Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
COPD Management in Community Pharmacy Results in Improved Inhaler Use, Immunization Rate, COPD Action Plan Ownership, COPD Knowledge, and Reductions in Exacerbation Rates
Authors Fathima M, Bawa Z , Mitchell B , Foster J , Armour C, Saini B
Received 27 October 2020
Accepted for publication 25 January 2021
Published 2 March 2021 Volume 2021:16 Pages 519—533
DOI https://doi.org/10.2147/COPD.S288792
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Mariam Fathima,1 Zeeta Bawa,1 Bernadette Mitchell,1 Juliet Foster,1 Carol Armour,1 Bandana Saini1,2
1Woolcock Institute of Medical Research, University of Sydney, Glebe, New South Wales, Australia; 2School of Pharmacy, University of Sydney, Camperdown, New South Wales, Australia
Correspondence: Bandana Saini Rm No. N348, Building No A15
The University of Sydney, Camperdown, New South Wales, 2006, Australia
Tel +61 2 93516789
Fax +61 2 93514791
Email [email protected]
Purpose: To evaluate the effectiveness of a pilot community pharmacy care model for patients with chronic obstructive pulmonary disease (COPD) to improve: 1) inhaler technique; 2) medication adherence; and 3) uptake of non-pharmacological treatment and prevention activities.
Patients and Methods: Forty “host” pharmacies in Sydney were invited to recruit eligible patients and to provide a counselling room/area in their pharmacy for service provision. Eligible patients were referred to two “consultant” pharmacists, specifically trained to deliver a specialized pharmacy COPD service which involved 3 in-pharmacy visits and 2 follow-up phone calls over a 6-month period. The service consisted of 1) inhaler technique assessment; 2) medication adherence assessment; and 3) referrals to the patient’s general practitioner (GP) to facilitate the uptake of non-pharmacological resources as well as to review COPD medications/devices, as required. Pre-post analyses were conducted using paired Student’s t-test and Wilcoxon Signed Rank Test for independent variables and chi-squared tests for proportional data.
Results: Nine “host” pharmacies recruited 40 patients, of whom 37 completed the baseline Visit and 27 completed all Visits. A total of 270 interventions were provided by the “consultant” pharmacists with most provided at Visit 1 (176). The most common interventions were addressing patient gaps in COPD knowledge and inhaler technique. A total of 119 referrals were made to GPs for various reasons, the most common being for a COPD action plan, pulmonary rehabilitation, or pneumonia vaccination. There were significant improvements pre-post intervention in inhaler use competence, COPD knowledge, immunization rate for pneumonia, exacerbation rate and COPD plan ownership.
Conclusion: In this pilot study, the specialized pharmacy-based COPD care model delivered by “consultant” pharmacists in community pharmacies provided significant health benefits for patients. Further research is needed to assess the model’s effectiveness in a larger population as well as when measured against standard care.
Keywords: inhaler technique, medication review, consultant pharmacist, COPD
Plain Language Summary
Chronic obstructive pulmonary disease (COPD) is an umbrella term that includes emphysema and chronic bronchitis. COPD is more common in older people and those with a history of smoking or exposure to lung irritants. The increasing breathlessness experienced by people living with untreated COPD has a negative impact on health outcomes and quality of life while increasing the risk for hospitalizations and premature death. There are many effective interventions for COPD that can be tailored to individual patient needs as follows:
- Inhaled medications
- Inhaler device technique training
- Smoking cessation
- COPD action plan to help recognise triggers, worsening symptoms and what to do to minimize risk of exacerbations and premature death
- Pulmonary rehabilitation (PR) program which involves assessment, education, and appropriate exercise training to suit individual patient needs
Community pharmacies are well placed to provide medication reviews and inhaler technique training as well as to support general practitioners (GPs) by referring and following up patients for COPD action plans and PR. In this study, the researchers demonstrated that a COPD service delivered by pharmacists provided education, medication counselling and inhaler technique assessment as well as continuity of care by regular follow-up to ensure that those living with COPD can access optimal treatment and available resources.
Introduction
Chronic obstructive pulmonary disease (COPD) is estimated to affect 7.5% of Australians aged ≥40 years and 29.2% aged ≥75 years.1 COPD is the fifth leading cause of death in Australia,2 with a hospitalization rate of 732 per 100,000 in those aged 45 and over.3 The financial impact of COPD in Australia was calculated to be in excess of $8 billion annually including direct health care costs of almost $1 billion,3,4 and indirect costs of more than $7 billion from lost productivity, welfare payments and home modifications.4,5
COPD exacerbations can be defined as “a sudden change from baseline in the patient’s key COPD symptoms (eg, dyspnoea, cough or sputum levels) that is beyond normal diurnal variations and warrants medication change and/or hospital admission”.6 Risk factors for such exacerbations include smoking, influenza or pneumococcal infection, malnutrition or obesity, seasonal changes, and comorbidities.6,7 COPD has a negative impact on quality of life, including sleep interruption and reduced ability to exercise, as well as causing significant pain and distress.3 Early interventions can significantly reduce exacerbations, improve quality of life, and reduce costs for the individual, the community, and the health care system.6,8
The management of COPD is complex and requires a combination of pharmacological and non-pharmacological interventions. Pharmacological treatments for COPD typically consist of bronchodilators and corticosteroids administered directly into the lungs via inhaler devices. Previous research has indicated that 60% of COPD patients do not take their medications as prescribed which can lead to adverse sequelae for patients.9–11 A systematic review, which measured retrospective prescription refill, found increased hospitalizations, mortality, poor quality of life and loss of productivity among patients who were non-adherent to their medication.12 Contributing factors to non-adherence include the inhaler device used, prescribed dosing schedule, routinization of drug therapy, knowledge and beliefs about the treatment, comorbidities, individual patient characteristics, patient preference, and patient–clinician relationship.13 Since the severity of COPD increases with age, non-adherence is compounded by further difficulties caused by visual impairment, cognitive decline, arthritis, poor coordination, and the weakening of the inspiratory muscle function.14
People with COPD often have significant comorbidities, such as cardiovascular disease, diabetes, obesity, depression, gastro-oesophageal reflux disease, osteoporosis, and lung cancer.15–22 Multiple medications for comorbidities increase the risk of side effects, duplication of therapy, underuse/overuse, and cumulative medication burden.18 Non-adherence in patients with COPD is frequently unintentional23 and can be improved by making changes that simplify their treatment regimen to better manage medications for multiple comorbidities as well as providing advice about minimising common adverse effects.24–27
Previous research has shown that competence of inhaler use in COPD patients was only 31%,28 and the proportion who made at least one error ranged from 50% to 83%, depending on the device used.29 This compromises both drug deposition in the lungs and the resulting clinical outcomes as well as creating additional costs for the individual and the health care system through medication wastage.30 There are a wide range of devices available, which can make the choice of optimal inhaler device for each patient challenging, many of whom are using several different inhaler devices.31 Changing multiple inhalers to a combined dosage device,25 or those that require similar usage technique,26 has been shown to improve not only competence in using the inhaler devices but also adherence.
