Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Contrast media-induced nephropathy: how has Italy contributed in the past 30 years? A systematic review
Authors Sessa M, Rossi C, Mascolo A, Scavone C, di Mauro G, Grassi R, Sportiello L, Cappabianca S, Rafaniello C
Received 20 June 2017
Accepted for publication 27 August 2017
Published 24 October 2017 Volume 2017:13 Pages 1463—1478
DOI https://doi.org/10.2147/TCRM.S144418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Maurizio Sessa,1,* Claudia Rossi,2,* Annamaria Mascolo,1 Cristina Scavone,1 Gabriella di Mauro,1 Roberto Grassi,2 Liberata Sportiello,1 Salvatore Cappabianca,2 Concetta Rafaniello1
1Section of Pharmacology “L Donatelli”, Department of Experimental Medicine, University of Campania “L Vanvitelli”, Naples, Italy; 2Section of Radiology and Radiotherapy, Department of Clinical and Experimental Medicine “Magrassi-Lanzara”, University of Campania “L Vanvitelli”, Naples, Italy
*These authors contributed equally to this work
Background and objective: The use of contrast media in Italy has exponentially increased in the past 3 decades. However, it is unknown whether there has been an increase in clinical research evaluating the risks associated with contrast media usage, especially regarding contrast-induced nephropathy. To fill this gap in knowledge, we performed a systematic review.
Study eligibility criteria: Meta-analyses, observational studies, and clinical trials assessing contrast media-induced nephropathy as the safety outcome, in which at least one author was affiliated with an Italian university/health care structure, were eligble.
Data sources: Ovid MEDLINE, Ovid Embase, Cochrane Methodology Register, and Web of Science were screened.
Participants: Men and women exposed to contrast media.
Results: In total, 60 original articles were retrieved with an incremental trend between 1990 and 2017. Cohort studies were the most common study design represented. In total, 45 of 60 (75.0%) studies were monocenter studies and 41 of 60 (68.3%) received no funding. In all, 91.7% of studies disclosed no conflicts of interest and 81.7% had no external collaboration. Most of the studies provided a level of evidence of III-2 (32/60; 53.3%) and II (23/60; 38.3%). In total, 50 of 60 studies (83.3%) were published in a scientific journal ranked in the first quartile of their subject area.
Conclusion: There was an increased number of studies evaluating contrast-induced nephropathy in Italy during the last three decades. These studies covered procedures to prevent contrast-induced nephropathy or aimed to identify risk factors, biomarkers, and scores, and their related prognosis.
Keywords: nephropathy, contrast media, Italy, systematic review, drug safety, adverse drug reaction, pharmacovigilance, post-marketing surveillance
Introduction
In the past 3 decades, in the Italian national territory, there has been significant widespread use of medical diagnostics. This phenomenon was mainly attributable to the introduction and progressive improvement of the accuracy and effectiveness of imaging methodologies, such as ultrasound, computed tomography, and magnetic resonance imaging techniques for which contrast media have played a key role.1–3 On the one hand, the increased use of contrast media has resulted in undoubted improvement in diagnostic efficiency; on the other hand, it has resulted in an increased pool of patients at risk of developing contrast media-related adverse drug reactions. Contrast media are identifiable as medication, and, as with any medication, their use is not risk free.1,4–13 Contrast media could determine the occurrence of many types of adverse reactions, such as acute or delayed hypersensitivity reactions14 and organ-specific adverse reactions such as nephrotoxicity,15 cardiovascular toxicity, neurotoxicity, and lung toxicity.16 As for any medicine, experiences arising from premarketing studies are generally not sufficient to delineate the risk/benefit ratio by virtue of their inherent limitations. Therefore, during their entire life cycle on the pharmaceutical market, it is necessary to evaluate further their benefit/risk profile, especially under real-life conditions not normally covered by clinical trials, including the use in frail populations. In this regard, an excellent example is represented by contrast-induced nephropathy. This event was found to have a higher incidence in frail patients such as those with advanced kidney disease, and it was found to be among the top five causes of acquired acute renal injury during hospital admission.17,18 To date, it is unknown whether, concurrent with the increased pool of patients at risk of developing contrast-induced nephropathy as determined by the increased use of contrast media in the Italian national territory, there was an evolution of clinical research evaluating contrast-induced nephropathy by Italian researchers. Given the lack of knowledge on this aspect, we performed a systematic review to provide further insight into the scientific contribution of Italian clinical research on contrast-induced nephropathy.
Methods
Eligibility criteria for considering studies in this review
This systematic review evaluated meta-analyses, observational studies, and clinical trials assessing contrast media-induced nephropathy as a safety outcome for which at least one author was affiliated with an Italian university/health care structure. Letters to the editor, abstracts sent to national or international conferences, and case reports were considered ineligible. Reviews published by Italian authors (systematic and not) were included to search their reference lists for undetected records. This systematic review was restricted to studies published in Italian and/or international journals and for which the full text was available in English and/or Italian languages. We defined a contrast medium as any substance listed in the V08 code of anatomical therapeutic chemical classification as proposed by the World Health Organization.19 Contrast-induced nephropathy was defined as a 25% increase in serum creatinine from the baseline or as an increase of 0.5 mg/dL (44 μmol/L) in the absolute serum creatinine value within 2–3 days from contrast medium administration.
Outcomes
The main outcome is the number of studies published per year from 1990 to 2017 and a narrative overview of the main findings. Secondary outcomes included the evaluation of 1) Italian regions involved based on the location of the universities/health care structures performing the study; 2) the journal in which the study was published; 3) the ranking of the journal; and 4) the subject area of the journal in which the study was published. Tertiary outcomes included the evaluation of 1) funding (yes/no); 2) the design of the study; 3) the level of evidence provided; 4) the presence of a conflict of interest; and 5) collaboration with universities located outside the national territory.
Search methods for the identification of studies
We searched Ovid MEDLINE (from January 1990 to January 2017), Ovid Embase (from January 1990 to January 2017), Cochrane Methodology Register (2015, Version 2), and Web of Science (January 2017) and screened all references listed in the reviews (systematic and not systematic) included. The research strategy developed for Ovid MEDLINE and adapted for the other databases is provided in Table S1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist is provided in Table S2.
Selection of studies
Two independent researchers (MS and AM) screened the titles and abstracts, when available, of all retrieved records for obvious exclusions, and the full text of the remaining articles. If disagreements arose during the evaluation, they were resolved by consensus.
Data extraction and management
Two independent researchers (MS and AM) performed data extraction and management. For all original articles included, information on the year of publication, affiliation of the authors, conflict of interest, funding, study design, outcomes, and journal of publication was extracted. To establish the level of evidence of each study, the scale proposed by Merlin et al20 was used. To establish the topic and ranking of the journal, the SCImago database was used (http://www.scimagojr.com/).
Results
After duplicates were removed, 2011 records underwent title and abstract screening, among which 984 were identified with research blocks while 1,027 were identified from reference lists of narrative and systematic reviews. For 22 reviews, it was not possible to obtain the full text. In all, 1,914 records were eliminated because they did not fulfill the eligibility criteria; the remaining 75 references (60 original articles and 15 reviews) underwent full-text evaluation and were considered eligible to be included in this systematic review (Figure 1).
  | Figure 1 Study flow diagram. |
These studies mainly focused on the evaluation of procedures to prevent contrast-induced nephropathy, the identification of risk factors, biomarkers, and scores, and the prognosis or provided head-to-head comparison among contrast media for the risk of developing contrast-induced nephropathy (Figure 2).
