Back to Journals » International Journal of Nanomedicine » Volume 12
Continuous delivery of propranolol from liposomes-in-microspheres significantly inhibits infantile hemangioma growth
Authors Guo XN, Zhu XS, Liu DK, Gong YB, Sun J, Dong CX
Received 21 March 2017
Accepted for publication 12 June 2017
Published 18 September 2017 Volume 2017:12 Pages 6923—6936
DOI https://doi.org/10.2147/IJN.S137634
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Xiaonan Guo,1,* Xiaoshuang Zhu,1,* Dakan Liu,1 Yubin Gong,1 Jing Sun,2 Changxian Dong1
1Department of Hemangioma and Vascular Malformation, Henan Provincial People’s Hospital, Zhengzhou, People’s Republic of China; 2Department of Pharmacy, Second Military Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Purpose: To reduce the adverse effects and high frequency of administration of propranolol to treat infantile hemangioma, we first utilized propranolol-loaded liposomes-in-microsphere (PLIM) as a novel topical release system to realize sustained release of propranolol.
Methods: PLIM was developed from encapsulating propranolol-loaded liposomes (PLs) in microspheres made of poly(lactic-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic-co-glycolic acid) copolymers (PLGA-PEG-PLGA). The release profile of propranolol from PLIM was evaluated, and its biological activity was investigated in vitro using proliferation assays on hemangioma stem cells (HemSCs). Tumor inhibition was studied in nude mice bearing human subcutaneous infantile hemangioma.
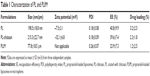
Results: The microspheres were of desired particle size (~77.8 µm) and drug encapsulation efficiency (~23.9%) and achieved sustained drug release for 40 days. PLIM exerted efficient inhibition of the proliferation of HemSCs and significantly reduced the expression of two angiogenesis factors (vascular endothelial growth factor-A [VEGF-A] and basic fibroblast growth factor [bFGF]) in HemSCs. Notably, the therapeutic effect of PLIM in hemangioma was superior to that of propranolol and PL in vivo, as reflected by significantly reduced hemangioma volume, weight, and microvessel density. The mean hemangioma weight of the PLIM-treated group was significantly lower than that of other groups (saline =0.28 g, propranolol =0.21 g, PL =0.13 g, PLIM =0.03 g; PLIM vs saline: P<0.001, PLIM vs propranolol: P<0.001, PLIM vs PL: P<0.001). The mean microvessel density of the PLIM-treated group was significantly lower than that of other groups (saline =40 vessels/mm2, propranolol =31 vessels/mm2, PL =25 vessels/mm2, PLIM =11 vessels/mm2; PLIM vs saline: P<0.001, PLIM vs propranolol: P<0.01, PLIM vs PL: P<0.05).
Conclusion: Our findings show that PLIM is a very promising approach to locally and efficiently deliver propranolol to the hemangioma site leading to a significant inhibition of infantile hemangioma.
Keywords: propranolol, liposomes, microsphere, controlled release, hemangioma
Introduction
Infantile hemangioma is a common childhood tumor composed of disorganized blood vessels and immature cells.1,2 Agents with reported activity in treating infantile hemangioma include corticosteroids, interferon α, vinca alkaloids, and, recently, propranolol.3–5 The commonly used, older drug propranolol has earned a role as a first-line therapy in the management of infantile hemangioma.6 With clinical use, propranolol has been found to be rapidly effective for infantile hemangioma, well-tolerated, and better than previous therapies in inducing regression.7 Although propranolol has been found to be effective for treating infantile hemangioma through oral administration, adverse effects have been described frequently, including symptomatic bradycardia, hypotension, hypoglycemia, and hypoglycemia-induced seizures.7,8 Further, three times daily oral administration of propranolol in the treatment of infantile hemangioma also significantly reduces the compliance of patients.6–8 These adverse effects and too frequent administration severely hamper the clinical use of propranolol in infantile hemangioma. Thus, it is urgent to reduce the adverse effects and high frequency of administration of propranolol in the treatment of infantile hemangioma.
Although the oral route of administration is the most widely used route and most acceptable for patients, it suffers from several disadvantages: slow absorption of most orally administered drugs; unpredictable absorption due to degradation by stomach acid and enzymes; wide distribution in the body, including in important organs, leading to unwanted side effects.9 In contrast, topical administration can directly target diseased regions with a desirable concentration, thus minimizing unpredictable absorption in other organs and tissues and unwanted side effects.10 To overcome the high frequency of administration such as daily administration of drugs, various continuous and controlled-release systems have been developed to release drugs, resulting in significant therapeutic outcome for longer times, with decreased drug quantities, and frequency of administration.11 Thus, we hypothesize that a topical continuous and controlled-release systems could be utilized to reduce the adverse effects and high frequency of administration of propranolol in the treatment of infantile hemangioma.