Evidence suggests that long-term management of people with COPD is suboptimal,3,6 not only due to non-adherence but also to low patient uptake of non-pharmacological evidence-based COPD management strategies,3,32,33 including smoking cessation,34 COPD action plan,3,6 and pulmonary rehabilitation (PR).35,36 With the increasing burden of COPD in Australia, integration of other health care professionals to support primary care37–39 may facilitate increased uptake of these non-pharmacological treatments. In addition, community pharmacists are well placed as medication experts to provide professional advice and information on a patient’s medications as well as general health-related education and counselling. Their services are highly accessible and often free of charge to consumers without the need to make an appointment.40 Research demonstrates that pharmacists are increasingly contributing to chronic disease management through the provision of health promotion, risk assessment and early intervention, medication management, ongoing treatment and monitoring programs to improve patient self-care, disease outcomes, quality of life, and patient satisfaction,41–44 many of which have been found to be cost-effective.45,46 Pharmacist delivered interventions focused on correcting inhaler technique and minimizing non-adherence may offer potential for significant improvements in clinical outcomes and quality of life for people with COPD.47,48 Indeed, Australian community pharmacy asthma management programs that utilize these interventions have consistently been shown to significantly improve patient outcomes in robust trials.49,50 However, pharmacist delivered management models for COPD that incorporate these interventions have not yet been tested in Australia.
The primary aim of the study, therefore, was to pilot test the efficacy of a community pharmacist delivered COPD care model to improve: 1) patient competency in using their inhaler(s); 2) adherence to their medications; and 3) uptake of other guideline-based non-pharmacological interventions and prevention activities.
Patients and Methods
Ethics Approval
The study received ethics approval from the University of Sydney’s Human Research Ethics Committee (HREC#2014/495). This study was conducted in accordance with the Declaration of Helsinki.51
Study Design
A 6-month pre-post pilot study was conducted in community pharmacies in metropolitan Sydney and consisted of locum “consultant” pharmacists trained to provide the service in a range of “host” pharmacies; a model currently employed for the delivery of Home Medicine Reviews in Australia. Assuming that 50% of recruited COPD patients use their inhalers correctly and that the intervention would enhance this to at least 80% (based on effect sizes reported in previous reviews/pharmacy studies52,53) as well as using a significance level of 0.05, a power of 90%, and assuming a 20% dropout rate, at least 25 patients were required to participate. Given previous studies with pharmacy asthma services suggested that a 3–5 patient per pharmacy recruitment rate was achievable, sampling strategies included recruiting at 10 “host” pharmacies with 2 “consultant” pharmacists providing the service.
Based on previous asthma studies41,49,50 and the COPD guidelines,6 a comprehensive COPD management model was developed and initially pre-tested with five practising community pharmacists, who had prior experience in asthma and COPD pharmacy-based practice research. Based on their feedback, the study protocol was streamlined to focus on the most effective interventions targeting key aspects of COPD care that would be feasible in a community pharmacy setting.
Outcome Measures
The primary outcome measure was the change in mean percentage of correct inhaler technique steps performed by patients from baseline to study end. Secondary outcomes included: medication adherence based on 6-month dispensing history as measured by Medication Refill Adherence (MRA) and the Foster score adherence questionnaire;54 quality of life using the COPD Assessment Test (CAT);55 breathlessness using the modified Medical Research Council (mMRC) dyspnoea scale;56 current smoking status using the Heaviness of Smoking Index Score (HSI);57 COPD knowledge using the COPD knowledge questionnaire;58 as well as self-reported COPD action plan ownership; attendance at a PR clinic; vaccination status; and exacerbation frequency requiring GP/hospital visits in the past 6 months.
Recruitment of Participants
“Host” Pharmacy Recruitment
Pharmacies that were willing to “host” the delivery of the COPD management service via a visiting “consultant” pharmacist were recruited using researchers’ professional networks in the Sydney region (New South Wales, Australia). Eligibility criteria for “host” pharmacies were: providing informed consent; having a private counselling area/room available for the “consultant” pharmacist to conduct patient visits; and providing relevant consented patient data for the “consultant” pharmacist to access using a secure, password-protected study database. Participating “host” pharmacies sent introductory letters to their local GPs to inform them about the service.
“Consultant” Pharmacist Recruitment
Two “consultant” pharmacists were recruited by the research team based on their practice experience and interest in COPD. Both were currently practicing clinical pharmacists who were not linked with any academic role or faculty position. They completed the online COPD pharmacy training module offered by the Lung Foundation Australia,59 which consists of COPD pathophysiology and management. A pass mark of 80% or more was required to complete the module successfully. To consolidate key learnings and deliver the interventions according to the study protocol, face-to-face training was also provided by COPD experts and included interactive activities, such as case studies and assessment of pharmacists’ inhaler demonstration technique as per the COPD management kits provided. After completing the training module, “consultant” pharmacists were provided with a suite of resources including patient information hand-outs, referral letter templates and a full set of all available inhaler devices (containing placebo only).
Patient Recruitment
The participating “host” pharmacies were provided with promotional material to advertise the service, including study posters and shelf “wobblers” (tags that hang from pharmacy shelves and move to attract customer attention). They also identified potential participants by making a list of patients taking COPD medications from their pharmacy dispensing records. Inclusion criteria for patients were: having an established COPD diagnosis; aged between 40 and 80 years; taking ≥5 medications or ≥12 doses/day of all medications combined; registered with a GP local to the “host” pharmacy; and competency in the English language [Note: taking ≥5 medications daily (ie using polypharmacy)] was set to match the Australian Home Medicine Review service eligibility criteria.60 Further, evidence suggests that this cut-off point of consuming ≥5 medications is associated with the risk of adverse sequelae such as falls, frailty, disability, and mortality in older adults, and hence presents a valid target for medication review provision.61 Exclusion criteria included having a cognitive impairment; diagnosis of cancer; or any other illness that may have hindered participation. Study information was provided and, if a patient agreed to participate, they gave their informed signed consent for their details to be forwarded to the “consultant” pharmacist who then made the appointment for the baseline visit. At this point, pre-appointment instructions were provided by the “consultant” pharmacist to the consented patient to request that they bring all their COPD medications/inhalers to the appointment (including any self-prescribed complementary medications), as well as to check beforehand the name of the patient’s regular GP and current postal area code (so that local COPD relevant resources could be identified by the “consultant” pharmacist in advance of the appointment).
