Back to Journals » OncoTargets and Therapy » Volume 9
Concurrent cisplatin-based chemoradiotherapy versus exclusive radiotherapy in high-risk cervical cancer: a meta-analysis
Authors Meng X, Liao Y, Liu X, Li S, Shi M, Zeng X
Received 30 September 2015
Accepted for publication 5 February 2016
Published 31 March 2016 Volume 2016:9 Pages 1875—1888
DOI https://doi.org/10.2147/OTT.S97436
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Xiang-Yu Meng,1,* Yi Liao,2,* Xiao-Ping Liu,3 Sheng Li,1 Ming-Jun Shi,4 Xian-Tao Zeng1
1Center for Evidence-Based and Translational Medicine, Zhongnan Hospital of Wuhan University, Wuhan, Hubei Province, People’s Republic of China; 2Department of Oncology, Nanfang Hospital, Southern Medical University, GuangZhou Province, People’s Republic of China; 3Department of Hematology and Oncology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei Province, People’s Republic of China; 4Institut Curie, Paris Sciences et Lettres Research University, Le Centre National de la Recherche Scientifique, Les Unités Mixtes de Recherche 144, F-75005, Paris, France
*These authors contributed equally to this work
Objective: To evaluate the efficacy and safety of cisplatin-based concurrent chemoradiotherapy (DDP-CCRT) in patients with high-risk cervical carcinoma (CC) compared with exclusive radiotherapy (RT).
Materials and methods: Databases were searched for randomized controlled trials (RCTs) and cohort studies comparing DDP-CCRT with RT alone. Risk of bias assessment for RCTs was performed using the Cochrane Collaboration’s tool, and the Newcastle–Ottawa quality scale was used to perform quality assessment for cohort studies. Meta-analysis was conducted using Review Manager 5 and Stata 12.0 software.
Results: Finally, eight RCTs and three cohort studies containing 2,130 subjects were included. Analysis on total failures revealed a statistically significant difference in favor of DDP-CCRT (risk ratio =0.77, 95% confidence intervals [CIs]: 0.67–0.89). No significant heterogeneity was detected for pooled analysis concerning overall survival; the result of which demonstrated the superiority of DDP-CCRT over RT alone (hazard ratio =0.68, 95% CI: 0.57–0.80), and stable and established accumulative effects were observed in cumulative meta-analysis. Similar results were observed for progression-free survival (hazard ratio =0.63, 95% CI: 0.50–0.76). In terms of treatment-related Grade 3 and 4 adverse events, our pooled analysis with a fixed-effects model showed significantly enhanced toxicity in the DDP-CCRT group compared with that in the RT group (odds ratio =3.13, 95% CI: 2.37–4.13).
Conclusion: Solid and stable beneficial effects are associated with DDP-CCRT, and its superiority over comparative RT in patients with high-risk CC is confirmed. DDP-CCRT should be considered one of the frontline treatment options for high-risk CC patients without contraindications. However, enhanced toxicity associated with DDP-CCRT should never be ignored.
Keywords: cervical carcinoma, concurrent chemoradiotherapy, meta-analysis, cisplatin, radiotherapy, survival
Introduction
Cervical cancer (CC) is one of the most common malignancies among females worldwide, with 400,000–500,000 new cases identified annually; the incidence of which varies across different countries and regions.1 In developing and undeveloped countries, a much more severe prevalence of this malignancy is associated with a generally worse economical and sanitary condition, lack of effective screening, as well as underimplemented prevention strategy, where a lot of women were exposed to the risk of, or already affected by, high-risk CC, which remains a major health problem for women in these countries, though important advancement and progress has been witnessed in the last few years.2,3
In CC patients without distant metastasis, several factors have been demonstrated as directly associated with a worse prognosis, including locally advanced disease, bulky tumor, deeply invasive disease, and pelvic lymph node or parametrial involvement. Patients with the aforementioned characteristics are at higher risk of recurrence and generally have a shorter survival period.4–8 Primarily applied conventional treatment modality for high-risk CC is radiotherapy (RT) with or without hysterectomy; however, inefficient local control and lymph node metastasis remain the major causes of treatment failure.6,9,10 Therefore, treatment strategy combining RT with chemotherapy has been evaluated in a lot of clinical trials, initially in several pilot studies published ~15 years ago, most of which were randomized controlled trials (RCTs).11–18 In these trials, concurrent chemoradiotherapy (CCRT) was the experimental treatment mode most widely assessed. Chemotherapy, at first, was applied exclusively as palliative care for patients with unfavorable prognosis. Among the drugs used for chemotherapy in advanced CC, cisplatin was one of the most effective agents.19 Thus, cisplatin was primarily selected as one of the drugs tested in trials investigating CCRT. Among early researches comparing cisplatin-based concurrent chemoradiotherapy (DDP-CCRT) with RT, results with apparent discrepancies were reported. Four studies reported positive results, with a maximum risk reduction of 49% for estimated 4-year overall survival (OS), which supported the superiority of DDP-CCRT.11,14,17,18 However, no significant benefits in favor of DDP-CCRT concerning survival outcomes and toxicity profile were revealed in two other studies.12,16 These differences might be attributed to different study designs, subjects enrolled, control settings, regimens used, and duration of follow-up.
