1007/s40618-016-0510-0
22. Ranke MB, Lindberg A, Chatelain P, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS international board. Kabi pharmacia international growth study. J Clin Endocrinol Metab 1999;84(4):1174–1183. [published Online First: 1999/04/13]. doi: 10.1210/jcem.84.4.5634
Supplementary materials
 |
Table S1 Height |
 |
Table S2 BM |
Reference
1. Cacciari E, Milani S, Balsamo A, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest 2006;29(7):581–593. [published Online First: 2006/09/08]. doi: 10.1007/bf03344156
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
