Back to Journals » Clinical Ophthalmology » Volume 11
Corneal thickness in dry eyes in an Iraqi population
Authors Ali NM, Hamied FM, Farhood QK
Received 9 August 2016
Accepted for publication 20 October 2016
Published 23 February 2017 Volume 2017:11 Pages 435—440
DOI https://doi.org/10.2147/OPTH.S119343
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Noora Mauwafak Ali,1 Furkaan M Hamied,1 Qasim K Farhood2
1Department of Surgery, College of Medicine, Al-Qadisiya University, Diwaniyah, 2Department of Surgery, College of Medicine, University of Babylon, Hillah, Iraq
Background: Dry eye disorder is a multifactorial disease of the tears and ocular surface that results in discomfort and visual disturbance. Corneal pachymetry becomes increasingly important in refractive surgery, for the accurate assessment of intraocular pressure, and in the preoperative assessment of other ocular surgeries.
Purpose: To assess the effect of dry eye disorder on the central corneal thickness (CCT) by comparing with CCT of normal eyes of age-matched individuals.
Patients and methods: The total number of eyes examined was 280 (140 dry eyes from 70 patients and 140 normal eyes from 70 individuals). Pentacam (Scheimpflug imaging system) was used for measuring the CCT of all eyes.
Results: Patients with dry eye syndrome had significantly lower CCT compared to the control group (P<0.01). Its mean was 536.5 versus 561.3, respectively.
Conclusion: CCT of dry eyes was significantly reduced when compared with age- and gender-matched population. This result can be attributed to chronic desiccation by the inflammatory mediators in dry eyes, leading to corneal thinning.
Keywords: central corneal thickness, pentacam, dry eye syndrome
Introduction
The tear film is an interface between the eye and the outer world, maintaining the health and function of the ocular surface.1
A three-layered tear film has an essential relationship with the superficial epithelial layers of the cornea and conjunctiva. The innermost layer of the tear film is a mucus layer of thickness 0.2 μm,2,3 which contain mucin, salts, immunoglobulins, glucose, leukocytes, cellular debris, and enzymes. This layer is secreted mainly by the conjunctival goblet cells.4,5 Overlying this layer, there is an aqueous phase of thickness 7 μm that consists of water, electrolytes, proteins, immunoglobulins, peptide growth factors, cytokines, vitamins, and antimicrobials (lysozymes, lactoferrin).4,5
The superficial layer is a lipid layer of thickness 0.1 μm, which is composed of oil secreted by the meibomian glands. It is the major barrier of evaporation from the ocular surface and reduces evaporation by 95%.6,7
The roles of the precorneal tear film are protection of cornea from drying, maintenance of the refractive power of cornea, protection against eye infection, and permission of oxygen to move from the air to the avascular cornea.2
Corneal epithelium
It is composed of stratified squamous epithelium and makes up approximately 5%–10% of the total corneal thickness. The epithelium and tear film form an optically smooth surface. Tight junctions between superficial epithelial cells prevent penetration of tear fluid into the stroma. Continuous proliferation of perilimbal basal epithelial cells gives rise to other layers that differentiate later into superficial cells. With maturation, these cells become coated with microvilli on their outermost surface and then desquamate into the tears.8
Under the epithelium is the Bowman’s layer which is the acellular superficial layer of the stroma and is formed from collagen fibers. The stroma makes up 90% of corneal thickness. It is arranged in regularly orientated layers of collagen fiber and keratocytes.3
Dua’s layer is 15 μm thick. It is the fourth of the six layers of the cornea between the corneal stroma and the Descemet’s membrane. The attachment of them appeared to be accomplished by this layer.9
Descemet’s membrane is a discrete sheet consisting of a fine latticework of collagen fibrils. The innermost layer is the endothelium which consists of a monolayer of polygonal cells. Endothelial cells maintain corneal deturgescence throughout life by pumping the excess fluid out of the stroma.3 The average central thickness of the normal human cornea is 540 μm.8
Dry eye