Liposomes, spherical lipid vesicles with single or multiple lipid bilayers, are the most widely used drug delivery system due to their high biocompatibility, favorable pharmacokinetic profile, feasible surface modification, long circulation time, and simplicity of technology.12 There are over fifteen liposomes that are approved by the US Food and Drug Administration (FDA) or are in phase III clinical trial.32 Liposomes have been widely and successfully used as a continuous and controlled-release system for various drugs, including protein drugs and small-molecule drugs (for example, interferon-γ and cisplatin).13,14 However, the sustained release of liposomes is not satisfactory, since the membrane of liposomes is soft and easily ruptured.15,16 Further, the cholesterol and phospholipid exchange between liposomes and other membranes could easily occur, resulting in the destabilization of liposomes and drug release from liposomes.15 Poly(lactic-co-glycolic acid) (PLGA) microspheres have been used as a controlled delivery system of many drugs such as chemotherapeutic agents. By varying the co-monomers ratio, the time release of the drugs could range from weeks to months.17 Importantly, PLGA is biocompatible and can be used in humans.17 Therefore, the use of PLGA microspheres for therapeutic delivery could provide a prolonged sustained delivery, thus maintaining high levels of the agent at the site of interest.
Further, researchers have realized that a suitable combination of liposomes and PLGA microspheres, which is defined as liposomes-in-microsphere (LIM), may be able to integrate the advantages of both agents and avoid their respective disadvantages.18,19 For example, for liposomes, which are successful for delivery of small-molecule drugs, PLGA polymer could coat the surface of liposomes to improve their stability, drug loading, and encapsulation efficiency. This is because both the lipid layers and polymer matrix would block the drug leakage. LIM is delivered by direct injection into the tumor or intra-arterially to the upstream of the tumor, and it would be entrapped in the tumor vasculature due to chemoembolization effects. On the other hand, the biocompatibility of PLGA microspheres could be improved by the presence of lipids on their surface. Even if intact liposomes are released from microspheres, the liposomes could still act reservoirs for sustained release of drugs. Two researchers have developed LIM for successful sustained release of various drugs that had previously suffered from the drawback of quick drug release from liposomes.18,19 Feng et al19 described the possible mechanism underlying the release of liposomes from PLGA microspheres as follows. Before release from the polymer matrix of microspheres, liposomes had to diffuse through tortuous water channels of microspheres. As time passed, the degradation of the polymer matrix caused expansion of the channels, thereby leading to a sustained release of liposomes.
To reduce the adverse effects and high frequency of administration of propranolol in the treatment of infantile hemangioma, we first utilized LIM as a topical controlled-release system to realize the sustained release of propranolol. We developed propranolol-loaded LIM (PLIM) by loading propranolol in liposomes and then loaded the liposomes in PLGA microspheres and characterized PLIM in the size and drug release. The anti-hemangioma activity of PLIM was examined both in vitro and in vivo.
Materials and methods
Materials
Poly(lactic-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic-co-glycolic acid) copolymer (PLGA-PEG-PLGA, 70,000-4,600-70,000, 1:1 lactic acid [LA]: glycolic acid [GA], molecular weight [MW] 11,900) was bought from PolySciTech, Akina, Inc. (West Lafayette, IN, USA). Poly(vinyl alcohol) (PVA, MW 30,000–70,000), chitosan (hydrochloride salt), and propranolol (hydrochloride salt) were purchased from Sigma-Aldrich (St Louis, MO, USA). Distearoyl-L-phosphatidylcholine (DSPC) and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Matrigel was purchased from Becton Dickinson (Franklin Lakes, NJ, USA). Primers were synthesized by Invitrogen (Shanghai, People’s Republic of China), and the sequences of the primers are listed in Table S1. All organic reagents were of analytical grade and purchased from Sinopharm (Shanghai, People’s Republic of China).
Infantile hemangioma tissue and cell culture
This study was approved by the Research Ethics Committee of Henan Provincial People’s Hospital (Zhengzhou, People’s Republic of China). Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards. Specimens of infantile hemangioma were obtained in our hospital, and the clinical diagnosis was confirmed in the Department of Pathology in our hospital. Hemangioma stem cells (HemSCs) isolation was performed as described previously.20,21 In brief, tissues with regions of proliferating infantile hemangioma were disassociated with collagenase, and then HemSCs were selected by CD133+ magnetic bead isolation (Miltenyi Biotec, Bergisch Gladbach, Germany). HemSCs were cultured in Endothelial Cell Growth Medium-2 (EGM-2; Lonza, Walkersville, MD) with 20% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, USA).
Quantitative real-time polymerase chain reaction
Total RNA was extracted from cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and the first-strand complementary DNA was reverse transcribed from RNA using a Reverse Transcription System kit (Promega, Madison, WI, USA). Real-time polymerase chain reaction (RT-PCR) was performed using a Light Cycler (Roche, Mannheim, Germany). The mRNA expression levels, which were normalized against β-actin, were calculated and expressed as 2CΔΔCT.