Service Model Interventions
The 6-month service consisted of 3 in-pharmacy visits (baseline, 3- and 6-months post-baseline) and 2 follow-up telephone calls (2- and 6-weeks post-baseline) between the “consultant” pharmacist and the patient. A comprehensive Patient Record File (PRF) was used for each patient. The PRF used standard operating procedures and clinical algorithms to enable uniform data collection and standard intervention delivery. In the PRF, all patient consent forms, completed patient COPD assessment instruments, interventions provided (by ticking pre-prepared checklists), and referrals made were recorded. Following the sequence of the PRF served to prompt standardised clinical information gathering, decision-making and recording by the two “consultant” pharmacist service providers.
At Visit 1 (Baseline), the “consultant” pharmacist recorded the patient’s demographic details, smoking status, vaccination status, PR attendance, medical and medication profile. They also assessed inhaler device technique and medication adherence, as well as recorded self-reported baseline measures for COPD action plan ownership, exacerbation history and patient use of health care services related to their COPD over the preceding 6 months. Based on the needs assessment of each patient, the “consultant” pharmacist provided a medication review (Figure 1A) and individualized COPD management interventions (Figure 1B).
 |
Figure 1 Assessment of patient needs and provision of COPD interventions: (A), pharmacological; and (B), non-pharmacological. Shaded boxes highlight the key clinical assessments conducted by the “consultant” pharmacists. The unshaded boxes to the right of the arrows provide a summarized version of interventions provided to address key issues that became apparent through the systematic assessment. PR=Pulmonary Rehabilitation. Abbreviations: GP, general practitioner; COPD, chronic obstructive pulmonary disease. Notes: *Foster JM, Smith L, Bosnic-Anticevich SZ et al. Identifying patient-specific beliefs and behaviours for conversations about adherence in asthma. Intern Med J. 2012;42(6):136–144.54 #Maples P, Franks A, Ray S, Stevens AB, Wallace LS. Development and validation of a low-literacy Chronic Obstructive Pulmonary Disease Knowledge Questionnaire (COPD-Q). Patient Educ Couns. 2010;81(1):19–22.58 |
1A: Medication Review
The patient’s inhaler technique was assessed by the “consultant” pharmacist by having the patient demonstrate the use of their own inhaler(s) on the day of the first appointment (Visit 1). The technique was scored using the inhaler device checklists developed by the National Asthma Council.62 Any patient scoring less than 100% was deemed as requiring an intervention in their inhaler device use, which involved the “consultant” pharmacist demonstrating the correct technique on a placebo device and highlighting where the patient had made errors (Figure 2). The patient then repeated the inhaler technique assessment incorporating adjustments to correct any errors. This process was continued at this initial visit until the patient achieved competency or had a maximum of 3 demonstrations. An instruction label was stuck on each of the patients’ inhalers (on the body of the device) or provided to the patients if they did not bring their inhalers to the appointment.63–65 These labels highlighted the steps that had been problematic for the patient to serve as a reminder during subsequent use. Patients who failed to demonstrate optimal technique after 3 demonstrations were provided with a GP referral for device change consideration.
 |
Figure 2 Inhaler technique assessment and education. This figure highlights the evidence-based three-step “show and tell” sequential process followed to assess and train patients at the baseline visit and, if required, at follow-up face-to-face visits. Based on the results published by: Basheti IA, Armour CL, Bosnic-Anticevich SZ, Reddel HK. Evaluation of a novel educational strategy, including inhaler-based reminder labels, to improve asthma inhaler technique. Patient Educ Couns 2008;72:26–33.85 |
All medication regimens were checked for drug interactions, adverse drug reactions and any under/over/non-treatment or duplication of therapy based on patient self-report as well as the patient’s previous 6-month dispensing history. Any issues requiring medication changes were referred to the patient’s GP. Patient adherence to their COPD medications was measured using the Foster score,54 and the MRA to assess patient’s adherence recall and behaviour, respectively. The MRA was calculated by dividing the total days’ supply on the dispensed history by 180 days (6 months) and multiplying by 100 to provide a percentage adherence value at baseline and at study end.66 For patients taking more than one COPD medication, a composite average medication adherence percentage was calculated,67 e.g., for a patient using 3 inhalers with 60%, 50% and 100% MRA, their composite average MRA would be 70%. Patients with a composite average MRA of ≤80% were considered non-adherent,68,69 and >100% were considered as overusing their medications and were provided with an adherence intervention.
The Brief Medication Questionnaire (BMQ)70 was utilized to identify barriers to medication use. The BMQ includes a 5-item Regimen Screen that asks patients how they took each medication in the past week, a 2-item Belief Screen that asks about drug effects and bothersome features, and a 2-item Recall Screen about potential difficulties remembering to take their medications. Barriers to non-adherence were addressed by interventions that provided practical strategies and motivational support, such as agenda-setting and addressing readiness to change.71
1B: COPD Non-Pharmacological Interventions
Patients’ COPD knowledge was assessed using a validated COPD knowledge questionnaire,58 and information was provided by the “consultant” pharmacist to address incorrect responses. Patients were also provided with written information about COPD and its management,72 as well as about local support groups for COPD patients to access emotional support and self-management strategies.
The number of exacerbations over the previous 6-months and the type of treatment required were recorded for referral purposes in those who did not have a COPD action plan. Information was provided by the “consultant” pharmacist on how to use the COPD action plan to identify worsening symptoms that may trigger an exacerbation when this was obtained post referral from their GP (Note: an exacerbation was defined as deteriorating symptoms requiring the patient to visit the GP or the hospital for treatment).
For current smokers, the level of nicotine dependence was assessed and a specialized smoking cessation support intervention, using clinical algorithms that included a smoking cessation action plan together with a referral to the GP for smoking cessation medication,73 were provided where appropriate and if acceptable to the patient.
