Back to Journals » Journal of Blood Medicine » Volume 12
Correlation of Serum Ferritin and Liver Iron Concentration with Transient Liver Elastography in Adult Thalassemia Intermedia Patients with Blood Transfusion
Authors Atmakusuma TD , Lubis AM
Received 16 February 2021
Accepted for publication 19 March 2021
Published 15 April 2021 Volume 2021:12 Pages 235—243
DOI https://doi.org/10.2147/JBM.S303703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Tubagus Djumhana Atmakusuma, Anna Mira Lubis
Division of Hematology-Medical Oncology, Department of Internal Medicine, Dr. Cipto Mangunkusumo General Hospital, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia
Correspondence: Tubagus Djumhana Atmakusuma
Division of Hematology-Medical Oncology, Department of Internal Medicine, Dr. Cipto Mangunkusumo General Hospital, Faculty of Medicine Universitas Indonesia, Jalan Diponegoro No. 71, Jakarta Pusat, DKI, Jakarta, Indonesia
Tel +622 13162497
Email [email protected]
Introduction: Iron overload is a common feature of thalassemia intermedia due to regular blood transfusion and increased gastrointestinal iron absorption. Early detection and adequate iron chelator can decrease morbidity and mortality from iron overload. Liver iron concentration (LIC) by MRI T2* is the best non-invasive way to measure body iron stores. However, this method is expensive and not available nationwide in Indonesia. The aim of this study was to identify liver iron overload and correlation of transferrin saturation, serum ferritin, liver MRI T2* and LIC with transient liver elastography in adult thalassemia intermedia patients.
Methods: This is a cross-sectional study of 45 patients with thalassemia intermedia with blood transfusion and with and without iron chelator therapy. The study was conducted at Cipto Mangunkusumo Hospital from August through October 2016. We performed measurements of transferrin saturation, serum ferritin level, transient liver elastography and liver MRI T2*. Pearson and Spearman correlation tests were used to evaluate the correlation between transient liver elastography with transferrin saturation, serum ferritin, liver MRI T2*and LIC.
Results and Discussion: This study showed that 64.4% of study subjects are β-Hb E thalassemia intermedia. Furthermore, 84.4% of study subjects have regular transfusion. Based on liver MRI T2*all subjects suffered from liver iron overload, 48.9% had severe degree. Median value of liver MRI T2* was 1.6 ms. Mean serum ferritin was 2831 ng/mL, with median transferrin saturation of 66%. Mean of LIC corresponding to liver MRI T2* and mean liver stiffness measurement was 15.36± 7.37 mg Fe/gr dry weight and 7.7± 3.8 kPa, respectively. Liver stiffness correlated with serum ferritin (r=0.651; p=0.000), liver MRI T2* (r=− 0.357; p=0.016), and LIC (r=0.433; p=0.003). No correlation was found between liver elastography and transferrin saturation (r=0.204; p=0.178).
Conclusion: Serum ferritin, liver MRI T2*and LIC correlated with liver elastography. No correlation was found between transferrin saturation and liver elastography.
Keywords: liver MRI T2*, LIC, serum ferritin, thalassemia intermedia, transient liver elastography
Introduction
Thalassemia is a red blood cells genetic disorder characterized by the abnormality formation of globin chain alpha, beta, or both. Thalassemia major (TM) is generally characterized by severe anemia and clinically appears in infancy (<2 years), requiring red blood cell transfusions on a regular basis for growth and live, while thalassemia trait is generally asymptomatic and do not require red blood cell transfusion.1,2 Thalassemia intermedia (TI) is a group of thalassemia phenotype spectrum which is between the major and trait thalassemia.
Indonesia is one of the countries with the highest prevalence of thalassemia genetic, called “thalassemia belt”.3 Based on data from Thalassemia Center in RSCM, there are 9031 TM patients in Indonesia,4 and this is expected to rise in line with the high number of thalassemia gene carriers who are asymptomatic, and generally they have not done premarital screening.