Temporal trend and characteristics of studies published between 1990 and 2017
The 60 original articles were published between 1990 and 2017 (Figure 3; Appendices S1 and S2) with a peak of studies published in 2014/2015. For 22 reviews, we did not have full-text access to retrieve reference lists (Appendix S3). By evaluating the Italian authors’ affiliations, the three most representative Italian regions were Lombardia (31/60; 51.7%), Campania (17/60; 28.3%), and Toscana (11/60; 18.3%; Figure 4). The subject area covered by the journal in which original articles were published ranged from nephrology (4/60; 6.7%) to cardiology and cardiovascular medicine (32/60; 53.3%; Figure S1). The cohort study was the most representative study design (31/60; 51.7%; Figure 5). In total, 45 of 60 (75.0%) studies were performed as a monocenter study, and 41 of 60 (68.3%) received no funding for executing the project. In all, 91.7% (55/60 studies) of studies disclosed no conflicts of interest, and 81.7% (49/60 studies) had no external collaboration (Figures S2 and S3). Most of the studies provided a level of evidence of III-2 (32/60; 53.3%) and II (23/60; 38.3%; Figure 6). The Journal of the American College of Cardiology (9/60; 15.0%), Circulation (5/60; 8.3%), and American Heart Journal (4/60; 6.7%) were the three scientific journals with the higher number of studies published among those included (Figure S4). In total, 50 of 60 (83.3%) studies were published in a scientific journal ranked in the first quartile of their subject area (Figure S5).
  | Figure 3 Temporal trend of the number of studies included by the year of publication. |
  | Figure 4 Distribution by Italian region of the included studies. |
  | Figure 5 Distribution by the study design of the included studies. |
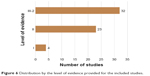  | Figure 6 Distribution by the level of evidence provided for the included studies. |
Studies evaluating the procedures to prevent contrast-induced nephropathy
3-hydroxy-3-methyl-glutaryl-coenzyme-A reductase inhibitors to prevent contrast-induced nephropathy
In this systematic review, nine studies investigated the administration of 3-hydroxy-3-methyl-glutaryl-coenzyme-A reductase inhibitors as a preventive measure against contrast-induced nephropathy, among which eight reported a protective effect of this drug class. In particular, Patti et al21 published the results of a randomized, multicenter, prospective, double-blind clinical trial aimed to assess the effect of short-term, high-dose atorvastatin pretreatment in preventing iobitridol-induced nephropathy in 241 patients who underwent percutaneous coronary intervention. The authors found that these patients had a 66% (odds ratio [OR]: 0.34; 95% confidence interval [CI]: 0.12–0.97) lower probability of developing contrast-induced nephropathy than patients exposed to a placebo. Similarly, Quintavalle et al22 investigated the impact of administering atorvastatin versus placebo to prevent contrast-induced acute kidney injury in 410 patients with chronic kidney disease exposed to iodixanol during coronary angiography or percutaneous coronary intervention. The authors found a lower incidence of contrast-induced acute kidney injury among patients pretreated with atorvastatin. Leoncini et al23 published the results of the PRATO-ACS trial evaluating the impact of high-dose rosuvastatin in preventing the development of contrast-induced nephropathy in 504 patients with non-ST-elevation acute coronary syndrome. All patients were exposed to iodinated contrast media and underwent angiography and/or percutaneous coronary intervention. The authors found that early high-dose rosuvastatin was associated with a protective effect on the risk of developing contrast-induced nephropathy and improved the short-term clinical outcome. Toso et al,24 using PRATO-ACS trial data, evaluated whether the beneficial impact of administering a high dose of rosuvastatin to prevent contrast-related nephropathy varied in relation to baseline high-sensitivity C-reactive protein levels. The authors found that the administration of high-dose rosuvastatin was more protective in reducing the probability of developing contrast-induced nephropathy in patients with higher baseline high-sensitivity C-reactive protein in both the short and intermediate term. Tropeano et al25 performed a post hoc analysis of PRATO-ACS trial to assess the impact of administering rosuvastatin on the prevention of contrast-induced nephropathy in two age groups (elder versus young). The authors found that the administration of rosuvastatin was beneficial for all patients in preventing contrast-induced nephropathy, especially in patients with high-sensitivity C-reactive protein.
One study found the absence of a beneficial effect of administering 3-hydroxy-3-methyl-glutaryl-coenzyme-A reductase inhibitors as a preventive measure for contrast-induced nephropathy. In particular, Toso et al26 published the results of their study that aimed to assess the efficacy of short-term high-dose atorvastatin administration in preventing contrast-induced nephropathy among patients who underwent coronary angiography and/or other cardiac surgery. In all, 304 patients were included, among whom 152 were randomized to receive atorvastatin and 152 were randomized to receive placebo. All patients received iodixanol as contrast media. The authors found that short-term pretreatment with a high dose of atorvastatin was not associated with additional protection to contrast-induced nephropathy.
In addition, three meta-analyses were performed to provide an aggregate estimation of the protective effect of 3-hydroxy-3-methyl-glutaryl-coenzyme-A reductase inhibitors in preventing contrast-induced nephropathy. In particular, Giacoppo et al27 evaluated the impact of pretreatment with 3-hydroxy-3-methyl-glutaryl-coenzyme-A reductase inhibitors among patients who underwent coronary catheterization. By including eight clinical trials in the meta-analysis, for a total population of 4,984 patients, the authors found that patients receiving statins had a 46% lower risk of developing contrast-induced nephropathy than patients who were not pretreated. Barbieri et al,28 in contrast, focused on the prevention of contrast-induced nephropathy among patients exposed to contrast media for coronary angiography/percutaneous interventions. By performing a meta-analysis on eight clinical trials, for a total population of 4,734 patients, the authors found that both high- and low-dose statin preadministrations were beneficial in preventing contrast-induced nephropathy. Marenzi et al29 published the results of a meta-analysis that aimed to assess whether short-term, preprocedural, intensive statin treatment may reduce contrast-induced nephropathy among patients undergoing coronary angiography and percutaneous coronary intervention with and without acute coronary syndrome. In total, the meta-analysis included nine randomized clinical trials for a total of 5,212 patients, among whom 2,593 were assigned to the high-dose statin regimen. The authors found that pretreatment with high-dose statin was associated with a 50% risk reduction in developing contrast-induced nephropathy (risk ratio [RR]: 0.50; 95% CI: 0.39–0.64).
N-acetylcysteine to prevent contrast-induced nephropathy
In all, nine studies investigated the impact of administering N-acetylcysteine for preventing contrast-induced nephropathy. In particular, Calabro et al30 published a study that aimed to evaluate the protective effect of saline hydration and N-acetylcysteine on the risk of developing contrast-induced nephropathy. The study included 322 patients exposed to iopromide for coronary artery angiography. The authors found that the administration of intravenous hydration and N-acetylcysteine was effective in reducing the risk of contrast-induced nephropathy. Briguori et al31 published the results of a study that aimed to compare the impact of administering hydration with and without N-acetylcysteine for preventing contrast-induced nephropathy in patients who underwent coronary and/or peripheral angiography and/or angioplasty. In total, 183 patients were enrolled, among whom 92 patients received N-acetylcysteine and intravenous saline prior to iopromide administration, and 91 patients received intravenous saline alone. The authors found that N-acetylcysteine could provide better protection than hydration alone. However, this effect was evident only when a small volume of contrast agent was used. Accordingly, Vallero et al32 found that the administration of N-acetylcysteine could not prevent contrast-induced nephropathy when a high volume of iodixanol was administered in 100 patients who underwent coronary angiography and/or transluminal angioplasty. Similarly, Alessandri et al33 compared the protective effect of the pretreatment with sodium chloride solution infusion versus co-treatment with sodium bicarbonate plus N-acetylcysteine in preventing contrast-induced nephropathy. The study included 296 patients exposed to iomeprol during coronary angiography. The authors found that the combination of N-acetylcysteine/sodium bicarbonate was more effective in reducing the risk of developing contrast-induced nephropathy among patients with severe chronic kidney disease. Marenzi et al34 investigated the impact of administering a double dose of N-acetylcysteine to prevent the development of contrast-induced nephropathy among patients undergoing primary angioplasty. In total, 354 patients were randomized to receive a single dose of N-acetylcysteine, a double dose of N-acetylcysteine, or placebo. All patients received iohexol as a contrast medium. The authors found that a double dose of N-acetylcysteine had a higher protective effect than the control groups regarding contrast-related nephrotoxicity in patients undergoing primary angioplasty. Similarly, Briguori et al35 evaluated the impact of administering a double dose of N-acetylcysteine to prevent the development of contrast-induced nephropathy. The study included 224 patients with chronic kidney disease who underwent coronary or peripheral procedures and who were exposed to iobitridol. These authors found that a double dose of N-acetylcysteine was more effective than a standard dose in preventing contrast-induced nephropathy.