It is a disorder of the tear film due to tear deficiency or excess tear evaporation.10 A new definition states that it is a multifactorial disease of the tears and ocular surface that results in the symptoms of discomfort, visual disturbance, and tear film instability, with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.11
Estimating the prevalence of dry eye syndrome is complicated by the absence of consensus on a single dependable diagnostic test. Several population-based epidemiologic studies have utilized questionnaires to assess the prevalence of dry eye symptoms. American and Australian studies revealed a prevalence of 5%–16%, while Asian studies revealed a higher prevalence of approximately 27%–33%.12
The most common risk factors for the development of dry eye disorders (DEDs) are: increasing age, female gender, menopause, hormone replacement therapy, smoking, autoimmune disorders, contact lens wear, medications, eye lid disorders, and environmental factors.13
Typical complaints include burning, itching, foreign body sensation, stinging, dryness, photophobia, ocular tiredness, and redness. Although the symptoms are usually nonspecific, careful awareness to details will help refine the diagnosis.12
The tear film should provide a complete tear layer throughout the blink interval. When break-up of the tear film occurs prior to the next blink, there is a discontinuity that degrades the visual image and produces irritation. The ratio of the tear break-up time (TBUT) to the interblink interval is called the ocular protection index, of which values >1 are considered pathologic.14,15
The hyperosmolarity condition is the result of either an inadequate secretion of fluid from the lacrimal glands (low aqueous flow) and/or excessive evaporation of the tear film. Normal tear osmolarity averages around 295 mOsmol/L, which is isotonic with blood.15
Inflammation has been observed in all stages of dry eye. Increased levels of inflammatory cytokines, especially interleukin-6, have been observed in the lacrimal glands, conjunctival epithelium, and/or tear fluid of patients with dry eye.11
Pachymetry
The measurement of central corneal thickness (CCT) has become increasingly important in ophthalmic practice. For example, refractive surgery is routinely planned according to the preoperative measurement of CCT, and accurate determination of intraocular pressure (IOP) may need to be modified according to CCT.16–18 Valid and repeated estimates of corneal thickness are necessary to determine the level of corneal edema and, thus, to ensure that a contact lens is appropriate for extended wear.18,19
In recent years, several optical technologies have been introduced that offer the advantages of a noncontact technique and objective determination of the center of the cornea.
Pentacam is a noninvasive and objective device that allows extensive evaluation of the corneal structure using a three-dimensional model showing the thickness, volume, and spatial section.20 This system is based on a rotating Scheimpflug camera, which scans and measures the complete cornea and anterior chamber parameters in approximately 2 seconds.21
Using pentacam in the current study for measuring the CCT allows fast, noncontact examination of the anterior segment, while the patient is sitting comfortable, without application of local anesthetics or causing corneal erosion.22 Also, it can detect any irregularities of the cornea and rule out corneal thinning due to ectasia. At the same time, CCT value obtained with pentacam is similar to those measured with U/S pachymeter;23 which was the most dependable method in the past years.
Aim of the study
The study aims to assess the effect of DED on CCT in a sample of Iraqi population.
Patients and methods
The study groups consisted of 280 eyes: 140 eyes of 70 patients diagnosed with dry eye disease aged between 23 and 65 years and 140 eyes of 70 healthy individuals (control group) aged between 20 and 66 years without any ophthalmic or systemic pathology.
Both eyes of each patient were analyzed. All eyes were investigated by the same pentacam device between September 2015 and March 2016 at Ibn Al Haitham teaching hospital.
Patients with previous ocular surgery or trauma, any previous or recent active eye infection or inflammation such as Stevens–Johnson disease, chemical burns, infectious or allergic conjunctivitis, glaucoma, pseudoexfoliation, active meibomian gland disease, lid disorders, corneal dystrophy such as Fuchs endothelial dystrophy, and patients using topical medications regularly were excluded from the study.