Preparation of PLIM
Liposomes (DSPC:cholesterol =1:1, total lipid concentration =2 mM) were prepared by the film hydration method (multilamellar liposomes [MLLs]) followed by membrane extrusion (unilamellar liposomes [ULLs]) as described before.22,23 Briefly, a certain amount of lipids dissolved in organic solvent (20 mL chloroform) was transferred to a small glass vial and dried by nitrogen gas to form a thin lipid film on the wall of the vial. After the film was stored in vacuum overnight to remove the residual organic solvent, it was hydrated with an appropriate volume of propranolol solution for 5 h at 65°C. Vortex mixing was then performed to form MLLs. ULLs were obtained by extruding the MLLs with a membrane extruder (LiposoFast™; Avestin, ON, Canada) using membranes with 200 nm pore sizes (Whatman® Nuclepore™ membrane; Thermo Fisher Scientific, Waltham, MA, USA) at 65°C for 10 cycles. Gel filtration (Sephadex G50) was carried out to separate the MLL or ULL from the nonencapsulated propranolol. To coat liposomes with chitosan, 2 mL liposome solution was mixed with 6 mL aqueous solution of chitosan (1.5%) and incubated at 4°C overnight.
PLIM was prepared by the modified double emulsion method. Propranolol-loaded liposomes (PL) solution (0.2 mL) was dispersed into an organic phase consisting of 150 mg PLGA-PEG-PLGA dissolved in 3 mL ethyl acetate, by vortex mixing, resulting in a water/oil (W/O) emulsion. The W/O emulsion was injected dropwise into 50 mL 2% PVA aqueous solution and was mechanically stirred at 1,000 rpm for 5 min. The W/O/W emulsion obtained was poured into 450 mL 2% PVA aqueous solution. Mechanical stirring at 500 rpm was performed for 3 h to extract/evaporate the organic solvent to form solid microspheres. The final product was obtained after filtration, washing, and freeze-drying.
For convenience, the following designations are used for abbreviations: propranolol-loaded liposomes (PL), PL-chitosan (PL coated with chitosan), liposomes-in-microsphere (LIM), and propranolol-loaded liposomes-in-microsphere (PLIM). Drug-free liposomes or microspheres are designated as blank liposomes or microspheres.
Size and zeta potential
After liposomes were dispersed in deionized water, their particle size and zeta potential were analyzed using a Zetasizer Nano S (Malvern instruments, Malvern, UK). The size distribution of microspheres was tested by a Malvern Mastersizer 2000 particle size analyzer (Malvern Instruments).
The drug encapsulation efficiency and loading of propranolol
The encapsulation efficacy and drug loading of propranolol in formulations were determined by high-performance liquid chromatography (HPLC) as described.
After 1 mL of liposome solution was vacuum dried, they were fully dissolved in 1 mL of methanol to obtain a clear solution sample for HPLC (L-2000; Hitachi, Tokyo, Japan) analysis. Alternatively, 5 mg PLIM was fully dissolved in 1 mL dichloromethane. The dichloromethane solution was evaporated in a vacuum evaporator, and then 1 mL of methanol was added to form a clear solution sample for HPLC analysis. The HPLC was equipped with an ODS C-18 column (Hypersil™, 250×4.6 mm, 5 μm), and the mobile phase consisted of methanol/0.02 M monopotassium phosphate (65:35, v/v). The flow rate was set at 1 mL/min. The detection wavelength and column temperature were set at 289 nm and 25°C, respectively. The injection volume was 20 μL. All the samples were filtered through a syringe filter (0.45 μm pore size; Millipore, Billerica, MA, USA) before HPLC analysis.
The encapsulation efficacy of propranolol in the formulations was calculated using the following formula: ME/MT ×100%. ME and MT are defined as the mass of encapsulated propranolol and the mass of totally added propranolol, respectively. The drug loading of propranolol was calculated using the following formula: ME/MN ×100%. ME and MN are defined as the mass of encapsulated propranolol and the mass of liposomes or PLIM, respectively.
In vitro drug release
An in vitro release study was performed to evaluate the propranolol release from PL or PLIM. Briefly, 2 mL PL solution or 10 mg PLIM was transferred to a dialysis membrane (MWCO 1000, Spectra/Por®; Spectrum Laboratories Inc., Los Angeles, CA, USA). The sealed tube was then introduced into a vial containing phosphate buffered saline (PBS) (60 mL; pH 7.4) with or without 10% FBS. The vial was secured in a water bath at 37°C with stirring (100 rpm). At predetermined time intervals, a 2 mL aliquot of dialysate was taken and replaced with 2 mL of fresh solution. The amount of propranolol in the dialysate was determined by HPLC as described earlier.