Unlike the TM, iron overload is still an important issue in TI, and associated with the increase of morbidity and mortality.5 Even if thalassemia intermedia patients do not get a blood transfusion, they are still at risk for iron overload, due to increased iron absorption in the gastrointestinal tract.6 Iron overload can be monitored by serum ferritin, which is a very quick, inexpensive, non-invasive, and generally widely available examination. But the value of this serum ferritin can be elevated in other conditions such as infection, inflammation, malignancies, etc. Liver iron concentration (LIC) is the gold standard in estimating the value of iron body load and can predict accurately total body iron.7–9
Liver biopsy is the gold standard in assessing the LIC directly. However, it is associated with several side effects and disadvantages such as pain, bleeding, infection, extensive sampling variability, and inter-observer variability. Therefore, a non-invasive examination has been developed in assessing LIC, such as magnetic resonance imaging (MRI). T2* MRI examination of liver is now a validated examination in assessing LIC.10,11 This examination is non-invasive, fast, accurate and reproducible.2 But it has not been equally distributed across thalassemia service center in Indonesia. In thalassemia patients, liver toxicity due to iron overload can lead to fibrosis, cirrhosis, and even liver cancer.
An accurate assessment of iron load in patients with thalassemia during long-term follow-up are essential, not only for the prevention of complications due to iron but also for monitoring the adequacy of iron chelation treatment. Until now, iron chelation adequacy treatment was assessed by measurement of serum ferritin and LIC.12 It is a serial measurement that makes it a very expensive examination. So far, we have not found yet a publication about the correlation between liver elastography and liver MRIT2* on IT, transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia (NTDT) patients. Liver elastography is used to measure liver fibrosis since it is a non-invasive method and correlates well with the degree of fibrosis.13–15 This method is a vital tool that can be added to the armamentarium of assessing chronic liver injury due to iron overload. The examination can be performed bedside, using a portable machine, and provide instantaneous results. The limitation of this method is the inability to assess fibrosis status when there is ascites or tumor present. This study is expected to assess the correlation of liver elastography and liver MRI T2* as standard tests to assess the LIC.
Methods
This cross-sectional study aimed to identify liver and blood iron overload in adult patients with TI and the correlation between transferrin saturation, serum ferritin, MRI T2* liver, and LIC assessed by MRI T2* liver examination with liver elastography value in Hematology–Oncology Division of the Internal Medicine Department Policlinic General Hospital National Center Cipto Mangunkusumo (RSUPNCM) in August 2016 to October 2016.
Patients diagnosed with thalassemia (thalassemia α, thalassemia β and thalassemia β-Hb E) using the method of Hb microcapillary electrophoresis, high-performance liquid chromatography (HPLC) or by DNA analysis, aged at least 18 years and do not have Hepatitis B or hepatitis C, human Immunodeficiency Virus (HIV), severe infection, massive ascites, hepatic failure, congestive heart failure, BMI>30 kg/m2, total bilirubin>3g/dl, claustrophobia (phobia of narrow or closed place) and willing to participate in this research were included in this study.
We conducted demographic data such as age, gender, ethnicity, age of first thalassemia diagnosis and age of first transfusion. Clinical data such as splenectomy status, iron chelation therapy, and drug inducers of hemoglobin F were collected through interviews and medical records. Complete physical examination, and venous blood sampling for peripheral blood complete test, liver function test (AST, ALT, total bilirubin, direct, indirect), the levels of serum ferritin and transferrin saturation were conducted in all subjects. On the same day, we conducted liver Elastography examination using FibroScan® by Echosens™ with M probe in Hepatology Procedure Room RSUPNCM, performed by two operators (consultants of gastro-entero-hepatology and one trainee) who do not have prior knowledge about the patient iron status. The elasticity was obtained though multiple measurements, averaged by the machine. Normal measurement ranges from 2 to 7 kPa. T2* MRI examination of the liver using 1.5 T MRI Siemens™ Avanto Magnetom® completed by CMR Tools software, carried out in the Department of Radiology RSUPNCM, with the funding coming from National Health Insurance (JKN) as routine checks, which were analyzed by two consultant radiologists who did not know the status of the iron and liver FibroScan results, with a maximum distance of 1 month.