Among other studies that investigated the protective effect of N-acetylcysteine in preventing the development of contrast-induced nephropathy, three studies provided a head-to-head comparison with other active ingredients. In particular, Briguori et al36 performed a study that evaluated the protective effect of N-acetylcysteine compared with that provided by fenoldopam. The study included 192 patients with chronic kidney disease who were exposed to iodixanol for coronary and/or peripheral procedures. The authors found that, compared with fenoldopam, N-acetylcysteine was more effective in preventing contrast-induced nephropathy. In addition, the same authors compared three preventive strategies (saline infusion plus N-acetylcysteine, sodium bicarbonate infusion plus N-acetylcysteine, and 0.9% saline plus ascorbic acid and N-acetylcysteine) for the development of contrast-induced nephropathy in 326 patients with chronic kidney disease exposed to iodixanol for coronary and/or peripheral procedures.37 The authors found that the combination of sodium bicarbonate plus N-acetylcysteine was more effective in preventing contrast-induced nephropathy than other strategies. Also, Briguori et al38 compared the nephrotoxicity of iso-(iodixanol) and low-osmolality (iobitridol) contrast agents in patients pretreated with 0.45% saline infusion and N-acetylcysteine. The study included 115 patients receiving iobitridol and 100 patients receiving iodixanol for coronary and/or peripheral procedures. The authors found that patients receiving iobitridol and those receiving iodixanol had a similar risk of experiencing contrast-induced nephrotoxicity if pretreated with hydration and N-acetylcysteine.
Calcium channel blocker administration for the prevention of contrast-induced nephropathy
Russo et al39 investigated the impact of calcium channel blocker administration in the prevention of contrast-induced changes in renal hemodynamics. The study included 30 patients randomly assigned to three groups: diatrizoate meglumine, iopamidol, and diatrizoate meglumine plus nifedipine. The authors found that calcium channel blockers could prevent changes in renal hemodynamics induced by diatrizoate meglumine, which, in turn, was associated with a higher risk of contrast-induced nephropathy.
Saline infusion to prevent contrast-induced nephropathy
In all, four studies investigated the impact of administering saline infusion to prevent contrast-induced nephropathy. In particular, Maioli et al40 investigated the role of hydration in preventing contrast-induced nephropathy in patients with ST-elevation myocardial infarction who underwent primary percutaneous coronary intervention. In total, 450 patients were randomized to receive hydration with sodium bicarbonate pre and post procedure (early hydration group), hydration with isotonic saline post procedure (late hydration group), or no hydration (control group). The authors found that the incidence of contrast-induced nephropathy was significantly lower in patients receiving hydration with sodium bicarbonate. La Manna et al,41 in contrast, investigated the efficacy of hydration strategies in preventing iso-osmolar nonionic contrast-induced nephropathy in patients at high risk of developing the event. The study included 784 patients and demonstrated the benefit of hydration strategies in preventing contrast-induced nephropathy. Marenzi et al42 evaluated the impact of hydration with intravenous saline infusion versus hydration with concurrent furosemide-forced diuresis in preventing contrast-induced nephropathy. The study included 170 patients with chronic kidney disease who received iomeprol during coronary angiography. This study found that the use of hydration with concurrent furosemide-forced diuresis was more effective in reducing the risk of developing contrast-induced nephropathy. In addition, Briguori et al43 evaluated the impact of administering saline infusion plus N-acetylcysteine and furosemide to achieve a high urine flow rate to reduce the risk of contrast-induced nephropathy. The study included 400 patients who received iodixanol for coronary and/or peripheral angiography/angioplasty. The authors found that saline infusion with furosemide was an efficient approach to reduce the risk of developing contrast-induced nephropathy. Moreover, the authors established that a urine flow rate greater than 450 mL/hour was the optimum target to prevent contrast-induced nephropathy.
Hemofiltration to prevent contrast-induced nephropathy
Among studies that investigated the impact of venovenous hemofiltration in preventing contrast-induced nephropathy, the study by La Manna et al44 investigated this topic in 12 patients with severe chronic renal impairment. All patients were exposed to iodixanol for coronarography. The authors found that venovenous hemofiltration was an efficient technique to reduce the risk of developing contrast-induced nephropathy in these patients. Guastoni et al,45 in contrast, assessed the impact of venovenous hemofiltration in preventing contrast-induced nephropathy among patients with chronic kidney disease who underwent coronary procedures. The study included 53 patients exposed to iopamidol and showed the beneficial effect of this technique in reducing the risk of developing contrast-induced nephropathy. Marenzi et al46 estimated the impact of hemofiltration in reducing the risk of developing contrast-induced nephropathy compared with isotonic saline hydration in 114 patients with chronic renal failure exposed to iopentol for coronary angiography or percutaneous coronary intervention. The authors found that hemofiltration was more effective than isotonic saline hydration to prevent the development of contrast-induced nephropathy. Moreover, Marenzi and Bartorelli47 published another study to investigate the role of hemofiltration versus isotonic saline hydration in preventing contrast-induced nephropathy. The study included 114 patients with chronic kidney disease who were exposed to contrast media for percutaneous coronary interventions. The authors found that hemofiltration and hydration were more effective in preventing contrast-induced nephropathy than isotonic saline hydration. The study performed by Marenzi et al48 was aimed to compare the impact of hydration with isotonic saline, isotonic saline followed by hemofiltration, and hemofiltration alone before and after contrast media exposure to prevent the development of contrast-induced nephropathy. The study included 92 patients with chronic kidney disease who were exposed to iopentol for invasive diagnostic/therapeutic cardiovascular procedures. The authors found that hemofiltration performed before and after percutaneous coronary interventions was the most effective protocol. Similarly, Briguori et al49 investigated the role of high urine output and fluid balancing in reducing the risk of contrast-induced nephropathy among patients with chronic kidney disease exposed to iodixanol for coronary and/or peripheral angiography and/or angioplasty. Patients were exposed to hydration with saline and N-acetylcysteine controlled by the RenalGuard System and furosemide compared with sodium bicarbonate solution and N-acetylcysteine. The authors found that RenalGuard therapy was superior in preventing contrast-induced nephropathy. Continuous venovenous hemofiltration was found to be an efficient preventive treatment also for the development of oligo-anuric acute renal failure in patients exposed to iopentol for percutaneous coronary interventions.50
Transradial intervention to prevent contrast-induced nephropathy
Ando et al51 published the protocol of an ongoing randomized clinical trial that aimed to assess whether patients who underwent percutaneous coronary intervention with transradial intervention had a lower probability of developing contrast-induced nephropathy than those who underwent transfemoral intervention. The authors will evaluate the effect of transradial intervention in reducing peri-procedural bleeding and its association with the risk of contrast-induced nephropathy in patients with acute coronary syndrome that will undergo diagnostic cardiac catheterization and percutaneous coronary intervention.