The diagnosis of dry eye was made based on the following steps:
- McMonnies (1986) questionnaire:2,24 A score of over 20 is indicative of dry eye, while a total score between 10 and 20 is suggestive of borderline DED.
- Slit-lamp examination: To exclude any other ocular disorder and to do the TBUT.
- TBUT: Two spots in <10 seconds were considered abnormal.
- Schirmer’s test 1, at least 10 minutes after the other tests: To evaluate basic and reflex tear volume. Patients who had a Schirmer’s test score ≤10 mm/5 minutes were considered to have dry eye.
Both groups were systematically studied using the routine questionnaire, that is, McMonnies (1986) questionnaire, which included the symptoms given in Figure 1.
  | Figure 1 McMonnies (1986) questionnaire. |
A score of over 20 is indicative of dry eye, while a total score between 10 and 20 is suggestive of borderline DED (Figure 1).
Both groups (patients and control) underwent full ophthalmologic evaluations, including visual acuity testing with correction, measurement of IOP by pneumatic tonometry, and slit-lamp biomicroscopic and fundoscopic examinations. The TBUT was performed with a sterile fluorescein strip that was placed in the lower eyelid fornix. The patient was instructed to blink three times and then look straight forward without blinking. The time interval between a complete blink and the first appearance of a dry spot or line in the precorneal tear film was measured under cobalt blue-filtered light. The mean of three consecutive TBUT measurements was used for analysis. Two spots in <10 seconds were considered abnormal.
In Schirmer’s test, the round edge of Schirmer’s strip was placed behind between the outer and middle third of the eyelid. After 10 minutes, the strip was removed and the wet portion of the strip was measured in millimeters. Patients who had a Schirmer’s test score ≤10 mm/5 minutes were considered to have dry eye. During the test, the patient should sit comfortable in a quiet place as much as possible.
CCT was performed using a high-resolution rotating Scheimpflug imaging system (HR Pentacam, Oculus, Germany). Both eyes of all subjects were scanned by the same examiner. The mean of three successive measurements was used for analysis.
Statistical methods
Statistical analysis was performed using Statistical Package for Social Sciences software Windows version 22. Descriptive statistics used the mean and standard deviation, while inertial statistics depended on paired t-test to reveal any significant association. The results were considered significant when the P-value was ≤0.05.
Ethical issue
This study was approved by the Scientific Council of Ophthalmology of Iraqi Board for Medical Specializations. The research followed the tenets of the Declaration of Helsinki, and each patient gave his or her written informed consent to participate in the study.
Results
Two hundred and eighty eyes were included in this study: 140 eyes from 70 patients and 140 eyes from 70 normal individuals in the control group. The female to male ratio was 1.5:1 in each group.
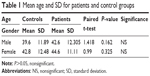
There were no statistical differences regarding age and gender between normal and dry eye patients, as shown in Table 1.
  | Table 1 Mean age and SD for patients and control groups |
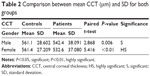
On comparing the CCT between the two groups, there was a highly significant decrease in mean CCT in the patients group especially among females, as shown in Table 2.
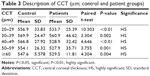
Table 3 shows a significant decrease in the mean CCT (P<0.01) for each age group of patients. The greater decrease was within the age group 40–49 years, in which the CCT was 528.9 μm.
The results of McMonnies questionnaire, the routine questionnaire for patients group, were 81.43% of patients got 10–20 as the score (borderline dry eye or marginal), while 18.57% got >20 as the score (indicative of dry eye).
Discussion
DED is one of the most common eye disorders, which increases with advancing age.25 The symptoms of dry eye are the most important feature of the syndrome for the patients.26
The dry eye is more common in females than in males, as shown in this study in which the number of female patients was 42, as compared to 28 males examined in our hospital (Table 2).