This study has obtained proper ethical clearance, established by Ethics Committee of The Faculty of Medicine, University of Indonesia; Ethical Approval Number: 600/UN2.F1/ETIK/2016. Appropriate informed consent to participate in this study has been obtained from the subjects prior to study commencement. This study was conducted in accordance with the Declaration of Helsinki. Data were analyzed with SPSS 20 for Windows. Pearson’s correlation coefficient (r) was performed to analyze the distribution of normal data or correlation coefficient.
Results
There were 45 subjects of the study, 28 (62.2%) were women, the median age of subjects was 33 (18–84) years (Table 1). A total of 42.4% of the subjects were in the range of 18–30 years age group. β-Thalassemia Hb E showed to be the highest proportion of thalassemia (64.4%). A total of 48.9% of the subjects diagnosed with thalassemia at the age of 20 years and the majority of subjects (71.1%) is TI with regular blood transfusions, with the median age of first receiving blood transfusion was 19 years old. Almost all subjects (95.6%) received iron chelation therapy, iron chelation with deferiprone is the most used.
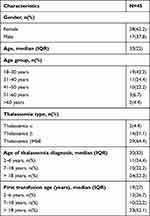 |
Table 1 Characteristic Data Based on Demographic Research Subjects |
Based on the assessment of iron load by checking transferrin saturation and serum ferritin, majority of the subjects had complications of iron overload. A total of 66.7% of the subjects had a transferrin saturation levels ≥55%, with a median value of 66%, with a range of 31–100%. A total of 93.3% of the research subjects had a mean serum ferritin >800 ng/mL, with the largest group of >2000 ng/mL, which is about 60% of the study subjects. T2* MRI examination of the liver obtained all the study subjects had hemosiderosis complications (median 1.6 ms) with 48.9% of subjects experienced severe hemosiderosis. LIC mean values obtained through T2* MRI examination was 15.36±7.37 mg iron/g dry weight liver. Elastography mean value and AST liver was 7.7±3.8 kPa and 33.3 U/L (Table 2).
 |
Table 2 Characteristic Thalassemia-Related Data of Study Subjects |
Liver Iron Concentration Based on the Liver MRI T2*
LIC values obtained from the calculation of MRI T2* liver showed as much as 49.9% of the study subjects study experienced severe complications of hemosiderosis (>15 mg iron/g of liver dry weight), and only 11.3% of research subjects with a light hemosiderosis (Table 3).
 |
Table 3 Liver MRI T2* and LIC Result |
Correlation Between Serum Ferritin and Liver Elastography Values
Based on Pearson correlation test, there was a positive correlation between serum ferritin and liver elastography values (r = 0.651; p <0.001; Figure 1). Pearson correlation of test results obtained a weak correlation between LIC and liver elastography value (r=0.433; p=0.003; Figure 2), with a positive direction.
 |
Figure 1 Correlation between serum ferritin and liver elastography. |
 |
Figure 2 Correlation between liver iron concentration (LIC) and liver elastography. |
Correlation Between Liver Elastography with Liver MRI T2*
Spearman correlation test results obtained weak correlation between the value of liver elastography and MRI T2* of liver (r=−0.357; p=0.016), with a negative direction (Figure 3).
 |
Figure 3 Correlation between MRI T2* and liver elastography. |
Discussion
Gastrointestinal iron absorption is increased due to chronic anemia and erythropoiesis, and also repeated blood transfusions which render thalassemia intermedia patients at high risk for the occurrence of iron overload.16 In the study, iron overload was assessed by serum ferritin, transferrin saturation and LIC by MRI T2*. Iron load varies greatly with time, depending on the intensity of the transfusion, administration of chelating iron and iron absorption in the gastrointestinal tract, which is heavily influenced by the severity of ineffective erythropoiesis and chronic anemia.17–24 Serum ferritin level also changes and varies in line with changes in body iron load.25–27 Because of that, in this experiment, we used the mean serum ferritin in the past year to avoid the presence of other factors that affect serum ferritin values such as infection, inflammation, etc.28–30 The mean serum ferritin showed a chronic condition compared with serum ferritin levels only. The mean ferritin per year was 2831±1828 ng/mL. A total of 60% of the subjects experienced a severe iron overload in ferritin values >2000 ng/mL.