Referrals to the patient’s GP were given for those who: 1) required a medication review; 2) did not have a COPD action plan; 3) had not previously attended a PR clinic; and 4) had overdue vaccinations for influenza and pneumonia. The GP referral provided by the “consultant” pharmacist to the patient had information on the reason for the referral, patients’ medication adherence status, the mMRC dyspnoea score,56 along with relevant areas highlighted for GP action.
Two telephone calls were made by the “consultant” pharmacists to the patients at 2 and 6 weeks to follow-up on any GP referrals provided at Visit 1. In addition, motivational smoking cessation advice and information about the next visit were also provided, as appropriate. Visits 2 and 3 were conducted at 3 and 6 months, respectively. Patients received targeted counselling based on follow-up of the issues raised at Visit 1 and outcome of any GP referrals. Typical topics discussed included inhaler technique, COPD action plan ownership, smoking status, and issues with any newly prescribed medication since the previous visit. The “consultant” pharmacists also provided medication adherence support, discussed potential or actual drug-related problems, and prompted the patient for PR attendance and immunization, if required. At Visit 3, in addition to discussing the issues raised at Visit 2, the patient completed the same set of questionnaires as at Visit 1 to obtain post-intervention scores for the outcome variables.
Data Collection and Analysis
All data were recorded on the custom-designed PRF and the Statistical Package for the Social Sciences (SPSS-V24)74 was used for data analyses. Descriptive and relational analyses were conducted with reference to data normality. For process evaluations, descriptive statistics such as mean, standard deviation, median and range were calculated. For all key reported outcome variables, normality tests were conducted using the Kolmogorov–Smirnov test. For normally distributed variables, pre-post comparisons were conducted using the paired Student’s t-test for two variables. For variables that were not normally distributed, the Wilcoxon Signed Rank Test was used to determine differences. Proportional data were analysed using the Chi-squared test for nominal variables. A 2-tailed 5% (0.05) level of significance was used for all statistical procedures.
Results
Forty community pharmacies were approached to “host” the COPD service and of these 17 consented to participate and 9 recruited participants. A total of 145 patients with COPD were identified from dispensing records as eligible and 40 patients consented, of which 37 attended Visit 1; 31 attended Visit 2; and 27 attended Visit 3. The reasons given for non-attendance included being unavailable, hospitalized, or moved to another area. Recruitment occurred from April to July 2018 and the service provision was completed between November 2018 and February 2019.
At baseline, patients had a mean age of 69 (51–87) years, and 51% were male. The mean body mass index was 26.4 ± 6.7 (18–51), which suggests that our sample was slightly overweight. A majority (70%) of patients reported being retired while 27% stated they were working. Most patients (89%) reported a smoking history of which 19% were current smokers, and one patient reported previous occupational exposure to cleaning products. A majority of patients were on medications for other chronic illnesses, including hypercholesterolemia (95%); hypertension or a heart condition (70%); anxiety, depression or insomnia (26%). The mean mMRC dyspnoea scores indicated a range of COPD severity among participants from “mild” to “very severe” and 58% reported one or more COPD exacerbations in the 6 months prior to the study (Table 1). COPD knowledge scores were suboptimal, with a mean score of 70% (Table 1). Only 5% had a COPD action plan, and only 27% had attended PR prior to the study (Table 1).
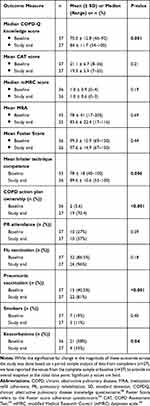 |
Table 1 Comparison of Outcome Measures at Baseline and Study End |
During the study, a total of 270 interventions were provided by the “consultant” pharmacists and the greatest number was at Visit 1 (176), with the most common being for addressing patient gaps in COPD knowledge and suboptimal inhaler technique. There was a total of 119 referrals made to GPs, with the greatest number (104 referrals) provided at Visit 1, the most common being for a COPD action plan, PR, or pneumonia vaccination.
Primary Outcome – Inhaler Technique
There were a wide range of inhaler devices used by patients and these are shown in Table 2. At Visit 1, a total of 72 demonstrations were provided by the pharmacists to 35 patients using 58 inhalers. Assessment of inhaler technique indicated a range of errors with the mean proportion of “correctly demonstrated steps” across the different inhalers ranging between 55% and 97% (Table 2). This resulted in 6 GP referrals for inhaler device change and 45 inhaler instruction labels to be stuck onto devices. The most common problematic steps were insufficient/inefficient exhaling to residual lung capacity prior to use and the inability to hold the breath for the recommended amount of time after using the inhaler.
 |
Table 2 Inhaler Technique – Mean Number, Range and Percentage of Correct Steps at Each Visit |
The mean percentage of correct steps combined across all inhalers significantly increased from 78% at baseline to 93.5% at Visit 2 and 89.6% at Visit 3. Although this improvement was greatest between Visits 1 and 2 and then declined slightly between Visits 2 and 3, this was still a highly significant improvement from baseline to study end (p=0.006; Table 1). A majority (88%) of patients were on 2 or more inhalers (range 1–4) at baseline with the most commonly used inhalers being Ellipta® (34%), Respimat® (31%) and Handihaler® (28%). The most commonly used devices (Ellipta® and Respimat®) remained unchanged at study end however the Turbuhaler® (27%) replaced the Handihaler® for third place towards the end of the study (Table 2). Two of the least commonly used devices at baseline also showed the highest percentage of correct steps at baseline, ie, Breezhaler® (97%) and the pMDI+Spacer (93%); however, the numbers are too small to make any valid comparisons (Table 2).
Secondary Outcome – Medication Adherence
Based on the composite average MRA cut-off ≤80%, a majority of patients at baseline were non-adherent (68%), of which 48% were underusing and 20% were overusing their COPD medications. There were no particular patterns around overuse/underuse related to medication class. Reasons for non-adherence included both intentional and non-intentional ones. For example, of those non-adherent, key reasons underpinning non-adherence included: forgetting to take their medication (3%); concerns about safety issues and side effects such as bad aftertaste (20%); doubts about efficacy (8%); taking multiple medications/needing medication review (40%); and problems getting repeats filled on time (30%). Strategies to address these issues were discussed with the patient, such as asking for family/carer support, using inhalers together (with a short break in between), changing to combination therapy in a single inhaler, inhaler technique demonstration by the “consultant” pharmacist, and rinsing the mouth after use to avoid side effects.