The mean age of patients and control groups was 43.84 versus 41.57, respectively, which is comparable to the mean age reported in similar studies: Sanchis-Gimeno et al27 (42.10 vs 40.15 for patients and controls, respectively), Sanchis-Gimeno et al28 in Spain (33.1 vs 35.9 for patients and controls, respectively).
The objective of this study was to investigate the effect of DED on the CCT by comparing the findings with those of normal age-matched individuals, because of the importance of this parameter in refractive surgery and the precise assessment of IOP in glaucoma patients.
The study showed that the CCT was significantly reduced in dry eyes, compared with normal eyes of age-matched population. The mean CCT in patients group was 536.5 μm, while it was 561.3 μm in the control group, and the difference was about 25 μm. This finding is consistent with two corneal morphometric studies,27,29 which reported that the dry eye can cause significant decrease in CCT values. The first study by Liu and Pflugfelder29 showed that the mean difference with normal eyes was approximately 35 μm; the CCT was 571±28 and 534±34 μm in normal and dry eye, respectively. The second study performed by Sanchis-Gimeno et al27 showed that the CCT in normal eyes was 558±30 μm, while it was 532±34 μm in dry eyes (P<0.001), and the mean difference between the two groups was approximately 26 μm.
Another study by Sanchis-Gimeno et al30 found 14 μm difference in mean CCT in a group of postmenopausal women with dry eye. They found that the mean CCT in dry eye was 533.10±4.74 μm, but it was 547.63±15.11 μm in age-matched control women (P<0.001).
Sanchis-Gimeno et al28 recorded significant decrease in mean CCT of dry eye relative to normal eyes (549±34 vs 527±30 μm) during their research to find out the differences in ocular dimensions between normal and dry eyes.
All the above studies used Orbscan Topography System (slit-scanning corneal topography) for the assessment of the CCT.
In a study done in Germany by Meyer et al31 with the aid of Scheimpflug photography, it was reported that CCT as well as corneal density were significantly reduced in patients with dry eye syndrome compared to the control group (P=0.0495).
Another recent study by Gunes et al32 reported results closer to the findings of our study, with the use of pentacam, on a sample of dry eyes in rheumatoid arthritis patients. The mean CCT was 529±32.8 versus 556±27.5 in dry and normal eyes, respectively.
Pentacam is a noninvasive and objective device that allows extensive evaluation of the corneal structure using a three-dimensional model showing the thickness, volume, and spatial section.22 This system is based on a rotating Scheimpflug camera which scans and measures the complete cornea and anterior chamber parameters in approximately 2 seconds,23 whereas the Orbscan measures the corneal thickness by analyzing the image of the anterior and posterior corneal reflecting surfaces based on slit-scanning technology and videokeratography.33
It was found in our study found that patients within the age group 50–59 years (24 patients [48 eyes]) were more in number than from other age groups. Also, the results showed that the differences in CCT were greater when comparing female patients with control females (532.6 vs 561.4 μm, respectively, Table 2).
The corneal thinning in dry eyes is attributed to an increase in tear film evaporation, or decreased tear turn over, resulting in increased osmolarity of the tear fluid.11,27 This may lead to a decrease in tear film thickness, which normally ranges from 3 to 40 μm.27 As a consequence, hyperosmolarity of tears contributes to the inflammatory cascade, which causes the distressed epithelial cells to produce high levels of the cytokine and matrix metallopeptidase (MMP)-9. In turn, this cytokine exacerbates the inflammatory cycle,11 and the concentration of MMP-9 is directly proportional to the severity of dry eye.34 There is evidence that these inflammatory events lead to apoptotic death of surface epithelial cells of cornea and conjunctiva, including the goblet cells.35 An experimentally induced dry eye model was observed later by another study when the cornea of the dry eye was examined36 in vivo by the use of confocal microscope. Hence, it was suggested that excessive apoptosis or shedding of the surface epithelium, if sustained and not compensated for any epithelial cycling, might lead to epithelial thinning or defects in severe chronic dry eye states.37
Another explanation for corneal thinning in dry eye is that there is an imbalance between MMP-1, which is responsible for the degradation of extracellular matrix in the stroma of cornea, and tissue inhibitors of this MMP. This imbalance occurs due to elevated level of cytokines, which subsequently leads to destructive keratolysis and corneal thinning or even ulceration in severe cases.32,38
This finding encourages many researchers to find out if tear substitute can protect the cornea from this thinning. So, a study in Turkey demonstrated that 1 week treatment with artificial tears can cause increase in CCT. This finding may be useful in dry eye diagnosis and follow-up, suggesting that pachymetry could be included in the routine management of dry eye patients.39
Conclusion and recommendation
Dry eye disease causes significant decrease in CCT. The link between CCT and dry eye should be considered when taking IOP measurements as well as in refractive surgery decision.