In this study, most of the subjects received blood transfusions on a regular basis; hence, the mean serum ferritin levels were high. Furthermore, the iron which was distributed to the reticuloendothelial system (RES), increased ferritin synthesis and was released into the circulation, leading to a high serum ferritin.31–35 One out of three subjects with serum ferritin <800 ng/mL (229 ng/mL) had LIC 7.81 mg iron/gram dry weight of the liver and did not receive iron chelation. This showed the importance for monitoring the serum ferritin level as well as charge of excess iron.36 Monitoring of LIC should be done regularly if possible, to achieve optimal iron chelation.37–39
Based on MRI T2* examination of the liver, we found all subjects experienced liver toxicity; 48.9% of the subjects experienced severe liver hemosiderosis (MRI T2*<1.8 ms). The proportion of subjects experienced severe liver hemosiderosis (LIC >15 mg iron/g of liver dry weight) was not much different (49.9%). Clinical spectrum of TI patients was very wide and varied, from mild and did not need blood transfusions, to severe with regular transfusions such as TM. However, non-transfusion-dependent thalassemia(NTDT) patients were still at risk of having excess iron load. The ineffective erythropoiesis and chronic anemia caused reduced hepcidin levels that lead to increase iron absorption in the gastrointestinal tract and increase iron release from macrophages of the RES.40–47
It must always be remembered that, in contrast to TM, serum ferritin can not be used to assess the severity of iron overload in thalassemia intermedia, especially IT NTDT.8,9 A significant association between serum ferritin and LIC has been clear in the TM patients who receive regular transfusions. This is similar to the study conducted by Taher, et al11 in Lebanon. In this study, where the majority of study subjects were IT with regular transfusion, with a mean serum ferritin 2831 (1828) ng/mL, we found a high iron overload in the liver, with an average LIC 15.36 (7.37) gr iron/gr liver dry weight. The high LIC value was predicted because of the high requirement for blood transfusions.48–58
In this study, a serial transferrin saturation examination was not obtained from medical records, so we could not see the trend of transferrin saturation in the past year. The other was the use of iron chelation in the study subjects, deferiprone was the most used iron-chelating agent in this study. It showed a declining optimal transferrin saturation within 2 hours after deferiprone ingestion and increased within 6 hours.59–63 Deferiprone time lapse between consumption and time of blood sampling for the examination, which was not uniform in each research subject, could affect transferrin saturation values, related to the concentration of iron chelation and a half-life in the blood. Severe hepatic impairment (liver damage/necrosis of hepatocytes) can also lead to an increase in ferritin and transferrin decline in production.64–68
In this research, study subjects were IT patients with the majority of blood transfusions on a regular basis. In this study, serum ferritin used is the mean serum ferritin for 1 year, which was considered to represent the trend and chronicity of the charge of iron overload. Then, compared with the measurements of serum ferritin during the course, this was different from the previous study using serum ferritin at the time of the study. In this study, moderate correlations were shown, between the mean serum ferritin and elastography. Iron overload and liver iron exposure in the long term might cause iron toxicity in liver which proceed into fibrosis. Liver elastography was used to assess liver fibrosis.69
In this study, the factors that can affect the liver fibrosis had been excluded, which were an infection of hepatitis B or C, as the long-term complications resulting from transfusions in patients with thalassemia. Thalassemia patients with excess iron load and hepatitis infection, liver fibrosis was not only due to iron toxicity but also infection such as hepatitis provided a major contribution.55–57 In contrast to the study by Pipaliya et al,13 a weak correlation in this study was predicted because of difference in MRI methods used. In the study by Pipaliya,13 MRI T2* was used. Besides, variation of patient populations especially in subjects with TM might influence the results.69
Conclusion
The proportion of liver iron overload in adult patients with thalassemia intermedia was 100% in General Hospital National Center Cipto Mangunkusumo (RSUPNCM) assessed by MRI T2* examination 100%. There was no correlation between transferrin saturation with a liver elastography level. There was a moderate correlation between serum ferritin by liver elastography value. Furthermore, it showed a weak correlation between LIC based on liver T2* MRI examination and liver elastography value. Adding to that, there was a weak negative correlation between the liver elastography and MRI T2* liver.