Seventeen patients were referred to their GP for a medication review at Visit 1. Nine of these patients, for whom dispensed history data was available at Visit 2, were prescribed a different COPD medication after baseline, and at Visit 3, this had increased to thirteen patients. Reasons for a change in COPD medication as a result of referral included inadequate symptom control, GP recommendation, easier to use, medication overuse, patient’s preference, improve adherence, decrease confusion based on medication duplication, new diagnosis, and general health.
The mean adherence based on MRA at study end for n=25 patients had increased from 78% at baseline to 83.6% at study end, while the self-reported mean adherence of 99.3% at baseline also changed to 97.6% at study end, however, these changes were not significant (Table 1).
Secondary Outcome – Non-Pharmacological
The assessment of COPD knowledge and its medications was measured as the mean percentage of correct answers and showed a significant improvement at study end compared with baseline (Table 1; p=0.001). The percentage of patients reporting exacerbations at Visit 1 was 58%, with 33% patients experiencing at least one exacerbation, 19% experiencing 2 exacerbations, and 5% experiencing 3 exacerbations, in the 6 months prior to baseline. There was a significant reduction in the number of exacerbations reported by patients between baseline and Visit 3 (Table 1; p=0.04). Only 5.6% patients reported possessing a COPD action plan at baseline which resulted in 34 GP referrals to initiate a COPD action plan and this led to a highly significant increase in COPD action plan ownership to 70% at study end (Table 1; p<0.001). Of the 8 patients who did not obtain a COPD action plan, 5 forgot to ask the doctor, 2 had lost it, and 1 said that the doctor did not think it was necessary as he saw the patient regularly. At baseline, 7 GP referrals for flu vaccination and 22 for pneumonia vaccination were provided. At study end, the level of immunization had significantly improved from baseline for pneumonia vaccination (Table 1; p<0.001), and although flu vaccination rate also improved this was not significant (Table 1).
The mean health-related quality of life as measured by the CAT test improved from the severe range (20–30) at baseline to the moderate range (10–20)55 at study end; however, this was not significant (Table 1). There was no difference in the median mMRC score between baseline and study end on a group level (Table 1).
A total of 10 patients reported having attended PR in the 6 months prior to baseline. Twenty-two patients were provided with PR information and referrals to be completed by their GP. By Visit 2, the patients’ GPs had completed the PR referral form for 13 patients but of these only 4 patients had attended PR by study end and this increase was not significant (Table 1). The main reasons for non-attendance included patient not interested or GP did not think it was a priority.
Seven patients were current smokers at baseline and, four of these agreed to be provided with a smoking cessation action plan and an over the counter nicotine replacement therapy. The other 3 were either not interested or opted to discuss it at a later visit. Two patients were referred to the GP for further assessment. Although there was an improvement in smoking cessation rate at study end, this was not significant (Table 1).
Discussion
This is the first study to evaluate a model using specialized “consultant” pharmacists to deliver a comprehensive COPD management service in “host” community pharmacies. The results from this study showed that the interventions delivered by the “consultant” pharmacist led to significant improvements in patient’s inhaler technique, COPD exacerbation rate, COPD knowledge, COPD action plan ownership, and immunization rate for pneumonia. In addition, there were improvements in sub-group dyspnoea levels, smoking cessation, flu vaccination, PR attendance, medication adherence and quality of life; however, these were not statistically significant.
“Consultant” pharmacists are available for designated time periods to deliver professional services without interruption and, while “host” pharmacies are ideally placed to identify and recruit patients with COPD from their customer base, they often find it difficult to find the time required for service delivery, primarily because their existing operational model is based around dispensing activities.75,76 Furthermore, previous services that utilized this model demonstrated that “consultant” pharmacists can facilitate the delivery of a higher number of interventions in a shorter period of time as well as achieving better patient outcomes compared to other similar studies conducted in primary care.36,77
The main outcome measure was inhaler technique improvement. The inhaler technique intervention, which consisted of provision of oral and written education along with a physical re-iterative demonstration between the patient and the “consultant” pharmacist until competence was achieved, has been successfully used previously to improve inhaler technique for asthma patients in both Australia and overseas.63–65 The improvement in inhaler technique was greatest between Visit 1 and Visit 2 but declined slightly at the study end, which supports the suggestion that inhaler technique declines over time due to patients forgetting the instructions and, therefore, education needs to be reinforced by regular follow-up visits.64,65 Furthermore, patients trained in inhaler technique and followed up for over three months have been shown to experience lower exacerbations compared to the control group,78 and this is consistent with the significant reduction in the exacerbation rate evident in this study. In addition, the significant increases in COPD knowledge and action plan ownership, both of which contain substantial components on how to reduce exacerbations, taken together with improved competency in inhaler technique would be expected to empower the patient to more effectively manage their COPD.
The significantly higher rate of pneumococcal vaccination observed in this study is consistent with other interventions which have shown improved vaccination rates.79 Although there was also a trend in the increase in influenza vaccination rate at study end, the fact that a high percentage of patients were already vaccinated prior to baseline may reflect the usual scope of practice of community pharmacists to provide or refer for influenza vaccinations every year and therefore allowed little room for improvement. The study also showed improvements in the quality of life score for COPD patients at study end and although this was not significant, it is encouraging that the mean score moved from the severe range to the moderate range of the scale.55 There was low uptake of PR in this study, and one reason for this may have been due to the complex GP referral pathway required by the PR centres.36 In addition, PR centres are mainly based in hospitals which may limit accessibility for some people. Low PR uptake is a universally observed issue worldwide in COPD management.80
The results of this study have demonstrated that this COPD management service delivered by trained community pharmacists has the potential to improve the health of people living with COPD by addressing errors in inhaler technique, reviewing medications and facilitating the uptake of non-pharmacological interventions currently available in primary care. Previous pharmacist delivered COPD models in overseas settings have proved to be cost-effective81,82 and, although a cost-effectiveness analysis was not conducted in this study, it is proposed that the increased visits to the GP as a result of the referrals would have been more than offset by the cost savings from reduced exacerbations together with less wastage of medicines, through the capacity of improved inhaler technique to optimise medication delivery for patients.
The integration of other health professionals has previously been shown to be complementary to the care provided by GPs and to enhance the capacity of primary care to improve outcomes for people living with COPD.83 In this study, a community pharmacy-based COPD service model, utilising “host” pharmacists to identify and recruit eligible patients and trained “consultant” pharmacists to deliver the service, has been shown to play a significant role in providing education, reviewing inhaler technique and medications as well as promoting PR uptake, improving vaccination status and smoking cessation.