The small sample size of this study, especially of those aged more than 60, may lead to bias. Also, further studies are needed to know the effect of artificial tears on the corneal thickness of dry eyes.
Disclosure
The authors report no conflicts of interest in this work.
References
Kaido M, Kawashima M, Ishida R, Tsubota K. Relationship of corneal pain sensitivity with dry eye symptoms in dry eye with short tear break-up time. Invest Ophthalmol Vis Sci. 2016;57(3):914–919. | ||
Patel S, Blades KJ. The Dry Eye, a Practical Approach. China: Elsevier Science; 2003:3. | ||
Bowling B. Dry Eye, Kanski’s Clinical Ophthalmology, A Systematic Approach. 8th ed. China: Elsevier group; 2016:120. | ||
Holly FJ, Lemp MA. Tear physiology and dry eyes; review. Surv Ophthalmol. 1997;22:69. | ||
Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Prog Ret Eye Res. 2004;23(4):449. | ||
Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149. | ||
McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97. | ||
American Academy of Ophthalmology. In: Weisenthal RW, Editor. External Disease and Cornea (Basic & Clinical Science Course). San Francisco: American Academy of Opthalmology; 2014–2015;8(3):72–78. | ||
Dua HS, Faraj LA, Said DG, Gray T, Lowe J. Human Corneal Anatomy Redefined. American Academy of Opthalmology. 2013;120(9):1778–1785. Available from: http://www.aaojournal.org/article/S0161-6420(13)00020-1/abstract. Accessed September 19, 2016. | ||
Lemp MA. Report of the National Eye Institute/Industry Workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232. | ||
2007 report of international dry eye workshop (DEWS). Ocular Surf. 2007;5(2):65–204. | ||
Yanoff M, Duker JS. Ophthalmology, Cornea and Ocular Surface Disease, Dry Eye. China: Elsevier group. 2014:274–276. | ||
Chong EM, Harissi-Dagher M, Dana R. Wetting of the ocular surface and dry eye disorder. In: Albert DM, Miller JW, Azar DT, Blodi BA, Cohan JE, Perkins T, Editors. Albert & Jakobiec’s Principles & Practice of Ophthalmology. Philadelphia: WB Saunders; 2008: 773–778. | ||
Ousler GW, Hagberg KW, Schindelar M, Welch D, Abelson MB. The ocular protection index. Cornea. 2008;27(5):509–513. | ||
Holland EJ, Mannis MJ, Lee WB. Ocular Surface Disease, Cornea, Conjunctiva and Tear Film. China: Elsevier Saunders. 2013;79:80. | ||
Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure: a review and meta-analysis approach. Surv Ophthalmol. 2000;44(5):367–408. | ||
Gordon MO, Beiser JA, Brandt JD, et al; the ocular hypertension treatment study group. The ocular hypertension treatment study; baseline factors that predict the onset of primary open angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. | ||
Brennan NA, Coles ML. Extended wear in perspective. Optom Vis Sci. 1997;74(8):609–623. | ||
Machat JJ, Slade S, Probst LE. The Art of LASIK. 2nd ed. Thorofare, NJ: SLACK Inc.; 1999. | ||
Ambrosio R Jr, Alonso RS, Luz A, Coca Velarde LG. Corneal-thickness spatial profile and corneal-volume distribution: tomographic indices to detect keratoconus. J Cataract Refract Surg. 2006;32(11):1851–1859. | ||