Cytotoxicity assays
The cytotoxic effect of propranolol against the HemSCs was measured using a Cell Counting Kit-8 kit (CCK-8 kit; Dojindo laboratories, Kumamoto, Japan). Briefly, HemSCs (10,000 per well) were seeded at subconfluency on a fibronectin-coated 24-well plate in EGM-2 with 20% FBS. Four hours later, the media was removed and the cells treated with various concentrations of propranolol, PL, and PLIM. After a period of time, the cell viability was determined using the CCK-8 kit. Briefly, the cell viability was evaluated by adding CCK-8 (10 μL) solution to each well of the plate. After incubation for 2 h, the absorbance was measured at 450 nm/630 nm using a BIO-TEK ELx800 Universal Microplate Reader (Bio-Tek, Winooski, VT, USA). The cell viability was calculated using the formula: (AE − AB)/(AC − AB) ×100%. AE, AC, and AB are defined as the absorbance of experimental samples, untreated samples, and blank controls, respectively. Untreated samples, defined as samples without any drug treatment, were used as negative controls.
VEGF-A and bFGF measurement by ELISA
The effect of propranolol on the production of vascular endothelial growth factor-A (VEGF-A) and basic fibroblast growth factor (bFGF) in HemSCs was measured using commercially available VEGF-A or bFGF enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). Briefly, HemSCs (200,000 per well) were seeded on a fibronectin-coated 6-well plate in EGM-2 with 20% FBS. Four hours later, the media was removed and the cells treated with various concentrations of propranolol, PL, and PLIM. After 96 hours, the VEGF-A and bFGF concentrations of the supernatant of the treated cells were measured using the VEGF-A or bFGF ELISA kits as described by the manufacturer’s instruction.
Animal studies
All mice were purchased from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). Before use in the following studies, the mice were placed in a pathogen-free environment and allowed to acclimate for a week. All procedures were approved by the Committee on Animals of the Second Military Medical University (Shanghai, People’s Republic of China), and all procedures were performed in accordance with the guidelines of the Committee on Animals of the Second Military Medical University (Shanghai, People’s Republic of China).
To study the effects of propranolol on HemSCs in vivo, a xenograft mouse model of infantile hemangioma was used. In brief, 1.5×106 HemSCs suspended in Matrigel (BD Matrigel™ Basement Membrane) was implanted subcutaneously into the flanks of female nude mice (6~8 weeks, ~20 g). When hemangioma had reached about 25 mm3 in size (day 0), mice were treated with single intratumoral (IT) injections of either formulations (PL or PLIM) or free propranolol (2 mg propranolol/kg). Treatments were carried out on days 0, 5, 10, 15, 20, and 25. The hemangioma was measured using a caliper every 5 days, and the hemangioma volume was calculated using the formula: (width2 × length)/2. The body weight of the mice was monitored every 5 days. On day 35, the mice were euthanized, and the hemangioma was weighed. The hemangioma was collected, fixed overnight at 4°C in 10% formalin, dehydrated, and embedded in paraffin for histological analysis. The microvessel density (MVD) analysis of the histological sections was performed as described before.24
Statistical analysis
Data in this study were analyzed using the software SPSS 13.0 (SPSS Inc., Chicago, IL, USA). For values that were normally distributed, a direct comparison between two groups was conducted using Student’s nonpaired t-test. One-way analysis of variance with the Dunnett’s or Newman Keuls posttest was used to compare the means of three or more groups. A P-value of <0.05 was considered statistically significant. *P<0.05; **P<0.01; ***P<0.001; NS represents not significant (P>0.05).
Results
Preparation of PLIM using lipid film-based and double emulsion methods
The detailed preparation procedure of PLIM is described in Figure 1, and it uses the lipid film-based and double emulsion methods. We chose DSPC as the phospholipids in liposome preparation since it is saturated and has high phase transition temperature (melting temperature: 55°C), thus contributing to the high stability of liposomes. Lipid film-based method was chosen to load propranolol in liposomes, since it is a convenient approach to encapsulate hydrophilic drugs in liposomes. Encapsulation of liposomes in microsphere is very critical for the successful preparation of PLIM. Although the hydrophilic head groups on liposomes could have some protective effects, liposomes could be readily damaged by the organic solvent used for the preparation of PLIM, since phospholipids are soluble in organic solvents. Chitosan modification on liposomes could be performed to keep the integrity of liposomes in microspheres. Double emulsions are complex systems, also called “emulsions of emulsions”, in which the droplets of the dispersed phase contain one or more types of smaller dispersed droplets themselves. Double emulsions are commonly used for the encapsulation of hydrophilic drugs which suffer from low encapsulation efficiency because of rapid drug partitioning into the external aqueous phase when using single emulsions. Thus, we chose the double emulsion method to encapsulate hydrophilic liposomes in the microsphere made of PLGA-PEG-PLGA.