In 2002, a meta-analysis summarized these pilot studies, which presented positive results and comments recognizing improved outcomes achieved by DDP-CCRT, as well as evident toxic effects possibly enhanced by treatment combination.20,21 However, the results of the meta-analysis showed that interventions for control groups were not totally consistent among the included studies.11–15,17,18 Without considering surgical treatment performed in two studies as part of the local interventions for both experimental and control arms,15,18 exclusive RT were set as control in four studies;12,14,15,18 however in another two studies,11,13 hydroxyurea, a widely used cytotoxic agent with antitumor activity targeting a variety of malignancies, was combined with RT as control group treatment.
Afterward, several similar studies comparing DDP-CCRT with RT alone were conducted, with different results reported.22–28 Although more than 2 decades have passed since the initial application of DDP-CCRT in treating high-risk CC patients, during which time new agents and modalities have been developed, tested, and utilized, DDP remains in the first-line drug list for this specific population. Most recently, an RCT conducted in Brazil again evaluated the difference in treatment effects between DDP-CCRT and exclusive RT in advanced CC, using patients with International Federation of Gynecology and Obstetrics (FIGO) Stage III disease as the targeted population.22 With accumulated and updated data from relevant studies available for a new pooled analysis, we performed this meta-analysis with refined design and analytical methods to provide more definitive evidence for clinical guidance.
Materials and methods
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.29
Eligibility criteria
According to the PICOS,29 the following criteria were used for study selection:
Participants: We included CC patients with high-risk factors; the definitions of which were provided in previous studies.4–8
Intervention: DDP-CCRT.
Comparator: CRT.
Outcomes: In this study, survivals were chosen as primary outcome measures for combined analyses, including OS and progression-free survival (PFS); OS was defined as the time from randomization till death caused by any cause, and PFS was defined as the time from randomization till local recurrence/progression, metastasis, or death by any cause. Secondary outcome measures consisted of treatment failure, early efficacy, and toxicity profile. Treatment failure included three aspects, ie, locoregional refractory/recurrent disease, distant metastasis, and combined local–distant failure. Early efficacy focused on complete response (CR) and partial response (PR). Treatment-related Grade 3 and 4 adverse events were chosen as the index to illustrate toxicity profile.
Study design: RCTs and comparative cohort studies.
We excluded the study based on the following exclusion criteria: 1) lack of full text – we contacted the author and got no response; 2) lack of important information – we contacted the author and got no response; 3) duplicate reports of single studies; and 4) radiation modalities were different in experimental and control groups. It should be noted that adjuvant surgical interventions consistent in both arms would not affect the eligibility of studies that met all the selection criteria. If complementary valuable information was found in multiple reports of one trial, it was possible that different reports of a single study were included with different parts of data extracted for subsequent analyses.
Information source and search strategy
Computerized databases, such as PubMed, Embase, and the Cochrane Library, were searched using keywords “cervical cancer”, “cervical carcinoma”, “cisplatin”, “chemoradiotherapy”, “radiotherapy” with all possible combinations, for studies comparing DDP-CCRT with RT alone. The full search strategy is included in the “Supplementary material” section. The reference lists of the studies identified, relevant systemic reviews, and practice guidelines were also examined for additional potentially related studies.