Our study had some limitations. Firstly, it was a pilot study with a small sample of patients based on a before-and-after design with no control group for comparison. Secondly, patients received the initial intervention mainly during the winter months compared with the study end period which occurred during the summer months. Since COPD exacerbations can be triggered by seasonal changes such as cold weather,84 the sustainability of the service across seasons would need to be addressed. Long-term, randomised-controlled trials are therefore needed in the future to confirm whether this pharmacist-led, patient needs-based COPD management service model is suitable for wider implementation.
Conclusion
Given the burden of COPD on the individual and the health care system, new models of care that utilize a multidisciplinary approach are needed to incorporate support and expertize for enhancing primary care. Pharmacists are well placed to have a positive impact on the health of people with COPD both as medication experts to assist with adherence and inhaler technique, as well as to refer for non-pharmacological interventions such as smoking cessation, PR, and COPD action plan ownership. This pilot study has shown that a “consultant” pharmacist model offers the potential to significantly improve health outcomes for people living with COPD. Further research is needed to re-test this model using a stakeholder co-developed implementation based hybrid design, where the intervention clinical effect, cost-effectiveness and implementation strategies can be tested at the same time.
Abbreviations
BMQ, brief medication questionnaire; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; GP, general practitioner; HSI, heaviness of smoking index; mMRC, modified Medical Research Council; MRA, medication refill adherence; PR, pulmonary rehabilitation; PRF, patient record file; SPSS, Statistical Package for the Social Sciences.
Ethics Approval and Informed Consent
The study received ethics approval from the University of Sydney’s Human Research Ethics Committee (HREC#2014/495). Study information was provided to all participants and, if they agreed to participate, their informed signed consent was obtained before proceeding.
Consent for Publication
All authors have provided their consent for publication of the final text in this manuscript.
Acknowledgments
Woolcock Emphysema Centre and all the participating pharmacies.
Author Contributions
MF was responsible for the study conception and design, acquisition, analysis, and interpretation of data. CA, BS and JMF also contributed to the study conception and design, the initial interpretation of findings, and critical revision of the manuscript. ZB performed the service with the pharmacists and interpreted the data. BM was involved in data interpretation and the first draft of the manuscript. All authors contributed to and have approved the final text. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Woolcock Emphysema Centre Seed Funding.
Disclosure
Dr Bernadette Mitchell reports grants from Woolcock Emphysema Centre, during the conduct of the study.
Dr Juliet Foster reports grants, personal fees, and non-financial support outside the submitted work from Astra Zeneca and Boehringer Ingelheim for independent medical education, and also a speaker fee from GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. Toelle B, Xuan W, Bird T, et al. Respiratory symptoms and illness in older Australians: the burden of obstructive lung disease (BOLD) study. Med J Aust. 2013;198(3):144–148. doi:10.5694/mja11.11640
2. National Health Survey: First Results, 2017-18. Australian Bureau of Statistics (ABS) 2018. CAT no. 4364. 0.55.001.Canberra.
3. Australian Institute of Health and Welfare 2019. Chronic obstructive pulmonary disease (COPD). Cat. no. ACM 35. Canberra: AIHW. Available from: https://www.aihw.gov.au/reports/chronic-respiratoryconditions/copd.
4. Economic impact of COPD and cost-effective solutions. Access Economics Pty Ltd for the Australian Lung Foundation; 2008. Available from: http://www.rnig.org.au/docs/EconomicImpactofCOPDandCostEffectiveSolutions-226.pdf.
5. Better outcomes for people with chronic and complex health conditions. Primary Health Care Advisory Group Final Report (2015). Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/76B2BDC12AE54540CA257F72001102B9/%24File/Primary-Health-Care-Advisory-Group_Final-Report.pdf.
6. Yang IA, Brown JL, George J, et al. The COPD-X Plan: Australian and New Zealand Guidelines for the management of chronic obstructive pulmonary disease. Version 2.61; 2018. Available from: http://copdx.org.au/copd-x-plan/.
7. COPD Factsheet. Lung Foundation Australia. Available from: https://lungfoundation.com.au/wp-content/uploads/2018/09/Factsheet-COPD-Sept2018.pdf.
8. Global initiative for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.1wms.pdf.
9. Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacol Epidemiol Drug Saf. 2007;16(4):441–448. doi:10.1002/pds.1321
10. Cecere LM, Slatore CG, Uman JE, et al. Adherence to long-acting inhaled therapies among patients with chronic obstructive pulmonary disease (COPD). COPD. 2012;9(3):251–258. doi:10.3109/15412555.2011.650241
11. Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with tiotropium and fluticasone propionate/salmeterol in chronic obstructive pulmonary diseases patients. Curr Med Res Opin. 2014;30(7):1427–1436. doi:10.1185/03007995.2014.908828
12. Van Boven JF, Chavannes NH, Van der Molen T, Rutten-van Molken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103–113. doi:10.1016/j.rmed.2013.08.044
13. Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365–1373. doi:10.1007/s00228-012-1279-5
14. Hughes CM. Medication non-adherence in the elderly – how big is the problem? Drugs Aging. 2004;21(12):793–811. doi:10.2165/00002512-200421120-00004
15. Calverley PM, Anderson JA, Celli B, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010;65(8):719–725. doi:10.1136/thx.2010.136077
16. Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi:10.1136/thx.2009.128082
17. Gershon AS, Mecredy GC, Guan J, Victor JC, Goldstein R, To T. Quantifying comorbidity in individuals with COPD: a population study. Eur Respir J. 2015;45(1):51–59. doi:10.1183/09031936.00061414
18. Hanlon P, Nicholl BI, Jani BD, et al. Examining patterns of multimorbidity, polypharmacy and risk of adverse drug reactions in chronic obstructive pulmonary disease: a cross-sectional UK Biobank study. BMJ Open. 2018;8(1):e018404. doi:10.1136/bmjopen-2017-018404