Studies evaluating risk factors for contrast-induced nephropathy
Contrast medium volume as a risk factor for contrast-induced nephropathy
In all, four studies investigated the role of the iodinated contrast medium volume as a risk factor for contrast-induced nephropathy. Bianchi et al evaluated the impact of the contrast medium volume and time interval between angiography and cardiac catheterization on the risk of developing contrast-induced nephropathy among pediatric patients with congenital disorder of the heart.52 The study included 277 pediatric patients exposed to iomeprol. The authors found that the contrast medium volume was an important risk factor for contrast-induced nephropathy. Marenzi et al53 investigated the association between the absolute, weight- and creatinine-adjusted contrast volume and probability of developing contrast-induced nephropathy in 561 patients with ST-segment elevation acute myocardial infarction receiving iomeprol or iohexol for percutaneous coronary intervention. This study found that the probability of developing contrast-induced nephropathy increased with the increase in contrast medium volume. Ando et al54 published the results of a study that aimed to investigate whether the composite evaluation of preprocedural variables and renal function-adjusted contrast volume could better predict the risk of contrast-induced nephropathy in a population of 470 patients with ST-segment elevation acute myocardial who underwent percutaneous coronary intervention. The contrast media investigated were iomeprol and iopromide. The authors found that the renal function-adjusted contrast volume was a risk factor for the development of contrast-induced nephropathy in the study population. Moreover, the authors found that a total amount of contrast media restricted to 2.5 times the baseline estimated glomerular filtration rate should be considered the threshold to not overcome in patients who have undergone percutaneous coronary intervention. Ranucci et al55 published the results of a study that was aimed to assess the impact of the contrast medium volume on the risk of developing acute renal failure in patients who underwent diagnostic angiography and cardiac surgery. In total, the study included 423 patients exposed to iobitridol or iodixanol. The authors found that a higher dose of iobitridol or iodixanol was associated with a higher risk of developing acute renal failure after cardiac surgery.
Hyperglycemia as a risk factor for contrast-induced nephropathy
Marenzi et al56 published the results of a prospective cohort study aimed to assess the relationship between hyperglycemia and the risk of contrast-induced nephropathy in 780 patients with ST-segment elevation myocardial infarction who received iomeprol during percutaneous coronary intervention. The authors found that patients with hyperglycemia had a 2.33 (95% CI: 1.66–3.29) higher risk of developing contrast-induced nephropathy and had poorer in-hospital outcomes than patients without hyperglycemia.
Elevated homocysteine as a risk factor for contrast-induced nephropathy
Barbieri et al57 identified a novel risk factor for contrast-induced nephropathy in 876 patients who were exposed to ioversol, iodixanol, or iopromide for coronary angiography or percutaneous coronary intervention. The authors found that elevated homocysteine levels were associated with a higher risk (OR: 1.68; 95% CI: 1.09–2.59) of developing contrast-induced nephropathy.
Low hemoglobin levels as a risk factor for contrast-induced nephropathy
In a prospective cohort study performed by Morabito et al58 with 585 patients who underwent diagnostic or interventional coronary angiography, it was found that a low hemoglobin level and contrast medium volume were risk factors for iomeprol/iopromide-induced nephropathy.
Female gender as a risk factor for contrast-induced nephropathy
Lucreziotti et al59 published the results of a study aimed to evaluate the potential impact of gender differences on the risk of developing contrast-induced nephropathy in patients who underwent percutaneous intervention for ST-segment elevation myocardial infarction. In total, 323 patients were enrolled, and all patients received iodixanol as the contrast medium. Female gender was associated with an increased probability of developing contrast-induced nephropathy (OR: 2.49; 95% CI: 1.22–5.07) compared with male gender.
Age greater than 75 years, hemodynamic variation, time to reperfusion, use of an intra-aortic balloon pump, and cardiac surgery on the same day of angiography as risk factors for contrast-induced nephropathy
Donahue et al60 assessed the risk of contrast-induced nephropathy associated with carotid artery stenting. The study included 126 patients with chronic kidney disease exposed to iodixanol. The authors found that hemodynamic variations such as hypotension were associated with a higher risk of developing contrast-induced nephropathy (OR: 4.01; 95% CI: 1.07–15.03). Ranucci et al61 published the results of a study aimed to assess the risk of contrast-induced nephropathy in patients who underwent angiography and cardiac surgery on the same day. The study included 4,440 patients exposed to iobitridol or iodixanol. The authors found that performing surgery on the same day of angiography was associated with an increased risk of developing contrast-induced nephropathy (OR: 1.58; 95% CI: 1.04–2.40). Marenzi et al62 published the results of an observational study aimed to assess the incidence, clinical predictors, and outcome of contrast-induced nephropathy in 208 patients who underwent primary percutaneous coronary intervention for acute myocardial infarction. The study found an incidence of developing contrast-induced nephropathy of 19% (40/208 patients). Age greater than 75 years (OR: 5.28; 95% CI: 1.98–14.05), time to reperfusion (OR: 2.51; 95% CI: 1.01–6.16), contrast medium volume (OR: 2.80; 95% CI: 1.17–6.68), and use of intra-aortic balloon pump (OR: 15.51; 95% CI: 4.65–51.64) were risk factors for developing contrast-induced nephropathy.
Percutaneous coronary intervention and renal angioplasty as a risk factor for contrast-induced nephropathy
Marraccini et al63 compared the risk of contrast-induced nephropathy between patients who underwent renal angioplasty (33 patients) or percutaneous coronary intervention (33 patients). All patients received iomeprol as the contrast medium. The authors found that renal angioplasty was associated with a lower risk of contrast-induced nephropathy than percutaneous coronary intervention.
Acute coronary syndrome as a risk factor for contrast-induced nephropathy
Crimi et al64 published a post hoc analysis of a randomized clinical trial (PRODIGY) to evaluate whether contrast-induced acute kidney injury was associated with poor outcome in patients with stable coronary artery disease compared with acute coronary syndrome. In total, 1,918 patients were included. The author found that the incidence of contrast-induced nephropathy was higher among patients with acute coronary syndrome than among those with stable coronary artery disease. However, its negative prognostic impact was greater in patients with stable coronary artery disease.
Contrast medium osmolarity as a risk factor for contrast-induced nephropathy
Russo et al65 investigated the early effects of the administration of diatrizoate or iopamidol on renal hemodynamic and tubular function in 14 patients with chronic kidney disease. The authors found a decline in the glomerular filtration rate secondary to renal hypoperfusion and proportional to the osmolality of the contrast media.
Monoclonal gammopathies are not a risk factor for contrast-induced nephropathy
Preda et al66 assessed the impact of monoclonal gammopathies on the risk of contrast-induced nephropathy by comparing 30 patients with monoclonal gammopathies and 20 oncological patients with a normal electrophoretic profile (control group). The authors found that the use of iodixanol was not associated with a significant increase in serum creatinine as well as a significant increase in the risk of contrast-induced nephropathy in patients with monoclonal gammopathies compared with the control group.
Studies evaluating the prognosis of contrast-induced nephropathy
Contrast-induced nephropathy as a prognostic factor for postprocedural bleeding and hypotension
Valente et al67 published the results of a prospective cohort study performed to assess the incidence of contrast-induced nephropathy and its prognostic implication at 1 month in 194 patients with electrocardiographic ST-segment elevation or acute coronary syndromes who underwent urgent percutaneous coronary intervention. Of 194 patients, 67 received iodixanol, and of 194 patients, 127 received iopromide as the contrast medium. The incidence of contrast-induced nephropathy was 10.8% (21/194 patients) with a statistically significant difference (p<0.05) between patients receiving iodixanol (15/67; 22.3%) and those receiving iopromide (6/127; 4.7%). By evaluating the prognostic implication of contrast-induced nephropathy, patients who developed contrast-induced nephropathy were associated with a higher incidence of postprocedural bleeding and hypotension.
Contrast-induced nephropathy as a prognostic factor for death, dialysis, and/or major cardiovascular events
Maioli et al68 published the results of a study that aimed to assess the temporal evolution of renal function in patients who experienced contrast-induced nephropathy. The study included 3,986 patients with preexistent moderate-to-severe renal dysfunction who were exposed to iodixanol for coronary angiography. The authors found that the persistence of renal damage following contrast-induced nephropathy was associated with increased death, dialysis, and/or major cardiovascular events compared with patients with transient renal damage or those who did not develop contrast-induced nephropathy.