Acknowledgments
This paper is based on a thesis written in 2016: Lubis AM, Atmakusuma TD, Kurnawan J, Harimurti K. Korelasi antara muatan besi berlebih darah dan hati dengan nilai elastografi hati pada pasien thalasemia intermedia dewasa yang mendapatkan transfusi darah [Correlation between blood and liver excess iron load and liver elastographic values in adult intermedia thalassemia patients receiving blood transfusions]. Thesis. 2016.69
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wood B, Molecular Higgs D. Basis of thalassemia syndromes. In: Beaumont C, Beris P, Beauzard Y, Brugnara C, editors. Disorders of Erythropoiesis, Erythrocytes, and Iron Metabolism. Italy: European School of Hematology; 2009:251–262.
2. Taher AT, Vichinsky E, Musallam K, Cappellini MD, Viprakasit V. Guidelines for the management of non-transfusion dependent thalassemia (NTDT). In: Thalassemia International Federation. Cyprus: Publisher Thalassemia International Federation; 2013:1–8.
3. Musallam KM, Rivella S, Vichinsky E, Rachmilewtz EA. Review articles. Non-transfusion dependent thalassemias. Hematol J. 2013;98(6):833–844. doi:10.3324/haematol.2012.066845
4. Hematology Oncology Indonesia Working Group. Data Registri Thalassemia Indonesia. Children Thalassemia Center RSUPNCM Jakarta; 2016.
5. Viprakasit V, Porter J. Iron overload and chelation. In: Capellini M, Cohen A, Porter J, Taher A, Viprakasit V, editors. Guidelines for the Management of Transfusion-Dependent Thalassemia (TDT).
6. Taher AT, Musallam KM, Beshlawy A, et al. Efficacy and safety of long-term (>7 year) alemtuzumab therapy for refractory T-cell large granular lymphocytic leukaemia. Br J Haematol. 2010;150(4):480–497. doi:10.1111/j.1365-2141.2010.08218.x
7. Musallam KM, Cappellini MD, Wood JC, et al. Elevated liver iron concentration is a marker of increased morbidity in patients with β-thalassemia intermedia. Hematol J. 2011;96(11):1605–1612. doi:10.3324/haematol.2011.047852
8. Musallam KM, Cappellini MD, Taher AT. Evaluation of the 5 mg/g liver iron concentration threshold and its association with morbidity in patients with β- thalassemia intermedia. Blood Cells Mol Dis. 2013;51(1):35–38. doi:10.1016/j.bcmd.2013.01.015
9. Pantalone GR, Lace D, Valenza F, et al. Hepatocellular carcinoma in patients with thalassemia syndrome: clinical characteristics and outcome in a long-term single center experience. Br J Haematol. 2010;150(2):226–248. doi:10.1111/j.1365-2141.2010.08179.x
10. Taher AT, Viprakasit V, Musallam KM, Cappellini MD. Critical review: treating iron overload in patients with non-transfusion-dependent thalassemia. Am J Hematol. 2013;88(5):409–415. doi:10.1002/ajh.23405
11. Taher AT, Musallam KM, Wood JC, Cappellini MD. Magnetic resonance evaluation of hepatic and myocardial iron deposition in thalassemia intermedia independent transfusion – compared to regularly transfused thalassemia major patients. Am J Hematol. 2010;85(4):288–290. doi:10.1002/ajh.21626
12. Puliyel M, Sposto R, Bardoukas V, et al. Ferritin trends do not predict changes in total body iron in patients with transfusional iron overload. Am J Hematol. 2014;89(4):391–394. doi:10.1002/ajh.23650
13. Pipaliya N, Solanke D, Parikh P, et al. Comparison of tissue elastography with magnetic resonance imaging T2 * and serum ferritin quantification in detecting liver iron overload in the patient with thalassemia major. Clin Gastroenterol Hepatol. 2016.