Characteristics of PL and PLIM
The data regarding size, zeta potential, polydispersity index (PDI), encapsulation efficacy (EE), and drug loading of PL and PLIM are summarized in Table 1. PL had a small size of 198 nm and low PDI of 0.18, indicating a homogeneous size distribution. It was noted that PL showed negative zeta potential of −7.5 mV. Generally, zeta potential of −5 mV to −8 mV would be appropriate for nanoparticles as it contributes to both dispersibility and cell accessibility of nanoparticle.25 Thus, it is expected that our prepared liposomes may accumulate in hemangioma more efficiently. The EE of propranolol in PL was not so high but generally acceptable (~40%), since liposomes tend to load hydrophobic drugs with high EE. However, the EE of hydrophilic drugs in liposomes is not high due to the small aqueous internal volume. We observed the changes of size and zeta potential in liposomes after chitosan coating. After chitosan coating, the size of liposomes was slightly increased from 198.5 nm of PL to 215.3 nm of PL-chitosan, and the zeta potential of liposomes was changed from −7.5 mV of PL to +32.1 mV of PL-chitosan. Since chitosan is a cationic polymer, the size increase and charge reversal in the liposomes after chitosan coating demonstrated that the chitosan coating actually occurred. PLIM had a size of 77.8 μm and low PDI of 0.26. The EE of propranolol in PLIM was 23.9%, lower than that of PL, since microspheres could not encapsulate all liposomes.
The drug release profile is a critical parameter for drug delivery systems. As shown in Figure 2A, PL showed a quick drug release of propranolol, as reflected by >50% release at day 1, and >90% release after day 4. The quick drug release in PL is readily comprehensible, since encapsulated drugs in liposomes tend to permeate across liposomal membrane quickly, and the instability of liposomes both in circulation and storage severely hampers the clinical use of liposomes.26–28 Notably, the drug release of propranolol was significantly retarded after the liposomes were encapsulated in microspheres (Figure 2A). As for PLIM, only 8% of propranolol was released at day 1 and 35% at day 4. Furthermore, the release of propranolol reached the plateau (~75%) after day 20. We also evaluated the drug release of PL and PLIM in the presence of PBS-containing serum, which could better mimic the actual environment in vivo (Figure 2B). PLIM showed a slight quicker release in PBS-containing serum than in PBS on days 1, 2, and 20 (P<0.05), suggesting that serum could destabilize PLIM to some extent. Once again, the drug release of propranolol was still significantly slower in PLIM than in PL in PBS-containing serum (Figure 2B). Taken together, the drug release of propranolol in PLIM was much slower than that in PL, suggesting that encapsulation of propranolol-loaded liposomes in microspheres successfully conquered the quick release of propranolol from liposomes.
Cytotoxicity toward HemSCs
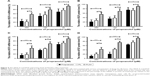
First, we evaluated the cytotoxicity of blank liposomes and blank LIM without loading propranolol. The result indicated that both blank liposomes and blank LIM showed little toxicity to HemSCs at the lipid concentration from 15~8,000 μg/mL or the polymer concentration from 40~20,000 μg/mL (corresponding to the concentration of propranolol used in the cytotoxicity assay), as reflected by the fact that the cell viability still exceeded 85%, even at the highest lipid or polymer concentration (Figure 3A and B). Next, we incubated free propranolol, PL, or PLIM at propranolol concentrations of 2~1,000 μM for 72 h or 120 h and evaluated the cell viability using the CCK-8 assay (Figure 3C and D).
As shown in Figure 3C and D, propranolol, PL, and PLIM showed a dose-dependent cytotoxicity toward HemSCs. Their half-maximal inhibitory concentration (IC50) values in HemSCs were calculated and are shown in Table 2. The 72 h IC50 values of propranolol, PL, and PLIM were found to be 110.5, 120.8, and 379.3 μM, respectively (suggesting that PL showed similar cytotoxic effect as propranolol, and PLIM was 3.4 times less effective than propranolol), and the 120 h IC50 values of propranolol, PL, and PLIM were found to be 40.8, 56.5, and 120.7 μM, respectively (suggesting that PL showed similar cytotoxic effect as propranolol, and PLIM was 2.95 times less effective than propranolol). Thus, the cytotoxic effect induced by propranolol was maintained when encapsulation in liposomes, and reduced after encapsulation in PLIM. We speculate that the reason may be attributed to the quick release of propranolol from PL (>90% of release at day 3) and slow release of propranolol from PLIM (30% of release at day 3).
The VEGF-A and bFGF expression level of HemSCs
VEGF-A and bFGF and are both heparin-binding growth factors capable of inducing angiogenesis in vitro and in vivo.29 We evaluated the expression of VEGF-A and bFGF using both mRNA and protein levels after treatment. The relative mRNA or protein level was expressed as the percentage of the mRNA or protein of treated group relative to the untreated group.