Contrast-induced nephropathy as a prognostic factor for adverse clinical outcome
Budano et al69 published the results of a study that assessed the impact of contrast-induced nephropathy on several clinical outcomes, including mortality. The study included 755 patients who were exposed to iodixanol for coronary angiography. The authors found that an increase in serum creatinine ≥0.5 mg/dL in patients experiencing contrast-induced nephropathy was a sensible threshold to determine the patients at higher risk of mortality and morbidity. Moreover, Narula et al70 presented the results of a study that aimed to evaluate the short- and long-term outcomes of contrast-induced nephropathy in patients exposed to iodixanol, iopamidol, ioxaglate, iohexol, iopromide, iotrolan, or ioversol for primary percutaneous coronary intervention. In total, 479 patients developed contrast-induced nephropathy that was associated with higher rates of major bleeding, death, target vessel revascularization for ischemia, or stroke at 30 days and 3 years from the event.
The Acute Kidney Injury Network (AKIN) definition provides better accuracy in predicting the prognosis in patients with contrast-induced nephropathy
Centola et al71 published the results of a study that aimed to assess the predictive accuracy of the long-term prognosis of two definitions of contrast-induced nephropathy. The study included 402 patients who underwent percutaneous coronary intervention. The authors found that the AKIN definition provided better accuracy in predicting long-term mortality associated with contrast-induced nephropathy than contrast-induced nephropathy defined as an increase in the serum creatinine level ≥25% or ≥0.5 mg/dL from baseline values within the first 72 hours after contrast exposure.
Studies evaluating biomarkers or scores to predict contrast-induced nephropathy
Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for contrast-induced nephropathy
Quintavalle et al72 assessed the diagnostic usefulness of NGAL as a predictor for contrast-induced nephropathy in patients exposed to iodixanol for coronary or peripheral angiography or angioplasty. The authors found that urine NGAL <20 ng/mL and serum NGAL <179 ng/mL at 6 hours were reliable markers of contrast-induced nephropathy. Moreover, serum NGAL ≥179 ng/mL at 6 hours could predict 1-year major adverse events.
Cystatin C as a biomarker factor for contrast-induced nephropathy
Among studies that investigated biomarkers to predict the development of contrast-induced nephropathy, the study published by Briguori et al73 focused on the role of cystatin C. The study included 410 patients with chronic kidney disease. All patients underwent coronary and/or peripheral angiography and/or angioplasty and were exposed to iodixanol. The authors found that cystatin C was a better biomarker than serum creatinine for the early diagnosis and prognosis of contrast-induced acute kidney injury. By contrast, the study performed by Ribichini et al74 in 166 patients found that the variation in the serum creatinine baseline was a biomarker more reliable than cystatin C for the early diagnosis of contrast-induced nephropathy.
The load-to-damage relationship method as a tool to predict contrast-induced nephropathy
Limbruno et al75 published the results of a new method entitled “load-to-damage relationship,” which was proposed to assess the dose-dependent nephrotoxicity induced by contrast media. The study included 113 patients who underwent coronary angiography and/or percutaneous coronary intervention, among whom 57 were exposed to iodixanol and 56 to iobitridol. The authors found a significant correlation between the normalized contrast load and change in creatinine for both iobitridol and iodixanol.
The “new score” as a clinical tool to predict contrast-induced nephropathy
Maioli et al76 published an article that aimed to develop a score system based on preprocedural clinical characteristics to predict the development of contrast-induced nephropathy in patients who underwent elective coronary angiography and percutaneous coronary intervention. The study included 1,218 patients receiving iodixanol, and the “new score” was found to be efficient in predicting the risk of developing contrast-induced nephropathy.
Serum creatinine increase as a biomarker for contrast-induced nephropathy
Ribichini et al77 investigated the prognostic value of the relative increase in the serum creatinine concentration in contrast-induced nephropathy. The study included 216 high-risk patients exposed to iodixanol for diagnostic angiograms and coronary interventions. It was found that the increase in serum creatinine concentration was an efficient predictor of contrast-induced nephropathy and 30-day renal damage.
Head-to-head comparison for the risk of developing contrast-induced nephropathy
Head-to-head comparison between iopromide and iodixanol for the risk of contrast-induced nephropathy
Bolognese et al78 evaluated the non-inferiority of iopromide in terms of nephrotoxicity compared with that of iodixanol in a randomized clinical trial involving 475 patients with ST-segment elevation acute myocardial infarction who underwent percutaneous coronary intervention, among whom 239 were randomized to receive iopromide. The study found that iopromide was not inferior to iodixanol in the occurrence of contrast-induced nephropathy. Carraro et al79 published the results of a study aimed to compare iodixanol and iopromide for the risk of contrast-induced nephropathy. The study included 64 patients who underwent intravenous urography. The study showed no difference in the levels of serum creatinine between the two groups, entailing a low risk of nephropathy in patients exposed to both iodixanol and iopromide.
Head-to-head comparison among iodixanol, iopamidol, iomeprol, ioversol, iohexol, and ioxaglate for the risk of contrast-induced nephropathy
Biondi-Zoccai et al80 published the results of a meta-analysis that aimed to compare the risk of contrast-induced nephropathy between iso- and low-osmolar contrast media. A total of 42 trials were included in the meta-analysis with 10,048 patients exposed to seven different iodine-based contrast media. The authors found a similar renal safety profile among iodixanol, iopamidol, iomeprol, and ioversol. Iohexol and ioxaglate, in contrast, were associated with an adverse renal-related clinical outcome. Finally, for iopromide, there was insufficient evidence to provide any recommendation.
Head-to-head comparison between iomeprol and iopamidol for major changes in vital signs and clinical and laboratory parameters among patients who underwent renal intra-arterial digital subtraction angiography for suspected renovascular stenosis
Simonetti et al81 compared the safety profiles of iomeprol and iopamidol in 40 patients with hypertension who underwent renal intra-arterial digital subtraction angiography for suspected renovascular stenosis. The study found that both iomeprol and iopamidol did not induce major changes in vital signs, clinical parameters (including contrast-induced nephropathy), and laboratory parameters in this study population.
Studies evaluating other topics related to contrast-induced nephropathy
No dose adjustment was necessary for iomeprol to prevent contrast-induced nephropathy
Lorusso et al82 evaluated the safety profile of iomeprol among 30 individuals, including six healthy volunteers, six patients with mild renal failure, four patients with severe renal failure, and eight patients with end-stage renal disease. The authors found that no dose adjustment was necessary for iomeprol to reduce the risk of adverse drug reactions, including contrast-induced nephropathy, also in patients with end-stage renal disease.
Gadolinium-induced nephrogenic systemic fibrosis
Lombardi et al could not provide evidence for gadolinium-induced nephrogenic systemic fibrosis by analyzing the EuroCRM registry, a multicenter, multinational, and multiethnical registry with consecutive enrollment of patients in 57 European centers83 due to the inability of this registry to detect non-acute renal adverse events.