14. Musallam KM, Motta I, Salvatori M, et al. Longitudinal changes in serum ferritin levels correlate with measures of hepatic stiffness transfusion-independent in patients with β-thalassemia intermedia. Blood Cells Mol Dis. 2012;49(3–4):136–139. doi:10.1016/j.bcmd.2012.06.001
15. Fraquelli M, Cassinerio E, Roghi A, et al. Transient elastography in the assessment of liver fibrosis in adult thalassemia patients. Am J Hematol. 2010;85(8):564–568. doi:10.1002/ajh.21752
16. Weatherall DJ. The definition and epidemiology of non-transfusion-dependent thalassemia. Blood Rev. 2012;26S:S3–6. doi:10.1016/S0268-960X(12)70003-6
17. Sengsuk C, Tangvarasittichai O, Chantanaskulwong P, et al. Association of iron overload with oxidative stress, hepatic damage, and dyslipidemia in transfusion-dependent β-thalassemia/HbE patients. Ind J Clin Biochem. 2014;29(3):298. doi:10.1007/s12291-013-0376-2
18. Viprakasit V, Tyan P, Rodmai S, Taher AT. Review. Identification and key management of non-transfusion-dependent thalassemia patients: not a rare but under-recognized potentially condition. Orphanet J Rare Dis. 2014;9(131):1–11. doi:10.1186/s13023-014-0131-7
19. Cappellini MD, Musallam KM, Cesarwtti C, Taher A. Thalassemia intermedia. In: Beaumont C, Beris P, Beauzard Y, Brugnara C, editors. Disorders of Erythropoiesis, Erythrocytes, and Iron Metabolism. Italy: European School of Hematology; 2009:287.
20. Maharani EA, Soedarmono Y, Nainggolan IM. Frequency of thalassemia carrier and Hb variant and the quality of stored donor blood. Med J Indones. 2014;23(4):209–212. doi:10.13181/mji.v23i4.766
21. Musallam KM, Cappellini MD, Wood JC, Taher AT. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev. 2012;26S:S16–9. doi:10.1016/S0268-960X(12)70006-1
22. Musallam KM, Taher AT, Rachmilewitz EA. Β-thalassemia intermedia: a clinical perspective. Cold Spring Harb Perspect Med. 2012;2(7):1–15. doi:10.1101/cshperspect.a013482
23. Taher AT, Musallam KM, Cappellini MD, Weatherall DJ. Optimal management of β thalassemia intermedia. Br J Haematol. 2011;152(5):512–523. doi:10.1111/j.1365-2141.2010.08486.x
24. Taher A, Isma’eel H, Cappellini MD. Thalassemia intermedia: revisited. Blood Cells Mol Dis. 2006;37(1):12–20. doi:10.1016/j.bcmd.2006.04.005
25. Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body of iron metabolism and the pathophysiology of iron overload. Int J Haematol. 2008;88(1):7–15. doi:10.1007/s12185-008-0120-5
26. Rivella S. The role of ineffective erythropoiesis in a non-transfusion-dependent thalassemia. Blood Rev. 2012;26S:S12–5. doi:10.1016/S0268-960X(12)70005-X
27. Batts KP. Iron overload syndromes and the liver. Mod Pathol. 2007;20(S1):S31–9. doi:10.1038/modpathol.3800715
28. Taher A, Hershko C, Cappellini MD. Iron overload in thalassemia intermedia: reassessment of iron chelation strategies. Br J Haematol. 2009;147(5):634–640. doi:10.1111/j.1365-2141.2009.07848.x
29. Musallam KM, Cappellini MD, Daar S, et al. Serum ferritin levels and morbidity risk in transfusion-independent in patients with β-thalassemia intermedia: the ORIENT Study. Hematol J. 2014;99(11):e218–21. doi:10.3324/haematol.2013.097220
30. Musallam KM, Cappellini MD, Daar S, Karimi M, El-Beshlawy A, Taher AT. Serum ferritin levels and morbidity in β-thalassemia intermedia: a 10-year cohort study. [Abstract]. Am Soc Hematol. 2012.