Lackner B, Schmidinger G, Pieh S, Funovics MA, Skorpik C. Repeatability and reproducibility of central corneal thickness measurement with pentacam, orbscan, and ultrasound. Optom Vis Sci. 2005;82(10):892–898. | ||
Rabsilber TM, Khoramnia R, Auffarth GU. Anterior chamber measurement using roating schempflug camera. J Cataract Refract Surg. 2006;32:456–459. | ||
Barkana Y, Gerber Y, Elbaz U, et al. Central corneal thickness measurement with the pentacam Schimpflug system, optical low coherence reflectometry pachymeter, and, u/s pachymetery. J Cataract Refract Surg. 2005;31(9):1729–1735. | ||
Christie C. Marginal dry eye and its management. Optician. 2006;21:231. | ||
Matsuo T, Tsuchida Y, Morimoto N. Trehalose eye drops in the treatment of dry eye syndrome. Ophthalmology. 2002;109(11):2024–2029. | ||
Tu EY, Rheinstorm S. Dry eye. In: Yanoff M, Duker JS. editors. Clinical Ophthalmology. St Louis, MO: Mosby; 2004:520–526. | ||
Sanchis-Gimeno JA, Herrera M, Alonso L, Rahhal MS, Martinez Soriano F. Morphometric differences between normal and dry eyes. Eur J Anat. 2005;9(3):143–148. | ||
Sanchis-Gimeno JA, Herrera M, Sánchez-del-Campo F, Martínez-Soriano F. Differences in ocular dimensions between normal and dry eyes. Surg Radiol Anat. 2006;28(3):267–270. | ||
Liu Z, Pflugfelder SC. Corneal thickness is reduced in dry eye. Cornea. 1999;18(4):403–407. | ||
Sanchis-Gimeno JA, Lleo-Perez A, Alonso L, Rahhal MS, Martinez-Soriano F. Reduced corneal thickness values in postmenopausal women with dry eye. Cornea. 2005;24(1):39–44. | ||
Meyer LM, Kronschläger M, Wegener AR. Schleimpflug photography detects alterations in corneal density and thickness in patients with dry eye disease. Ophthalmologe. 2014;111(10):914–919. | ||
Gunes A, Inal EE, Tok L, Tok O. Evaluation of central and peripheral corneal thicknesses in patients with rheumatoid arthritis. Arq Bras Oftalmol. 2015;78(4):236–240. | ||
Ho T, Cheng AC, Rao SK, Lau S, Leung CK, Lam DS. Central corneal thickness measurements using orbscan II, visante, ultrasound and pentacam pachymetry after laser in situ keratomelusis for myopia. J Cataract Refract Surg. 2007;33(7):1177–1182. | ||
Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203–3209. | ||
Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflug-felder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44(1):124–129. | ||
Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjögren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48(5):2017–2022. | ||
Fabiani C, Barabino S, Rashid S, Dana MR. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res. 2009;89(2):166–171. | ||
Riley GP, Harrall RL, Watson PG, Cawston TE, Hazleman BL. Collagenase (MMP-1) and TIMP-1 in destructive corneal disease associated with rheumatoid arthritis. Eye. 1995;9(pt 6):703–718. | ||
Karadayi K, Ciftci F, Akin T, Bilge AH. Increase in central corneal thickness in dry and normal eyes with application of artificial tears: a new diagnostic and follow-up criterion for dry eye. Ophthalmic Physiol Opt. 2005;25(6):485–491. | ||
McMonnies CW. Key questions in a dry eye history. J Am Optom Assoc. 1986;57:512–517. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.