Discussion
As expected, in Italy, during the past 3 decades, there was an increase in the number of studies clinically evaluating contrast-induced nephropathy. In the majority of studies, the sample under investigation was composed of patients who underwent angiography or percutaneous coronary intervention and who were exposed to an iodinated contrast media. Mainly, these contrast media were used to investigate coronary arterial trees or were used during surgical treatment of ischemic heart syndromes. In fact, cardiologists were those who mainly performed clinical research on this topic. This paradoxical result was already observed in other countries, and it was described in the scientific literature.84 The paradox beyond this result was based on angiographers being responsible for the development and popularization of percutaneous coronary arteriography.85 However, despite the burst that interventional radiography has obtained in the past 3 decades, radiologists for cardiac catheterizations have demonstrated marginal interest. This phenomenon has led to partial monopolization of the clinical research on this topic by cardiologists.84 By evaluating studies included in this systematic review, we found that Italian researchers performed studies providing a medium/high level of evidence. In this regard, due to the quality of evidence provided within the topic investigated, the journals in which these studies were published were ranked in the first quartile of their sectors, suggesting their role as lead journals. The most representative study design was the cohort study, which configures as a type of observational study. This result could be explained by the majority of studies not having any financial contribution for their execution, mainly configured as nonprofit research. Therefore, because observational studies have a lower cost than randomized clinical trials in their execution, they could be the optimal choice to investigate the topic.85 A great variability was observed among Italian regions regarding the level of contribution given to this topic. We hypothesized that a plausible explanation beyond this result is given by the distribution of health care structures that can perform this type of procedure in the Italian national territory. According to the National Association of Hospital Cardiologists census performed in 2005, cardiological centers that declared the performance of hemodynamic and contrastographic studies, and or coronary angioplasty, were highly representative in Lombardia, Campania, and Toscana, and these centers were found to have a higher number of procedures performed per inhabitant than those observed in bordering regions.86 As expected, an important quota of studies was aimed to evaluate procedures to prevent the development of contrast-induced nephropathy. We believe that this result is a natural consequence of the increased pool of patients at risk of developing contrast-induced nephropathy and of the negative prognosis associated with the occurrence of this event.87 In particular, these studies evaluated both pharmacological and non-pharmacological approaches to prevent contrast-induced nephropathy. Among studies investigating pharmacological approaches, those investigating the protective effect of 3-hydroxy-3-methyl-glutaryl-coenzyme-A reductase inhibitors or N-acetylcysteine to prevent contrast-induced nephropathy were highly numerous, especially during the past years. We believe that a plausible explanation for this result is the controversial and not fully recognized role of these treatments as preventive measures for contrast-induced nephropathy,88 which required more evidence to clarify these associations. In this regard, to date, it remains unclarified the biological mechanisms determining the hypothetical protective effects of these treatments. For statins, it was supposed that their pleiotropic properties – particularly their antioxidant, anti-inflammatory, and antithrombotic properties – could mediate their protective effects for contrast-induced nephropathy. Supporting this hypothesis, studies that evaluated the pathogenesis of contrast-induced nephropathy have shown that contrast media can induce nephrotoxicity mainly due to their cellular toxicity, which causes endothelial dysfunction and renal cell apoptosis. In addition, these effects seem to be related to renal medullary hypoxia through oxidative stress, reduction in vasodilator agents such as nitric oxide and prostaglandins, or an increase in vasoconstrictor agents such as adenosine and endothelin.89 Statins by reducing the secretion of endothelin and production of reactive oxygen species and by increasing the production of nitric oxide should, theoretically, stabilize the endothelium of renal vessels and prevent the development of ischemic nephropathy in human beings, as shown in experimental models.17 A similar effect was also attributed to N-acetylcysteine, which, due to its antioxidant activities, should stabilize the renal vessel endothelium and prevent the development of ischemic nephropathy mediated by reactive oxygen species.89 Two other topics that have been highly investigated are the protective effect of hydration and hemofiltration on contrast-induced nephropathy that was mainly aimed to compare different protocols and/or the saline solution concentration to establish the optimal treatment to prevent contrast-induced nephropathy. This is in accordance with the actual unsolved questions that arose in guidelines and scientific literature regarding these preventive measures.88 In particular, it was supposed that hydration should prevent contrast-induced nephropathy mainly due to its ability to reduce contrast media precipitation within the tubule lumen and its associated necrosis of epithelium, resulting in a reduction in intraluminal obstruction. Moreover, it was supposed that hydration should improve the distribution of sodium to the distal nephron that, in turn, could reduce the activation of the renin–angiotensin system via the macula densa, resulting in the preservation of renal blood flow.90 For hemofiltration, however, it was supposed that, through this technique, it was possible to remove the contrast medium quickly from the blood stream and, consequently, prevent pathophysiological mechanisms for contrast-induced nephropathy.90 Other topics related to preventive measures for contrast-induced nephropathy that were poorly investigated were the preventive use of diuretics and calcium channel blockers. We believe that a plausible explanation is that during these 3 decades, concerns arose concerning their effectiveness as preventative measures for contrast-induced nephropathy, and clinical guidelines never recognized their role as preventive measures.88–90 An important quota of studies, however, was aimed to evaluate risk factors, biomarkers, scores, and prognosis and provided a head-to-head comparison for contrast-induced nephropathy for the development of contrast-induced nephropathy. This result was expected as a typical activity performed in the postmarketing phase. In fact, clinical trials have inherent limitations, such as selected populations that typically make it impossible to evaluate the impact of several risk factors such as comorbidities, seniority, and co-treatments on the safety and efficacy of a medication. Moreover, during clinical trials, scores were rarely developed or biomarkers were rarely identified to predict the development of a specific event such as contrast-induced nephropathy. However, in the postmarketing phase, because the medication is also administered in non-selected populations and in less strictly monitored environments, it is possible to investigate the aforementioned topic.91 In this regard, the controversial and not fully recognized role of the identified risk factors, and the absence of validated biomarkers and scores, as well as the long-term prognosis or head-to-head comparison for contrast-induced nephropathy, made the execution of clinical research logical to better clarify these aspects.88
Conclusion
In Italy, during the past 3 decades, a significant increase was observed in the number of studies clinically evaluating contrast-induced nephropathy. These studies investigated several critical aspects connected to the prevention and treatment of this event, such as procedures to prevent contrast-induced nephropathy, identification of risk factors, biomarkers, and scores, and its related prognosis. Moreover, head-to-head comparison between contrast media was provided for the risk of the development of contrast-induced nephropathy.
Disclosure
The authors report no conflicts of interest in this work.
References
Sessa M, Rossi C, Mascolo A, et al. Suspected adverse reactions to contrast media in Campania Region (Italy): results from 14 years of post-marketing surveillance. Expert Opin Drug Saf. 2015;14(9):1341–1351. | ||