19. Ogale SS, Lee TA, Au DH, Boudreau DM, Sullivan SD. Cardiovascular events associated with ipratropium bromide in COPD. Chest. 2010;137(1):13–19. doi:10.1378/chest.08-2367
20. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
21. Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):506–512. doi:10.1513/pats.200707-096ET
22. Van der Molen T. Co-morbidities of COPD in primary care: frequency, relation to COPD, and treatment consequences. Prim Care Respir J. 2010;19(4):326–334. doi:10.4104/pcrj.2010.00053
23. Plaza V, Lopez-Vina A, Entrenas LM, et al. Differences in adherence and non-adherence behaviour patterns to inhaler devices between COPD and asthma patients. COPD. 2016;13(5):547–554. doi:10.3109/15412555.2015.1118449
24. Elliott RA, Boyd MJ, Salema NE, et al. Supporting adherence for people starting a new medication for a long-term condition through community pharmacies: a pragmatic randomised controlled trial of the New Medicine Service. BMJ Qual Saf. 2016;25(10):747–758. doi:10.1136/bmjqs-2015-004400
25. Yu AP, Guérin A, Ponce de Leon D. Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14(4):486–496. doi:10.3111/13696998.2011.594123
26. Bosnic-Anticevich S, Chrystyn H, Costello R, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis. 2016;12:59–71. doi:10.2147/COPD.S117196
27. Price D, Lee AJ, Sims EJ, et al. Characteristics of patients preferring once-daily controller therapy for asthma and COPD: a retrospective cohort study. Prim Care Respir J. 2013;22(2):161–168. doi:10.4104/pcrj.2013.00017
28. Sanchis J, Gich I, Pedersen S, et al. Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150(2):394–406. doi:10.1016/j.chest.2016.03.041
29. Sriram KB, Percival M. Suboptimal inhaler medication adherence and incorrect technique are common among chronic obstructive pulmonary disease patients. Chron Respir Dis. 2016;13(1):13–22. doi:10.1177/1479972315606313
30. Lewis A, Torvinen S, Dekhuijzen PN, et al. The economic burden of asthma and chronic obstructive pulmonary disease and the impact of poor inhalation technique with commonly prescribed dry powder inhalers in three European countries. BMC Health Serv Res. 2016;16(1):251. doi:10.1186/s12913-016-1482-7
31. Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag. 2019;15:461–472. doi:10.2147/TCRM.S160365
32. Australian Institute of Health and Welfare. (2012). Summary - Vaccination uptake among people with chronic respiratory disease. Available from: https://www.aihw.gov.au/reports/chronic-respiratory-conditions/vaccination-uptake-among-people-with-chronic-respi/contents/summary.
33. Liang J, Abramson MJ, Russell G, et al. Interdisciplinary COPD intervention in primary care: a cluster randomised controlled trial. Eur Respir J. 2019;53(4):1801530. doi:10.1183/13993003.01530-2018
34. Bai JW, Chen XX, Liu S, Yu L, Xu JF. Smoking cessation affects the natural history of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3323–3328. doi:10.2147/COPD.S150243
35. Bereznicki B, Walters H, Walters J, Peterson G, Bereznicki L. Initial diagnosis and management of chronic obstructive pulmonary disease in Australia: views from the coal face. Intern Med J. 2017;47(7):807–813. doi:10.1111/imj.13418
36. Johnston KN, Young M, Grimmer KA, Antic A, Frith PA. Barriers to, and facilitators for, referral to pulmonary rehabilitation in COPD patients from the perspective of Australian general practitioners: a qualitative study. Prim Care Respir J. 2013;22(3):319–324. doi:10.4104/pcrj.2013.00062
37. Britt H, Miller GC, Charles J, et al. General practice activity in Australia 2009–10. General practice series no. 27. Cat. no. GEP 27. 2010. Canberra: AIHW. Available from: https://www.aihw.gov.au/getmedia/ab96aa0b-a070-4026-a57e-bc96d329aae5/12118.pdf.aspx?inline=true.
38. Walters J. COPD - Diagnosis, management and the role of the GP. Aust Fam Physician. 2010;39(3):100–103.
39. The Royal Australian College of General Practitioners. General Practice: Health of the Nation 2018. Available from: https://www.racgp.org.au/download/Documents/Publications/Health-of-the-Nation-2018-Report.pdf.
40. Mentha K (2011). Retail Pharmacy: ready to take its own medicine. 11-03, p21. Korda Mentha Publication. Available from: https://www.yumpu.com/no/document/read/41213812/retail-pharmacy-a-ready-to-take-its-medicine-kordamentha.
41. Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax. 2007;62(6):496–502. doi:10.1136/thx.2006.064709
42. Bajorek B, LeMay K, Magin P, Roberts C, Krass I, Armour CL. Implementation and evaluation of a pharmacist-led hypertension management service in primary care: outcomes and methodological challenges. Pharm Pract (Granada). 2016;14(2):723. doi:10.18549/PharmPract.2016.02.723
43. Willis A, Rivers P, Gray LJ, Davies M, Khunti K. The effectiveness of screening for diabetes and cardiovascular disease risk factors in a community pharmacy setting. PLoS One. 2014;9(4):e91157. doi:10.1371/journal.pone.0091157
44. Ingram SJ, Kirkdale CL, Williams S. Moving anticoagulation initiation and monitoring services into the community: evaluation of the Brighton and hove community pharmacy service. BMC Health Serv Res. 2018;18(1):91. doi:10.1186/s12913-018-2901-8
45. Gordois A, Armour CL, Brillant M, et al. Cost-Effectiveness Analysis of a Pharmacy Asthma Care Program in Australia. Dis Manage Health Outcomes. 2007;15(6):387–396. doi:10.2165/00115677-200715060-00006
46. Hendrie D, Miller TR, Woodman RJ, Hoti K, Hughes J. Cost-effectiveness of reducing glycaemic episodes through community pharmacy management of patients with type 2 diabetes mellitus. J Prim Prev. 2014;35(6):439–449. doi:10.1007/s10935-014-0368-x
47. Tommelein E, Mehuys E, Van Hees T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. Br J Clin Pharmacol. 2014;77(5):756–766. doi:10.1111/bcp.12242
48. Hesso I, Gebara SN, Kayyali R. Impact of community pharmacists in COPD management: inhalation technique and medication adherence. Respir Med. 2016;118:22–30. doi:10.1016/j.rmed.2016.07.010
49. Armour CL, Reddel HK, LeMay KS, et al. Feasibility and effectiveness of an evidence-based asthma service in Australian community pharmacies: a pragmatic cluster randomized trial. J Asthma. 2013;50(3):302–309. doi:10.3109/02770903.2012.754463
50. Fuller JM, Saini B, Bosnic-Anticevich S, Garcia Cardenas V, Benrimoj SI, Armour C. Testing evidence routine practice: using an implementation framework to embed a clinically proven asthma service in Australian community pharmacy. Res Social Adm Pharm. 2017;13(5):989–996. doi:10.1016/j.sapharm.2017.05.019
51. World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. World Health Organization. Bull World Health Organ. 2001;79(4):373–374.