Data extraction
All the titles and abstracts obtained from the results of the search strategy were screened to select potentially eligible articles. After full-text papers were independently reviewed by two investigators, the eligibility of these articles was further verified to ensure that they met all the selection conditions. Data from studies eligible for meta-analysis were independently extracted by two investigators. These data included the name of the first author, year of publication, country, study design, number of patients, clinical–pathological stage, and follow-up information. For survival outcomes, hazard ratio (HR) with corresponding 95% confidence interval (CI) was extracted. Risk ratio (RR) calculated by Cox model was considered identical to HR. If HR was not available in the text, survival curves provided in the article would be used to calculate HR and 95% CI, according to previously published methods.30–32 Disagreement was thoroughly verified by a third investigator.
Risk of bias assessment
Risk of bias was assessed according to prespecified criteria from the Cochrane Collaboration’s tool.32,33 Briefly, the following items were evaluated: sequence generation, allocation concealment, blinding, completeness of outcome data, and other source of bias.
The quality of cohort studies was assessed using the Newcastle–Ottaw scale (NOS). This quality assessment tool covered the following aspects: selection of study groups (0–4 stars), comparability between groups (0–2 stars), and ascertainment of outcome of interest (0–3 stars) for cohort studies, respectively.
Statistical analysis
Statistical analyses were performed using the Review Manager 5 and Stata SE 12.0 software. HRs and 95% CIs were computed for survival statistics. Heterogeneity among studies was assessed using the χ2 test and I2 statistic, and a statistical heterogeneity of <25%, 25%–50%, and >50% was defined as low, moderate, and high, respectively.34 If no significant heterogeneity was detected, a fixed-effects model would be used; otherwise, a random-effects model would be used. We carried out cumulative meta-analyses based on the order of publication year for survival outcomes. Subgroup analyses and metaregression analyses were conducted to further explore potential confounding bias of studies reporting survival. For all the statistical analyses, a P-value <0.05 was set as the level of significance. The publication bias was examined using the funnel plot; the results of which were further verified with Begg’s test.35
Results
Characteristics of included studies
A total of 316 citations were retrieved, and is represented by a search flowchart as shown in Figure 1. After a refined selection according to the inclusion criteria, eight RCTs and three cohorts trials were finally included.12,15,16,18,22–28 A total of 2,230 patients were involved, 1,098 of whom had received radiation and DDP-CCRT regimen. The majority of the eligible trials reported primary outcome measures, ie, OS and PFS. Details of the eleven included articles are provided in Table 1.
  | Figure 1 Flow diagram of meta-analysis. |
Quality assessment
As listed in Table 2, according to the Cochrane Collaboration bias assessment tool, all of the eight RCTs were adequately randomized (Figure S1). Only one study22 described the allocation concealment. All of the included RCTs had not reported their blinding methods (either for participants or for appraisers). In addition, incomplete data were provided in one study,18 while another study26 selectively reported tumor response.
  | Table 2 Risk of bias assessment |
As evaluated by the NOS tool, the three cohort studies were considered high quality (8 stars, 7 stars, and 8 stars for Chen et al’s,28 Han and Kong’s,27 and Torbe et al’s23 reports, respectively).
Early efficacy
Data on early efficacy, ie, tumor response, were reported in five studies.12,23,25–27 Among these studies, Nagy et al25 only provided data on CR, while Torbe et al23 only reported CR + PR. Statistically significant heterogeneity was observed, with an I2 of 77% and 74% for CR and CR + PR, respectively. The pooled analysis with a random-effects model showed that statistical significance was reached in none of these comparisons (RR and 95% CI were 1.02 [0.77–1.34] and 0.96 [0.83–1.11] for CR and CR + PR, respectively; Figure 2).
Treatment failures
Patterns of treatment failure were evaluated in four RCTs and one cohort study.12,22,24,25,28 Apart from the trial of Zuliani et al,22 which only provided the total number of treatment failures, the other four studies separately provided the number of local failure, distant failure, and combined failure (local + distant). In particular, in Nagy et al’s25 report, combined failures were observed in none of the involved patients. The I2 value was 34%, 63%, 0%, and 0% for total failure, local failure, distant failure, and combined failure, respectively. In our meta-analyses, although no significant differences were demonstrated for the three subtotal comparisons (local: RR =0.75, 95% CI: 0.47–1.20 [Figure 3B]; distant: RR =0.93, 95% CI: 0.68–1.27 [Figure 3C]; and combined: RR =0.84, 95% CI: 0.41–1.73 [Figure 3D]), analysis for total treatment failures revealed a statistically significant difference in favor of CRT (RR =0.77, 95% CI: 0.67–0.89; Figure 3A).