As can be seen in Figure 4A, propranolol, PL, and PLIM exerted a dose-dependent inhibition of VEGF-A mRNA expression in HemSCs. It was noted that at the concentrations of propranolol ranging from 30 to 500 μM, propranolol and PL were significantly more efficient at inhibiting the expression of VEGF-A mRNA than PLIM (P<0.05). For example, propranolol and PL at 500 μM inhibited the expression of VEGF-A mRNA by ~80%, whereas PLIM at the same concentration only inhibited the expression by ~40%. Consistently, propranolol and PL were significantly more effective at inhibiting the expression of VEGF-A protein than PLIM at concentrations of propranolol ranging from 30 to 500 μM (P<0.05, Figure 4B). At 500 μM, propranolol and PL inhibited the expression of VEGF-A protein by ~80%, whereas PLIM only inhibited the expression of VEGF-A protein by ~55%. For bFGF, propranolol and PL were also significantly more effective at inhibiting the mRNA and protein expression than PLIM (P<0.05, Figure 4C and D). Taken together, propranolol and PL were more effective at inhibiting the mRNA and protein expression of VEGF-A and bFGF than PLIM. We speculate that the reason may be attributed to the quick release of propranolol from liposomes and slow release of propranolol from PLIM.
The inhibition of subcutaneous hemangioma growth by treatments in vivo
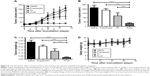
We next examined the therapeutic effect of propranolol, PL, and PLIM in mice bearing subcutaneous hemangioma. As shown in Figure 5A, by day 35 (the end time point), PLIM treatment had resulted in an 84% decrease in hemangioma volume, whereas PL and propranolol treatment had only resulted in a 44% and 13% decrease in hemangioma volume, respectively. By the end of the experiment, compared to the initial hemangioma volume (25 mm3), the hemangioma volume in the propranolol-treated mice had increased by 7.2-fold and the hemangioma volume in the PL-treated mice had increased by 4.8-fold. The hemangioma volume in the PLIM-treated mice did not increase significantly. The hemangioma volume of the PLIM-treated group was significantly smaller than that of other groups (saline =215 mm3, propranolol =187 mm3, PL =121 mm3, PLIM =35 mm3; PLIM vs saline: P<0.001, PLIM vs propranolol: P<0.001, PLIM vs PL: P<0.001) (Figure 5B).
The hemangiomas excised at the endpoint were weighed (Figure 5C). The mean hemangioma weight of the PLIM-treated group was significantly lower than that of other groups (saline =0.28 g, propranolol =0.21 g, PL =0.13 g, PLIM =0.03 g; PLIM vs saline: P<0.001, PLIM vs propranolol: P<0.001, PLIM vs PL: P<0.001). Propranolol inhibited hemangioma growth to a greater extent than saline (P<0.05). The mean hemangioma weight was significantly lower in the PL-treated group than in the propranolol-treated group (P<0.05) and the saline-treated group (P<0.01).
The toxicity of all treatments was measured by observing any behavioral changes posttreatment and by monitoring mice weight. The results showed that all of the treatments were well-tolerated by mice bearing hemangioma. None of the treated mice showed any noticeable behavioral change and no significant change in weight compared to the saline control, suggesting that propranolol, PL, and PLIM did not induce noticeable behavioral change of mice and have no detrimental effect on the weight of mice (Figure 5D).
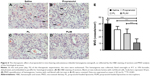
At the end of the experiment, the hemangioma was stained with hematoxylin and eosin (Figure 6A–D), and the MVD analysis of the histological sections was performed (Figure 6E). Compared with the saline-injected mice, propranolol caused significant inhibition of vascularization of the hemangioma (P<0.05). The mean MVD of the PLIM-treated group was significantly lower than that of other groups (saline =40 vessels/mm2, propranolol =31 vessels/mm2, PL =25 vessels/mm2, PLIM =11 vessels/mm2; PLIM vs saline: P<0.001, PLIM vs propranolol: P<0.01, PLIM vs PL: P<0.05), suggesting that PLIM was the most effective at inhibiting the vascularization of hemangioma among all the groups.
Discussion
Infantile hemangioma is a benign vascular tumor affecting approximately 4%~10% of infants. Although benign, rapid growth of the tumor could result in serious morbidity, mortality, and numerous complications. To reduce the adverse effects and high frequency of administration of propranolol in the treatment of infantile hemangioma, we first utilized PLIM as a topical controlled-release system to realize the sustained release of propranolol. Our study demonstrated that PLIM showed a sustained release of propranolol, achieving superior therapeutic efficacy compared to propranolol and significantly reducing the frequency of administration of propranolol.
The selection of which antihemangioma drug to use is critically important for superior activity of our prepared PLIM. The choice of propranolol in our prepared PLIM is rational and would achieve superior effects as expected. The superior effectiveness and reduced side effects of propranolol, coupled with the immediate availability of the medication in a pediatric formulation, have led to a rapid and widespread adoption of propranolol for infantile hemangioma.5 As it should be, the application of propranolol for infantile hemangioma will be more beneficial and widespread if its frequency of administration and side effect could be reduced.