31. Hershko C. Pathogenesis and management of iron toxicity in thalassemia. Ann NY Acad Sci. 2010;1202(1):1–9. doi:10.1111/j.1749-6632.2010.05544.x
32. Pietrangelo A. Mechanism of iron hepatotoxicity. Hepatol. 2016;65(1):226–227. doi:10.1016/j.jhep.2016.01.037
33. Britton RS, Leicester KL, Bacon BR. Iron toxicity and chelation therapy. Int J Haematol. 2002;76(3):219–228. doi:10.1007/BF02982791
34. Taher A, Rassi F, Isma’eel H, Koussa S, Inati A, Cappellini MS. Correlation of liver iron concentration determined by R2 magnetic resonance imaging with serum ferritin in patients with thalassemia intermedia. Hematol J. 2008;93(10):1584–1586. doi:10.3324/haematol.13098
35. Pakbaz Z, Fischer R, Fung E, Nielsen P, Harmatz P, Vichinsky E. Serum ferritin underestimates liver iron concentration in patients with thalassemia transfusion independent as compared to regularly transfused thalassemia and sickle cell patients. Pediatr Blood Cancer. 2007;49(3):329–332. doi:10.1002/pbc.21275
36. Shah R, Treban A, Das R, Marwaha R. Serum ferritin in thalassemia intermedia. Indian J Hematol Blood Transfus. 2014;30(4):281–285. doi:10.1007/s12288-013-0267-y
37. Taher AT, Porter JB, Kattamis A, et al. Defining serum ferritin thresholds to predict clinically relevant liver iron concentrations for guiding deferasirox therapy when MRI is unavailable in patients with non-transfusion-dependent thalassaemia. Br J Haematol. 2015;168(2):284–290. doi:10.1111/bjh.13119
38. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill US. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American association for the study of liver disease. Hepatology. 2011;54(1):328–343. doi:10.1002/hep.24330
39. Garbowski M, Carpenter J, Smith G, et al. Biopsy-based calibration of T2 * magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferris can. J Cardiovasc Magn Reson. 2014;16(40):1–11. doi:10.1186/1532-429X-16-40
40. Szurowska E, Sikorska K, Swieszewska E, et al. The role of MR imaging in the detection of hepatic iron overload in patients with cirrhosis of different origins. BMC Gastroenterol. 2010;10(13):1–14.
41. Timothy G, Pierre CP, Chua-anusom W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105(2):855–856. doi:10.1182/blood-2004-01-0177
42. Echeverria J, Castiella A, Emparanza J. Quantification of iron concentration in the liver by MRI. Insights Imaging. 2012;3(2):173–180. doi:10.1007/s13244-011-0132-1
43. Henninger B, Kremser C, Rauch S, et al. Evaluation of MR imaging with T1 and T2 * mapping for the determination of hepatic iron overload. Eur Radiol. 2012;22(11):2478–2486. doi:10.1007/s00330-012-2506-2
44. Gandon Y, Oiivie D, Guyader D, Aube C, Oberti F, Sebille V. Non-invasive assessment of hepatic iron stores by MRI. Lancet North Am. 2004;363(9406):357–362. doi:10.1016/S0140-6736(04)15436-6
45. Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2 * mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease Patients. Blood. 2005;106(4):1460–1465. doi:10.1182/blood-2004-10-3982
46. Sarigianni M, Liakos A, Vlachaki E, et al. Accuracy of magnetic resonance imaging in the diagnosis of liver iron overload: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13(1):55–63. doi:10.1016/j.cgh.2014.05.027
47. Angelucci E, Giovagnoni A, Valeri G, et al. Limitations of magnetic resonance imaging in measurement of hepatic iron. Blood. 1997;90(12):4736–4742. doi:10.1182/blood.V90.12.4736
48. Verissimo MP, Loggetto SR, Fabron A
49. Castiella A, Zapata E. Non-invasive prediction methods for liver fibrosis in hemochromatosis: one step beyond. World J Hepatol. 2010;2(7):251–255. doi:10.4254/wjh.v2.i7.251