Survival outcomes
As shown in Figure 4A, there were eight studies12,15,16,22–25,28 evaluating the OS, and no significant heterogeneity (I2=0.0%, P=0.636) was found among these studies. The pooled analysis with a fixed-effects model showed a statistically significant difference in favor of CRT group over RT group (HR =0.68, 95% CI: 0.57–0.80). Although several RCTs with large sample size were designed to evaluate the OS after 2002, cumulative meta-analysis indicated that stable pooled effect was already established with the three earliest RCTs12,15,16 (a total of 618 patients were involved) published in 1997, 2000, and 2002 (interim HR =0.68, 95% CI: 0.48–0.88), and further cumulative analyses with subsequent trials added resulted only in slightly changed point estimates and continuously narrowed 95% CIs (Figure 4B).
Similar results were observed for PFS. Minor heterogeneity among the included six studies12,15,16,22–24 was observed (I2=21.4%, P=0.273). Our meta-analysis demonstrated significantly better PFS in the CRT group (HR =0.63, 95% CI: 0.50–0.76; Figure 5A). The cumulative meta-analysis of three RCTs12,15,16 conducted before 2002 revealed an apparently better outcome of CRT group (interim HR =0.65, 95% CI: 0.47–0.84). Further cumulative analyses with subsequent trials added resulted only in narrowed 95% CIs and slightly changed pooled effect size (Figure 5B).
Adverse effects
To evaluate the adverse effects, the treatment-related Grade 3 and 4 adverse events were chosen as the effect size index, and totally five trials12,15,18,23,25 with no significant heterogeneity (I2=0.0%, P=0.79) were included. Among them, only one study15 provided the number of patients with Grade 4 toxicity. Our pooled analysis with a fixed-effects model showed significantly enhanced toxicity in the CRT group compared with the RT group (OR =3.13, 95% CI: 2.37–4.13; Figure 6).
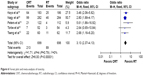  | Figure 6 Forest plot and meta-analysis of toxicity. |
Sensitivity analysis and metaregression analysis
Sensitivity analysis was performed to verify the reliability and stability of the evidence when significant heterogeneity was detected. In this study, high heterogeneity was detected for the tumor response outcome, and sensitivity analysis by excluding certain study did not significantly change the result. Because of the limited number of trials included for tumor response evaluation and potential inconsistency in study characteristics, such as study design, sample size, and CCRT and RT regimen, we did not perform any subgroup analysis to further explore the source of heterogeneity. For survival outcomes, several subgroup analyses were performed (Table 3). In terms of OS, important inconsistency was detected for subcomparisons stratified by region and study design, showing that no significant pooled difference existed between DDP-CCRT and RT in studies conducted in Asia or studies with non-RCT design (HR =0.89, 95% CI: 0.46–1.32, and HR =0.92, 95% CI: 0.43–1.40, respectively). However, in terms of PFS, no subgroup analysis showed interesting results. The metaregression analysis was only feasible for OS, and the L’Abbé graph adjusted by age showed an overall trend that with the increasing age, patients’ survival was shortened more frequently in the CRT group than the RT group (Figure 7).
Publication bias
The funnel plots of included articles were symmetrical, indicating the absence of publication bias, which was later confirmed by Begg’s test (P=0.376; Figure 8).