Changing the route of administration of drugs is a practical route to enhance the therapeutic efficacy and reduce the side effects of drugs.5 In this study, to overcome the side effects of propranolol and its high frequency of administration, we developed a practical sustained-release system defined as PLIM to release propranolol, which resulted in significant therapeutic outcome for longer times with decreased drug quantities and frequency of administration. The data presented here confirmed that PLIM showed a prolonged release of propranolol for 40 days. In contrast, PL released >90% of propranolol after only 4 days. Although PLIM was less efficiently in inhibiting the proliferation of HemSCs than free propranolol and PL in vitro, the therapeutic effect of PLIM in hemangioma was superior to that of free propranolol and PL in vivo, as reflected by significantly reduced hemangioma volume, weight, and MVD. Since propranolol is restricted to cell culture plates in vitro, the cytotoxic effect of propranolol depends on the concentration of propranolol. Obviously, the slow release of propranolol from PLIM would result in reduced cytotoxic effect. However, after IT injection, propranolol and PL would undergo quick elimination of propranolol from hemangioma, resulting in poor therapeutic efficacy toward hemangioma. In contrast, the prolonged and sustained release of propranolol from PLIM would significantly retard the angiogenesis in hemangioma. To our knowledge, we have shown for the first time that propranolol achieved a sustained release from microspheres after IT injection.
The safety of drug delivery systems is an important issue for their clinical use.30 In this study, PLIM did not show any major systemic toxicity. Since the safety of propranolol, liposomes, chitosan, and PLGA has been approved by FDA, the use of PLIM for therapeutic purposes in clinic is promising. As it should be, the detailed in vivo distribution and more safety data of PLIM should be investigated in further studies. Further, the IT injection strategy of our prepared drug delivery system is safe in clinic. Based on our clinical experience for ten-year treatment of infantile hemangioma, we can safely conclude that needle injection into hemangioma does not induce bleeding even in big hemangiomas, and the slight bleeding that occurs can be easily suppressed by hand compression. Further, the IT injection of drugs such as corticosteroids is a very common strategy in the treatment of infantile hemangioma.31
Our data have helped to elucidate the mechanism of the antihemangioma activity of PLIM. After IT injection of PLIM into hemangioma, liposomes have to diffuse through tortuous water channels of microspheres. As time passes, the degradation of the polymer matrix causes expansion of the tortuous water channels, thereby leading to a sustained release of liposomes. When the liposomes are released, the liposomes could still act as a reservoir for sustained release of propranolol, and propranolol is slowly released from liposomes after liposomal membrane is disrupted. After release of propranolol from PLIM, the free propranolol will bind to the β-adrenergic receptor, resulting in inhibition of proliferation of HemSCs and reduced expression of angiogenesis factors including VEGF-A and bFGF. The reduced expression of VEGF-A and bFGF will significantly retard the angiogenesis in hemangioma. Further, after PLIMs are delivered by direct injection into hemangioma, they would be entrapped in the hemangioma vasculature due to chemoembolization effects. After the vasculature of hemangioma is blocked, the oxygen and nutrients will be difficult to be delivered to the hemangioma, resulting in regression of hemangioma. In contrast, when free propranolol is injected into hemangioma, it would be eliminated quickly by blood circulation, resulting in poor therapeutic efficacy of hemangioma.
One limitation in our study is that the therapeutic efficacy of propranolol-loaded microspheres needs to be compared with that of PLIM. In fact, we tried hard to prepare propranolol-loaded microspheres in the initial stage of our study, but the encapsulation efficiency of propranolol in microspheres is extremely low (<1%). Nevertheless, we fully recognized that it is important to compare propranolol-loaded microspheres and PLIM. To validate the superior traits of PLIM, future preclinical studies should be directed to compare propranolol-loaded microspheres and PLIM in a wide range of studies.
Conclusion
The adverse effects and too frequent administration severely hamper the clinical use of propranolol in the treatment of infantile hemangioma. In this study, we firstly utilized PLIM as a topical controlled-release system to realize the sustained release of propranolol. Our study demonstrated that PLIM showed sustained release of propranolol, achieving superior therapeutic efficacy compared to propranolol and significantly reducing the frequency of administration of propranolol. Our findings show that PLIM is a very promising approach to locally and efficiently deliver propranolol to the hemangioma, site leading to a significant inhibition of the infantile hemangioma.
Acknowledgments
This work was supported by the Henan Provincial Science and Technology Plan Project (project number: 142102310080). We acknowledge the helpful comments on this article received from our reviewers.
Disclosure
The authors report no conflicts of interest in this work.