52. Wong LY, Chua SS, Husin AR, Arshad H. A pharmacy management service for adults with asthma: a cluster randomised controlled trial. Fam Pract. 2017;34(5):564–573. doi:10.1093/fampra/cmx028
53. Normansell R, Kew KM, Mathioudakis AG. Interventions to improve inhaler technique for people with asthma. Cochrane Database Syst Rev. 2017;3(3):CD012286. doi:10.1002/14651858.CD012286.pub2
54. Foster JM, Smith L, Bosnic-Anticevich SZ, et al. Identifying patient-specific beliefs and behaviours for conversations about adherence in asthma. Intern Med J. 2012;42(6):136–144. doi:10.1111/j.1445-5994.2011.02541.x
55. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
56. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnoea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
57. Chabrol H, Niezborala M, Chastan E, de Leon J. Comparison of the Heavy Smoking Index and of the Fagerstrom Test for Nicotine Dependence in a sample of 749 cigarette smokers. Addict Behav. 2005;30(7):1474–1477. doi:10.1016/j.addbeh.2005.02.001
58. Maples P, Franks A, Ray S, Stevens AB, Wallace LS. Development and validation of a low-literacy Chronic Obstructive Pulmonary Disease Knowledge Questionnaire (COPD-Q). Patient Educ Couns. 2010;81(1):19–22. doi:10.1016/j.pec.2009.11.020
59. COPD Pharmacy Online Training. Available from: https://lungfoundation.com.au/events/copd-pharmacy-online-training/.
60. Chen TF. Pharmacist-Led Home Medicines Review and Residential Medication Management Review: the Australian Model. Drugs Aging. 2016;33(3):199–204. doi:10.1007/s40266-016-0357-2
61. Varghese D, Ishida C, Haseer Koya H. Polypharmacy. 2020 Aug 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020.
62. Inhaler technique in adults with asthma or COPD. (2018). National Asthma Council. Available from: https://assets.nationalasthma.org.au/resources/Inhaler-Technique-info-paper-20180607-web.pdf.
63. Basheti IA, Obeidat NM, Reddel HK. Effect of novel inhaler technique reminder labels on the retention of inhaler technique skills in asthma: a single-blind randomized controlled trial. NPJ Prim Care Respir Med. 2017;27(1):9. doi:10.1038/s41533-017-0011-4
64. Bosnic-Anticevich S, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma. 2010;47(3):251–256. doi:10.3109/02770900903580843
65. Hammerlein A, Muller U, Schulz M. Pharmacist-led intervention study to improve inhalation technique in asthma and COPD patients. J Eval Clin Pract. 2011;17(1):61–70. doi:10.1111/j.1365-2753.2010.01369.x
66. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. doi:10.1345/aph.1H018
67. Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15(7):457–464.
68. Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi:10.1136/bmj.38875.675486.55
69. Vestbo J, Anderson JA, Calverley PMA, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(1):939–943. doi:10.1136/thx.2009.113662
70. Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. 1999;37(2):113–124. doi:10.1016/S0738-3991(98)00107-4
71. Rollnick S, Butler CC, Kinnersley P, Gregory J, Mash B. Motivational Interviewing. BMJ. 2010;2010(340):c1900. doi:10.1136/bmj.c1900
72. Australian Lung Foundation. COPD-The Basics’ booklet; 2018. Available from: https://lungfoundation.com.au/wp-content/uploads/2018/09/Book-COPD-The-Basics-Sep2018.pdf.
73. The Royal Australian College of General Practitioners (RACGP); 2011. Supporting smoking cessation: a guide for health professionals. Melbourne, Australia. [Updated 2014]. Available from: http://www.racgp.org.au/download/documents/Guidelines/smoking-cessation.pdf.
74. IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY; 2016.
75. Emmerton LM, Smith L, LeMay KS, et al. Experiences of community pharmacists involved in the delivery of a specialist asthma service in Australia. BMC Health Serv Res. 2012;12(1):164. doi:10.1186/1472-6963-12-164
76. Hughes S, LeMay KS, Saini B, Armour CL. Pharmacy asthma services - will evidence alone sustain them? Australian Pharmacist. 2015;34(1):67–72.
77. Zwar NA, Bunker JM, Reddel HK, et al. Early intervention for chronic obstructive pulmonary disease by practice nurse and GP teams: a cluster randomized trial. Fam Pract. 2016;33(6):663–670. doi:10.1093/fampra/cmw077
78. Göriş S, Taşci S, Elmali F. The effects of training on inhaler technique and quality of life in patients with COPD. J Aerosol Med Pulm Drug Deliv. 2013;26(6):336–344. doi:10.1089/jamp.2012.1017
79. Zwar NA, Hermiz O, Comino E, et al. Care of patients with a diagnosis of chronic obstructive pulmonary disease: a cluster randomised controlled trial. Med J Aust. 2012;197(7):394–398. doi:10.5694/mja12.10813
80. Rochester CL, Vogiatzis I, Holland AE, et al. ATS/ERS Task Force on Policy in Pulmonary Rehabilitation. An Official American Thoracic Society/European Respiratory Society Policy Statement: enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–1386. doi:10.1164/rccm.201510-1966ST
81. Wright D, Twigg M, Barton G, Thornley T, Kerr C. An evaluation of a multi-site community pharmacy–based chronic obstructive pulmonary disease support service. Int J Pharm Pract. 2015;23(1):36–43. doi:10.1111/ijpp.12165
82. Van Boven JFM, Tommelein E, Boussery K, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir Res. 2014;15(1):66. doi:10.1186/1465-9921-15-66
83. Dennis S, Reddel HK, Middleton S, et al. Barriers and outcomes of an evidence-based approach to diagnosis and management of chronic obstructive pulmonary disease (COPD) in Australia: a qualitative study. Fam Pract. 2017;34(4):
84. Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2014;(2014(9):1101–1110. doi:10.2147/COPD.S54475
85. Basheti IA, Armour CL, Bosnic-Anticevich SZ, Reddel HK. Evaluation of a novel educational strategy, including inhaler-based reminder labels, to improve asthma inhaler technique. Patient Educ Couns. 2008;72(1):26–33. doi:10.1016/j.pec.2008.01.014
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