  | Figure 8 Funnel plot of studies reporting OS. |
Discussion
After the results of five pivotal trials were published in 1999,11,14,15,17,18 with an alert issued by the National Cancer Institute recommending chemoradiation instead of radiation alone for the treatment of CC, DDP-CCRT has been extensively applied and investigated in clinical practice.36 A meta-analysis published in 2002 summarized the comparison between cisplatin-based CRT and RT; however, several important problems exist in this very study.20 First, for OS comparisons, the RR of each study was calculated with the number of events by the end of the research without considering the time-to-event information; thus, the results of the combined analyses were not as reliable as those using HR as effect size index. Second, apparent inconsistency existed among the control group settings of each study. Although major heterogeneity was not detected, this inconsistency may possibly introduce certain confounding effects into the results of pooled analyses; moreover, from our perspective, studies comparing DDP-CCRT with RT + HU should be considered comparisons between two different types of CRT rather than comparisons between DDP-CCRT and exclusive RT, and therefore should not be included for meta-analysis comparing DDP-CCRT with exclusive RT. On the other hand, including such studies into metacomparisons between DDP-CCRT and exclusive RT would preclude a reasonable interpretation for the pooled data. Third, the quality of original studies and risk of bias was not assessed. And finally, statistical analysis was not performed for data on toxicity. After the publication of this 2002 meta-analysis, updated data with different results addressing this issue are available for an updated evaluation. In 2010, the Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC) published an individual patient data (IPD) meta-analysis to reduce uncertainties about the effects of CCRT for CC; the results of which supported the superiority of platinum-based (OS: HR =0.83, P=0.017) and nonplatinum-based chemoradiotherapy (OS: HR =0.77, P=0.009) over RT.37 In this IPD meta-analysis, pooled analyses were conducted for studies investigating CCRT versus the same RT (13 trials), and toxicity comparisons were conducted. Although this CCCMAC work used IPD from single studies (published and unpublished) to obtain more reliable results, certain interesting points should be noted. For example, the latest study included in this IPD study was finished in 2005; however, according to the results of our search strategy, additional data from at least two studies published during 2005–2010 with 737 patients were potentially available for combined analysis.25,28 In addition, as to studies finished before 2005, at least one study with 122 patients was potentially useful for combined analysis.12 Therefore, it is very likely that potentially available data, especially later ones, were not fully utilized in this IPD study. Why were certain potentially eligible studies not included in this IPD study? A possible reason was that the CCCMAC was unable to obtain IPD for those studies. Of course, IPD meta-analysis is a proposed method to perform meta-analyses, especially for those investigating time-to-event end points. However, obtaining complete original data of individual patients is never easy and always time consuming, and so cannot be widely performed by researchers. In addition, statistical methods and tools for IPD meta-analysis were more complicated than those of traditional meta-analysis. If certain data (especially recent ones) available for traditional meta-analysis could not be used for IPD due to methodological barriers, the results of IPD may be no more reliable and no superior than traditional meta-analysis.
Therefore, noting the limitations in these two aforementioned meta-analyses, we performed this study with more recent data and refined the study design to make a complementary contribution to the evidence-based medicine on CC treatment. The beneficial effects of cisplatin, which is the most widely used agent for CCRT in CC patients, whether alone or in combination with other drugs, were not independently reported in the CCCMAC study.37 Our study focused on the comparisons between DDP-CCRT and exclusive RT to form a definitive conclusion for this issue. Moreover, only patients with high-risk disease, such as locally advanced cancer or bulky tumor, were investigated in this study. To the best of our knowledge, this is the first metacomparison for DDP-CCRT versus exclusive RT focusing on this specific subgroup of patients with CC. Furthermore, for the first time, cumulative meta-analyses addressing this topic were performed in our study, with stable and reliable results shown in the cumulative processing for survival outcomes.
In this study, combined analysis for the early efficacy (tumor response) was for the first time performed, and the results revealed major heterogeneity, which could not be successfully interpreted by sensitivity analysis and subgroup analysis. Since only four studies were included for analysis concerning CR12,25–27 as well as CR + PR,12,23,26,27 the heterogeneity detected could be attributed to relatively limited sample size. Obviously, the end point of tumor response was not as fully investigated as survival outcomes in previously published trials. Tumor response definitions based on the World Health Organization criteria were adopted in two studies, and the tumor response was evaluated by physical examination and tumor size measured with imaging techniques.12,27 In Nagy et al’s25 report, the tumor response was evaluated pathologically. Evaluation criteria were not clarified in two studies; however, it could be inferred that the tumor response was evaluated clinically.23,26 Different evaluation criteria may have contributed to the heterogeneity. Only Ke et al26 reported a significantly better early efficacy in the DDP-CCRT group, and the pooled effects demonstrated no significant differences between CRT and RT for CR and CR + PR both. Because only 28 patients were enrolled in Ke et al’s26 study and the new agent Taxotere® was combined with DDP, the particular finding revealed in Ke et al’s26 study needs further investigation.
As to treatment failures, significant difference in favor of DDP-CCRT was detected exclusively for the total failure. The result for total treatment failure was consistent with the result for PFS, and both supported the superiority of DDP-CCRT. Interestingly, on the other hand, negative results were observed for all the three subtotal comparisons, ie, for local failure, distant failure, and combined failure. Of course, local recurrence-free survival and distant recurrence-free survival could be better indices to reflect the status of disease control; however, since few data on these two indices were available, regrettably a meta-analysis could not be performed. Further investigation addressing posttreatment local and distant relapsed disease is needed.