References
Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008;25(2):168–173. | ||
Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341(3):173–181. | ||
Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131(1):99–108. | ||
Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118(3):882–887. | ||
Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131(1):128–140. | ||
Cheng CE, Friedlander SF. Infantile hemangiomas, complications and treatments. Semin Cutan Med Surg. 2016;35(3):108–116. | ||
Léaute-Labrèze C, Boccara O, Degrugillier-Chopinet C, et al. Safety of oral propranolol for the treatment of infantile hemangioma: a systematic review. Pediatrics. 2016;138(4):e20160353. | ||
Laken PA. Infantile hemangiomas: pathogenesis and review of propranolol use. Adv Neonatal Care. 2016;16(2):135–142. | ||
Pouton CW, Porter CJ. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60(6):625–637. | ||
Almeida H, Amaral MH, Lobao P, Frigerio C, Sousa Lobo JM. Nanoparticles in ocular drug delivery systems for topical administration: promises and challenges. Curr Pharm Des. 2015;21(36):5212–5224. | ||
Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282(1–2):1–18. | ||
Gao J, Xia Y, Chen H, et al. Polymer-lipid hybrid nanoparticles conjugated anti-EGFR antibody for targeted drug delivery to hepatocellular carcinoma. Nanomedicine (Lond). 2014;9(2):279–293. | ||
Van Slooten ML, Boerman O, Romøren K, Kedar E, Crommelin DJ, Storm G. Liposomes as sustained release system for human interferon-gamma: biopharmaceutical aspects. Biochim Biophys Acta. 2001;1530(2–3):134–145. | ||
Xiao C, Qi X, Maitani Y, Nagai T. Sustained release of cisplatin from multivesicular liposomes: potentiation of antitumor efficacy against S180 murine carcinoma. J Pharm Sci. 2004;93(7):1718–1724. | ||
Bittman R, Clejan S. Kinetics of cholesterol and phospholipid exchange between mycoplasma membranes and lipid vesicles. Isr J Med Sci. 1987;23(5):398–402. | ||
New RRC. Liposomes: A Practical Approach. Oxford, UK: Oxford University Press; Revised edition. ISBN-10: 0199630771. ISBN-13: 978-0199630776. | ||
Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28(1):5–24. | ||
Dass CR, Walker TL, Kalle WH, Burton MA. A microsphere-liposome (microplex) vector for targeted gene therapy of cancer. II. In vivo biodistribution study in a solid tumor model. Drug Deliv. 2000;7(1):15–19. | ||
Feng SS, Ruan G, Li QT. Fabrication and characterizations of a novel drug delivery device liposomes-in-microsphere (LIM). Biomaterials. 2004;25(21):5181–5189. | ||
Wong A, Hardy KL, Kitajewski AM, et al. Propranolol accelerates adipogenesis in hemangioma stem cells and causes apoptosis of hemangioma endothelial cells. Plast Reconstr Surg. 2012;130(5):1012–1021. | ||
Khan ZA, Boscolo E, Picard A, et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118(7):2592–2599. | ||
Yang K, Lu Y, Xie F, et al. Cationic liposomes induce cell necrosis through lysosomal dysfunction and late stage autophagic flux inhibition. Nanomedicine (Lond). 2016;11(23):3117–3137. | ||
Gong Z, Chen D, Xie F, et al. Co-delivery of propranolol and doxorubicin using nanoliposomes for targeting both liver cancer cells and cancer stem cells. Nanomedicine (Lond). 2016;11(19):2565–2579. | ||
Greenberger S, Yuan S, Walsh LA, et al. Rapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangioma. J Invest Dermatol. 2011;131(12):2467–2476. | ||
Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 2010;6(1):12–21. | ||
Su X, Song H, Niu F, et al. Co-delivery of doxorubicin and PEGylated C16-ceramide by nanoliposomes for enhanced therapy against multi-drug resistance. Nanomedicine (Lond). 2015;10(13):2033–2050. | ||
Xie F, Xu W, Yin C, Zhang G, Zhong Y, Gao J. Nanomedicine strategies for sustained, controlled, and targeted treatment of cancer stem cells of the digestive system. World J Gastrointest Oncol. 2016;8(10):735–744. | ||
Song H, Su X, Yang K, et al. CD20 antibody-conjugated immunoliposomes for targeted chemotherapy of melanoma cancer initiating cells. J Biomed Nanotechnol. 2015;11(11):1927–1946. | ||
Mason JC, Lidington EA, Ahmad SR, Haskard DO. bFGF and VEGF synergistically enhance endothelial cytoprotection via decay-accelerating factor induction. Am J Physiol Cell Physiol. 2002;282(3):578–587. | ||
Nystrom AM, Fadeel B. Safety assessment of nanomaterials: implications for nanomedicine. J Control Release. 2012;161(23):403–408. | ||
Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362(11):1005–1013. | ||
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;27;9(2)pii:E12. |
Supplementary material
  | Table S1 Real-time-PCR primers |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.