Lusic H, Grinstaff MW. X-ray computed tomography contrast agents. Chem Rev. 2013;113(3):1641–1666. | ||
Lencioni R, Chiesura-Corona M, Drudi FM, Morana G, Feltrin GP, Gavelli G. [Clinical experimentation with contrast media in Italy]. Radiol Med. 2001;101(3):109–110. | ||
Gazzetta Ufficiale; Repubblica Italiana [Italian Official Journal; Italian Republic]. Decreto Legislativo 19 febbraio 2014, n. 17 Attuazione della direttiva 2011/62/UE, che modifica la direttiva 2001/83/CE, recante un codice comunitario relativo ai medicinali per uso umano [Legislative Decree 19 February 2014, n. 17 Implementation of Directive 2011/62/EU amending Directive 2001/83/EC on the Community code relating to medicinal products for human use]. Available from: http://www.gazzettaufficiale.it/eli/id/2014/03/07/14G00027/sg. Accessed October 7, 2017. Italian. | ||
Sessa M, Mascolo A, Andersen MP, et al. Effect of chronic kidney diseases on mortality among digoxin users treated for non-valvular atrial fibrillation: a nationwide register-based retrospective cohort study. PLoS One. 2016;11(7):e0160337. | ||
Sessa M, Rafaniello C, Sportiello L, et al. Campania region (Italy) spontaneous reporting system and preventability assessment through a case-by-case approach: a pilot study on psychotropic drugs. Expert Opin Drug Saf. 2016;15(Suppl 2):9–15. | ||
Sessa M, Rossi C, Rafaniello C, et al. Campania preventability assessment committee: a focus on the preventability of the contrast media adverse drug reactions. Expert Opin Drug Saf. 2016;15(Suppl 2):51–59. | ||
Scavone C, Sessa M, Scavone C, et al. New era in treatment options of chronic hepatitis C: focus on safety of new direct-acting antivirals (DAAs). Expert Opin Drug Saf. 2016;15(Suppl 2):85–100. | ||
Parretta E, Rafaniello C, Magro L, et al. Improvement of patient adverse drug reaction reporting through a community pharmacist-based intervention in the Campania region of Italy. Expert Opin Drug Saf. 2014;13(Suppl 1):S21–S29. | ||
Rafaniello C, Ferrajolo C, Sullo MG, et al. Risk of gastrointestinal complications associated to NSAIDs, low-dose aspirin and their combinations: results of a pharmacovigilance reporting system. Pharmacol Res. 2016;104:108–114. | ||
Mascolo A, Rafaniello C, Sportiello L, et al. Dipeptidyl peptidase (DPP)-4 inhibitor-induced arthritis/arthralgia: a review of clinical cases. Drug Saf. 2016;39(5):401–407. | ||
Sessa M, Sullo MG, Mascolo A, et al. A case of figurate urticaria by etanercept. J Pharmacol Pharmacother. 2016;7(2):106–108. | ||
Mascolo A, Sessa M, Scavone C, et al. New and old roles of the peripheral and brain renin-angiotensin-aldosterone system (RAAS): focus on cardiovascular and neurological diseases. Int J Cardiol. 2017;227:734–742. | ||
Brockow K. Immediate and delayed cutaneous reactions to radiocontrast media. Chem Immunol Allergy. 2012;97:180–190. | ||
Berg KJ. Nephrotoxicity related to contrast media. Scand J Urol Nephrol. 2000;34:317–322. | ||
Namasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: an update. Curr Probl Diagn Radiol. 2006;35(4):164–169. | ||
Geenen RWF, Kingma HJ, van der Molen AJ. Contrast-induced nephropathy: pharmacology, pathophysiology and prevention. Insights Imaging. 2013;4:811–820. | ||
Thomsen HS, Morcos SK, Barrett BJ. Contrast-induced nephropathy: the wheel has turned 360 degrees. Acta Radiol. 2008;49(6):646–657. | ||
Skrbo A, Zulic I, Hadzic S, Gaon ID. Anatomsko-terapijsko-kemijska klasifikacija lijekova. The article is in Croatian [Anatomic-therapeutic-chemical classification of drugs]. Med Arh. 1999;53:57–60. Croatian. | ||
Merlin T, Weston A, Tooher R. Extending an evidence hierarchy to include topics other than treatment: revising the Australian “levels of evidence”. BMC Med Res Methodol. 2009;9:34. | ||
Patti G, Ricottini E, Nusca A, et al. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty – contrast-induced nephropathy] trial). Am J Cardiol. 2011;108:1–7. | ||
Quintavalle C, Fiore D, De Micco F, et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation. 2012;126(25):3008–3016. | ||
Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: results from the PRATO-ACS Study (Protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome). J Am Coll Cardiol. 2014;63(1):71–79. | ||
Toso A, Leoncini M, Maioli M, et al. Relationship between inflammation and benefits of early high-dose rosuvastatin on contrast-induced nephropathy in patients with acute coronary syndrome: the pathophysiological link in the PRATO-ACS study (Protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome undergoing coronary intervention). JACC Cardiovasc Interv. 2014;7(12):1421–1429. | ||
Tropeano F, Leoncini M, Toso A, et al. Impact of Rosuvastatin in contrast-induced acute kidney injury in the elderly: post hoc analysis of the PRATO-ACS trial. J Cardiovasc Pharmacol Ther. 2016;21(2):159–166. | ||
Toso A, Maioli M, Leoncini M, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol. 2010;105(3):288–292. | ||
Giacoppo D, Capodanno D, Capranzano P, Aruta P, Tamburino C. Meta-analysis of randomized controlled trials of preprocedural statin administration for reducing contrast-induced acute kidney injury in patients undergoing coronary catheterization. Am J Cardiol. 2014;114(4):541–548. | ||
Barbieri L, Verdoia M, Schaffer A, Nardin M, Marino P, De Luca G. The role of statins in the prevention of contrast induced nephropathy: a meta-analysis of 8 randomized trials. J Thromb Thrombolysis. 2014;38(4):493–502. | ||
Marenzi G, Cosentino N, Werba JP, Tedesco CC, Veglia F, Bartorelli AL. A meta-analysis of randomized controlled trials on statins for the prevention of contrast-induced acute kidney injury in patients with and without acute coronary syndromes. Int J Cardiol. 2015;183:47–53. | ||
Calabro P, Bianchi R, Crisci M, et al. Use and efficacy of saline hydration and N-acetyl cysteine to prevent contrast-induced nephropathy in low-risk populations undergoing coronary artery angiography. Intern Emerg Med. 2011;6(6):503–507. | ||
Briguori C, Manganelli F, Scarpato P, et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2002;40:298–303. | ||
Vallero A, Cesano G, Pozzato M, et al. Nefropatia indotta da mezzi di contrasto: nessun vantaggio dall’uso in profilassi dell’N-acetilcisteina (NAC) [Contrast nephropathy in cardiac procedures: no advantages with prophylactic use of N-acetylcysteine (NAC)]. G Ital Nefrol. 2002;19:529–533. Italian. | ||
Alessandri N, Lanzi L, Garante CM, et al. Prevention of acute renal failure post-contrast imaging in cardiology: a randomized study. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 1):13–21. | ||
Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354(26):2773–2782. | ||
Briguori C, Colombo A, Violante A, et al. Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur Heart J. 2004;25(3):206–211. | ||
Briguori C, Colombo A, Airoldi F, et al. N-Acetylcysteine versus fenoldopam mesylate to prevent contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2004;44(4):762–765. | ||
Briguori C, Airoldi F, D’Andrea D, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217. | ||
Briguori C, Colombo A, Airoldi F, et al. Nephrotoxicity of low-osmolality versus iso-osmolality contrast agents: impact of N-acetylcysteine. Kidney Int. 2005;68(5):2250–2255. | ||
Russo D, Testa A, Della Volpe L, Sansone G. Randomised prospective study on renal effects of two different contrast media in humans: protective role of a calcium channel blocker. Nephron. 1990;55(3):254–257. | ||
Maioli M, Toso A, Leoncini M, Micheletti C, Bellandi F. Effects of hydration in contrast-induced acute kidney injury after primary angioplasty: a randomized, controlled trial. Circ Cardiovasc Interv. 2011;4(5):456–462. | ||
La Manna G, Pancaldi LG, Capecchi A, et al. Risk for contrast nephropathy in patients undergoing coronarography. Artif Organs. 2010;34(6):E193–E199. | ||
Marenzi G, Ferrari C, Marana I, et al. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (induced diuresis with matched hydration compared to standard hydration for contrast induced nephropathy prevention) trial. JACC Cardiovasc Interv. 2012;5(1):90–97. | ||
Briguori C, Visconti G, Donahue M, et al. RenalGuard system in high-risk patients for contrast-induced acute kidney injury. Am Heart J. 2016;173:67–76. | ||
La Manna G, Pancaldi L, Dalmastri V, et al. Post-coronarography application of continuous veno-venous hemofiltration in the prevention of contrast nephropathy in patients with complex multisystem deficiency. In Vivo. 2008;22(1):123–129. | ||
Guastoni C, Bellotti N, Poletti F, et al. Continuous venovenous hemofiltration after coronary procedures for the prevention of contrast-induced acute kidney injury in patients with severe chronic renal failure. Am J Cardiol. 2014;113(4):588–592. | ||