Significantly improved OS and PFS in DDP-CCRT-treated patients were demonstrated in our pooled analysis. Moreover, solid and stable beneficial effects were established through cumulative meta-analyses for the first time in our study. The pooled HR and 95% CI for OS and PFS were 0.68 (0.57–0.80) and 0.63 (0.50–0.76), respectively; according to the results of our cumulative meta-analyses, OS and PFS data from additional trials will only result in minimally changed HR and narrowed 95% CI. In the 2010 IPD meta-analysis, the pooled HR and 95% CI of OS were 0.84 (0.72–0.98) and 0.76 (0.62–0.94), respectively, for platinum-based CCRT and nonplatinum-based CCRT against control, and the pooled HR and 95% CI of overall PFS were 0.78 (0.70–0.87) for CCRT against RT. Comparing these statistics, it is not unreasonable to speculate that the DDP may have better efficacy than other agents. Of course, this speculation should be further verified in future trials. Subgroup analyses were conducted for OS. According to analysis stratified by region, no significant difference of pooled OS between DDP-CCRT and RT was found for studies performed in Asian countries. Because only two studies with a total of 293 patients were categorized into this subgroup, including one RCT and one cohort trial both reporting negative results,12,28 the pooled result may not be solid. However, interestingly, in the report of Han and Kong,27 although survival curve and data on HR were not provided, no significant differences were found for the 3-year OS rate and 5-year OS rate between DDP-CCRT and RT. Moreover, in the CCCMAC IPD meta-analysis, only two Asian studies38,39 concerning platinum-based CCRT with a total of 169 patients were included, both with negative OS results.37 It is very interesting to note that almost all the Asian studies reported negative results. The racial difference could be an important underlying factor contributing to the evident inconsistency between the results of Asian studies and those of Western studies, and additional studies with emphasis on this issue are in need.
Limitations
First, concealment and blinding methods were not reported in most of the included RCTs. In fact, for the comparisons between DDP-CCRT and RT, concealment and blinding was almost impossible. Second, limited data on early efficacy, treatment failure, and toxicity profile were available for meta-analysis; therefore, to obtain more reliable results, further evaluation with additional data is necessary. Third, the value of HR and 95% CI for survival outcomes was not reported in certain studies; calculated statistics using time-to-event survival data or survival curves were utilized for meta-analysis. Although the statistic methods for these calculations are already established, minor deviations were inevitable during the process. However, according to our cumulative meta-analysis, the influence of these deviations was of minor importance.
Conclusion
To summarize, although high-risk CC patients form a heterogeneous population, solid and stable beneficial effects associated with DDP-CCRT compared with comparative RT alone in this special subgroup are further confirmed in our meta-analysis. For treatment decisions, CC patients with high-risk characteristics (eg, bulky tumor, deeply invasive disease, advanced disease, etc) could be considered an entity that would benefit from DDP-CCRT, which should be considered one of the frontline treatment options for high-risk CC patients without contraindications. On the other hand, enhanced toxicity associated with DDP-CCRT should never be ignored. According to the results of our cumulative meta-analyses, further comparisons on OS and PFS between DDP-CCRT and RT are of minor importance. Investigations on tumor response, survival comparisons stratified by tumor response, large RCTs on potential racial difference, and intercomparisons between different CCRT regimens could be subjects for future studies.
Disclosure
The authors report no conflicts of interests in this work.