50. Papastertergiou V, Tsochatzis E, Burroughts AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25(3):218–231.
51. Adhoute X, Foucher J, Laharie D, et al. Diagnosis of liver fibrosis and other FibroScan using noninvasive methods in patients with hemochromatosis: a prospective study. Gastroenterol Clin Biol. 2008;32(2):180–187. doi:10.1016/j.gcb.2007.12.021
52. Talwalkar J, Kurtz D, Schoenleber S, West C, Montori V. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5(10):1214–1220. doi:10.1016/j.cgh.2007.07.020
53. Paparo F, Cevasco L, Zefiro D, et al. Diagnostic value of real-time elastography in the assessment of hepatic fibrosis in patients with liver iron overload. Eur J Radiol. 2013;82(12):e755–61. doi:10.1016/j.ejrad.2013.08.038
54. Marco V, Bronte F, Cabibi D, et al. Noninvasive assessment of liver fibrosis in thalassemia major patients by transient elastography (TE) – lack of interference by iron deposition. Br J Haematol. 2009;148(3):476–479. doi:10.1111/j.1365-2141.2009.07996.x
55. Taher AT, Cappellini MD, Musallam KM. Recent advances and treatment challenges in patients with non-transfusion-dependent thalassemia. Blood Rev. 2012;26S:S1–2. doi:10.1016/S0268-960X(12)00028-8
56. Telfer PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B. Hepatic iron concentration combined with long term monitoring of serum ferritin to predict complication of iron overload in thalassemia major. Br J Haematol. 2000;110(4):971–977. doi:10.1046/j.1365-2141.2000.02298.x
57. Children Thalassemia Center RSUPNCM. Data MRI T2 * liver and heart in Dept. pediatrics 2014 Jakarta. Anak: Thalassemia Center; 2014
58. Olivieri NF, Gary MB, Doreen M, Matitiahu B, Laurence MB. Iron-chelation therapy with oral deferiprone in patients with major thalassemia. N Engl J Med. 1995;332(14):918–922. doi:10.1056/NEJM199504063321404
59. Galanello R, Piga A, Forni GL, et al. Phase II clinical evaluation of deferasirox, a once daily oral chelating agent, in pediatric patients with B-thalassemia major. Hematol J. 2006;91:1343–1351.
60. Adams P, Barton J. A diagnostic approach to hyperferritinemia with a non-elevated transferrin saturation. J Hepatol. 2011;55(2):453–458. doi:10.1016/j.jhep.2011.02.010
61. Al-Refaie FN, Silva CE, Wonke B. Hoffbrand AV changes in transferrin saturation after treatment with the oral iron chelator deferiprone in patients with iron overload. J Clin Pathol. 1996;48(2):110–114. doi:10.1136/jcp.48.2.110
62. Sinakos E, Vasilios P, Efthimia V, Ioanna T, Maria RG. Is liver stiffness really unrelated to liver iron concentration? Br J Haematol. 2010;150(2):247–248. doi:10.1111/j.1365-2141.2010.08183.x
63. Ezzat H. Utility of transient elastography (FibroScan) in estimating hepatic iron concentration in comparison to MRI in patients with transfusion dependent hemoglobinopathies. [Abstract]. Blood. 2014;124(21):4888. doi:10.1182/blood.V124.21.4888.4888
64. Majd Z, Haghpanah S, Ajami G, et al. Serum ferritin levels correlation with heart and liver MRI and LIC in patients with transfusion-dependent thalassemia. Iran Red Crescent Med J. 2015;17(4):e24959. doi:10.5812/ircmj.17(4)2015.24959
65. Origa R, Barella S, Argiolas GM, Bina P, Agus A, Galanello R. No evidence of cardiac iron in 20 never or minimally transfused patients with thalassemia intermedia. Hematol J. 2008;93(7):1095–1096. doi:10.3324/haematol.12484
66. Aessopos A, Kati M, Farmakis D. Heart disease in thalassemia intermedia: a review of the underlying pathophysiology. J Hematol. 2007;92(5):658–665. doi:10.3324/haematol.10915
67. Aessopos A, Farmakis D, Deftereos S, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127(5):1523–1530. doi:10.1378/chest.127.5.1523
68. Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systematic review and meta-analysis. Hepatology. 2015;61(1):29. doi:10.1002/hep.27382
69. Lubis AM, Atmakusuma TD, Kurnawan J, Harimurti K. Korelasi antara muatan besi berlebih darah dan hati dengan nilai elastografi hati pada pasien thalasemia intermedia dewasa yang mendapatkan transfusi darah. [Correlation between blood and liver excess iron load and liver elastographic values in adult intermedia thalassemia patients receiving blood transfusions] [Thesis]; 2016. Indonesian.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