Marenzi G, Marana I, Lauri G, et al. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003;349(14):1333–1340. | ||
Marenzi G, Bartorelli AL. Hemofiltration in the prevention of radiocontrast agent induced nephropathy. Minerva Anestesiol. 2004;70(4):189–191. | ||
Marenzi G, Lauri G, Campodonico J, et al. Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med. 2006;119(2):155–162. | ||
Briguori C, Visconti G, Focaccio A, et al; REMEDIAL II Investigators. Renal insufficiency after contrast media administration trial II (REMEDIAL II): renalguard system in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124(11):1260–1269. | ||
Marenzi G, Bartorelli AL, Lauri G, et al. Continuous veno-venous hemofiltration for the treatment of contrast-induced acute renal failure after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2003;58:59–64. | ||
Ando G, Cortese B, Frigoli E, et al. Acute kidney injury after percutaneous coronary intervention: rationale of the AKI-MATRIX (acute kidney injury-minimizing adverse hemorrhagic events by TRansradial access site and systemic implementation of angioX) sub-study. Catheter Cardiovasc Interv. 2015;86(5):950–957. | ||
Bianchi P, Carboni G, Pesce G, et al. Cardiac catheterization and postoperative acute kidney failure in congenital heart pediatric patients. Anesth Analg. 2013;117(2):455–461. | ||
Marenzi G, Assanelli E, Campodonico J, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009;150(3):170–177. | ||
Ando G, de Gregorio C, Morabito G, Trio O, Saporito F, Oreto G. Renal function-adjusted contrast volume redefines the baseline estimation of contrast-induced acute kidney injury risk in patients undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(4):465–472. | ||
Ranucci M, Ballotta A, Kunkl A, et al. Influence of the timing of cardiac catheterization and the amount of contrast media on acute renal failure after cardiac surgery. Am J Cardiol. 2008;101(8):1112–1118. | ||
Marenzi G, De Metrio M, Rubino M, et al. Acute hyperglycemia and contrast-induced nephropathy in primary percutaneous coronary intervention. Am Heart J. 2010;160(6):1170–1177. | ||
Barbieri L, Verdoia M, Schaffer A, et al. Elevated homocysteine and the risk of contrast-induced nephropathy: a cohort study. Angiology. 2015;66(4):333–338. | ||
Morabito S, Pistolesi V, Benedetti G, et al. Incidence of contrast-induced acute kidney injury associated with diagnostic or interventional coronary angiography. J Nephrol. 2012;25(6):1098–1107. | ||
Lucreziotti S, Centola M, Salerno-Uriarte D, et al. Female gender and contrast-induced nephropathy in primary percutaneous intervention for ST-segment elevation myocardial infarction. Int J Cardiol. 2014;174(1):37–42. | ||
Donahue M, Visconti G, Focaccio A, et al. Acute kidney injury in patients with chronic kidney disease undergoing internal carotid artery stent implantation. JACC Cardiovasc Interv. 2015;8(11):1506–1514. | ||
Ranucci M, Ballotta A, Agnelli B, et al; Surgical and Clinical Outcome Research (SCORE) Group. Acute kidney injury in patients undergoing cardiac surgery and coronary angiography on the same day. Ann Thorac Surg. 2013;95(2):513–519. | ||
Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. | ||
Marraccini P, Bianchi M, Fommei E, et al. Contrast medium nephrotoxicity after renal artery and coronary angioplasty. Acta Radiol. 2010;51(4):462–466. | ||
Crimi G, Leonardi S, Costa F, et al. Incidence, prognostic impact, and optimal definition of contrast-induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention. Insights from the all-comer PRODIGY trial. Catheter Cardiovasc Interv. 2015;86(1):E19–E27. | ||
Russo D, Minutolo R, Cianciaruso B, Memoli B, Conte G, De Nicola L. Early effects of contrast media on renal hemodynamics and tubular function in chronic renal failure. J Am Soc Nephrol. 1995;6(5):1451–1458. | ||
Preda L, Agazzi A, Raimondi S, et al. Effect on renal function of an iso-osmolar contrast agent in patients with monoclonal gammopathies. Eur Radiol. 2011;21(1):63–69. | ||
Valente S, Lazzeri C, Giglioli C, et al. Contrast-induced nephropathy in urgent coronary interventions. J Cardiovasc Med (Hagerstown). 2006;7(10):737–741. | ||
Maioli M, Toso A, Leoncini M, Gallopin M, Musilli N, Bellandi F. Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation. 2012;125:3099–3107. | ||
Budano C, Levis M, D’Amico M, et al. Impact of contrast-induced acute kidney injury definition on clinical outcomes. Am Heart J. 2011;161(5):963–971. | ||
Narula A, Mehran R, Weisz G, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014;35(23):1533–1540. | ||
Centola M, Lucreziotti S, Salerno-Uriarte D, et al. A comparison between two different definitions of contrast-induced acute kidney injury in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. 2016;210:4–9. | ||
Quintavalle C, Anselmi CV, De Micco F, et al. Neutrophil gelatinase-associated lipocalin and contrast-induced acute kidney injury. Circ Cardiovasc Interv. 2015;8:e002673. | ||
Briguori C, Visconti G, Rivera NV, et al. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121(19):2117–2122. | ||
Ribichini F, Gambaro G, Graziani MS, et al. Comparison of serum creatinine and cystatin C for early diagnosis of contrast-induced nephropathy after coronary angiography and interventions. Clin Chem. 2012;58(2):458–464. | ||
Limbruno U, Picchi A, Micheli A, et al. Refining the assessment of contrast-induced acute kidney injury: the load-to-damage relationship. J Cardiovasc Med (Hagerstown). 2014;15(7):587–594. | ||
Maioli M, Toso A, Gallopin M, et al. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown). 2010;11:444–449. | ||
Ribichini F, Graziani M, Gambaro G, et al. Early creatinine shifts predict contrast-induced nephropathy and persistent renal damage after angiography. Am J Med. 2010;123(8):755–763. | ||
Bolognese L, Falsini G, Schwenke C, et al. Impact of iso-osmolar versus low-osmolar contrast agents on contrast-induced nephropathy and tissue reperfusion in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (from the Contrast Media and Nephrotoxicity Following Primary Angioplasty for Acute Myocardial Infarction [CONTRAST-AMI] Trial). Am J Cardiol. 2012;109(1):67–74. | ||
Carraro M, Malalan F, Antonione R, et al. Effects of a dimeric vs a monomeric nonionic contrast medium on renal function in patients with mild to moderate renal insufficiency: a double-blind, randomized clinical trial. Eur Radiol. 1998;8(1):144–147. | ||
Biondi-Zoccai G, Lotrionte M, Thomsen HS, et al. Nephropathy after administration of iso-osmolar and low-osmolar contrast media: evidence from a network meta-analysis. Int J Cardiol. 2014;172(2):375–380. | ||
Simonetti G, Guazzaroni M, Carpanese L, Canalis GC, Urigo F. A double-blind comparative study of the safety and efficacy of iomeprol in renal intra-arterial digital subtraction angiography. Eur J Radiol. 1994;18(Suppl 1):S73–S76. | ||
Lorusso V, Taroni P, Alvino S, Spinazzi A. Pharmacokinetics and safety of iomeprol in healthy volunteers and in patients with renal impairment or end-stage renal disease requiring hemodialysis. Invest Radiol. 2001;36(6):309–316. | ||
Bruder O, Schneider S, Pilz G, et al. 2015 update on acute adverse reactions to gadolinium based contrast agents in cardiovascular MR. Large multi-national and multi-ethnical population experience with 37788 patients from the EuroCMR Registry. J Cardiovasc Magn Reson. 2015;17:58. | ||
Levin DC, Abrams HL, Castaneda-Zuniga WR, et al. Lessons from history. Why radiologists lost coronary angiography and what can be done to prevent future similar losses. Invest Radiol. 1994;29:480–484. | ||
Thiese MS. Observational and interventional study design types; an overview. Biochem Medica (Zagreb). 2014;24(2):199–210. | ||
De Luca L, Lucci D, Bovenzi F, Perrone Filardi P, Santoro G, Schweiger C. 5°Censimento delle strutture cardiologiche in Italia [Fifth Census of cardiological structures in Italy]. G Ital Cardiol. 2008;10(Suppl 3–6):5S–83S. Italian. | ||
Vercellino M, Bezante GP, Balbi M. Contrast medium induced nephropathy: new insights into prevention and risk management. Cardiovasc Hematol Agents Med Chem. 2009;7(2):166–180. | ||
Davenport MS, Cohan RH, Ellis JH. Contrast media controversies in 2015: imaging patients with renal impairment or risk of contrast reaction. AJR Am J Roentgenol. 2015;204(6):1174–1181. | ||
Safirstein R, Andrade L, Vieira JM. Acetylcysteine and nephrotoxic effects of radiographic contrast agents – a new use for an old drug. N Engl J Med. 2000;343(3):210–212. | ||
Stacul F, Adam A, Becker CR, et al; CIN Consensus Working Panel. Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol. 2006;98(6A):59K–77K. | ||
Kao LS, Tyson JE, Blakely ML, Lally KP. Clinical research methodology I: introduction to randomized trials. J Am Coll Surg. 2008;206:361–369. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