References
Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49(15): 3262–3273. | ||
Patra S, Panda D. Cervical cancer screening in developing countries. Indian J Cancer. 2010;47(3):344–345. | ||
Haar C, Swift S. The prevention of cervical cancer in developing nations. J S C Med Assoc. 2013;109(4):132–134. | ||
Perez CA, Grigsby PW, Nene SM, et al. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer. 1992;69(11):2796–2806. | ||
Kudaka W, Nagai Y, Toita T, et al. Long-term results and prognostic factors in patients with stage III-IVA squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy from a single institution study. Int J Clin Oncol. 2013;18(5):916–921. | ||
Huang YT, Wang CC, Tsai CS, et al. Long-term outcome and prognostic factors for adenocarcinoma/adenosquamous carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80(2):429–436. | ||
Fuller AF Jr, Elliott N, Kosloff C, Hoskins WJ, Lewis JL Jr. Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage IB and IIA carcinoma of the cervix. Gynecol Oncol. 1989;33(1):34–39. | ||
Eralp Y, Saip P, Sakar B, et al. Prognostic factors and survival in patients with metastatic or recurrent carcinoma of the uterine cervix. Int J Gynecol Cancer. 2003;13(4):497–504. | ||
Wolfson AH. Conventional radiation therapy of cervical cancer. Semin Surg Oncol. 1999;16(3):242–246. | ||
Saibishkumar EP, Patel FD, Sharma SC, Karunanidhi G, Sankar AS, Mallick I. Results of external-beam radiotherapy alone in invasive cancer of the uterine cervix: a retrospective analysis. Clin Oncol. 2006;18(1):46–51. | ||
Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17(5):1339–1348. | ||
Tseng CJ, Chang CT, Lai CH, et al. A randomized trial of concurrent chemoradiotherapy versus radiotherapy in advanced carcinoma of the uterine cervix. Gynecol Oncol. 1997;66(1):52–58. | ||
Thomas G, Dembo A, Ackerman I, et al. A randomized trial of standard versus partially hyperfractionated radiation with or without concurrent 5-fluorouracil in locally advanced cervical cancer. Gynecol Oncol. 1998;69(2):137–145. | ||
Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–1153. | ||
Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–1613. | ||
Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20(4):966–972. | ||
Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137–1143. | ||
Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154–1161. | ||
Dunst J, Sauer R. Simultaneous radiochemotherapy with cisplatin improves survival in cervical cancer. Strahlenther Onkol. 1999;175(6):257–258. German. | ||
Lukka H, Hirte H, Fyles A, et al. Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer – a meta-analysis. Clin Oncol. 2002;14(3):203–212. | ||
Dunst J, Haensgen G. Simultaneous radiochemotherapy in cervical cancer: recommendations for chemotherapy. Strahlenther Onkol. 2001;177(12):635–640. German. | ||
Zuliani AC, Esteves SC, Teixeira LC, Teixeira JC, de Souza GA, Sarian LO. Concomitant cisplatin plus radiotherapy and high-dose-rate brachytherapy versus radiotherapy alone for stage IIIB epidermoid cervical cancer: a randomized controlled trial. J Clin Oncol. 2014;32(6):542–547. | ||
Torbe B, Falco M, Torbe A, Ciepiela P, Kurzawa R. Radiotherapy versus radiochemotherapy with cisplatin in treatment of cervical cancer. Med Oncol. 2010;27(1):1–8. | ||
Stehman FB, Ali S, Keys HM, et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197(5):503.e501–e506. | ||
Nagy V, Coza O, Ordeanu C, et al. [Radiotherapy versus concurrent 5-day cisplatin and radiotherapy in locally advanced cervical carcinoma. Long-term results of a phase III randomized trial]. Strahlenther Onkol. 2009;185(3):177–183. German. | ||
Ke QH, Zhou SQ, Du W, et al. Early efficacy of taxotere and cisplatin chemo-radiotherapy for advanced cervical cancer. Asian Pac J Cancer Prev. 2012;13(2):617–619. | ||
Han C, Kong WM. [Retrospective study of chemoradiotherapy based on cisplatin compared with radiotherapy alone for cervical cancer]. Zhonghua Fu Chan Ke Za Zhi. 2007;42(11):723–726. Chinese. | ||
Chen SW, Liang JA, Hung YC, et al. Concurrent weekly cisplatin plus external beam radiotherapy and high-dose rate brachytherapy for advanced cervical cancer: a control cohort comparison with radiation alone on treatment outcome and complications. Int J Radiat Oncol Biol Phys. 2006;66(5):1370–1377. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. | ||
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. | ||
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. | ||
Higgins JPT AD, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. Accessed February 22, 2016. | ||
Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. | ||
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. | ||
Thomas GM. Improved treatment for cervical cancer – concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340(15):1198–1200. | ||
Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev. 2010;1:Cd008285. | ||
Chen HW, Jei J, Wei L. A randomized trial of hyperthermo-radiochemotherapy for uterine cancer. Chinese Journal of Oncology. 1997;24:249–251. | ||
Onishi H, Yagamushi M, Kuriyama K, et al. Effect of concurrent intra-arterial infusion of platinum drugs for patients with stage III of IV uterine cervical cancer treated with radiotherapy. Cancer Journal of Scientific American. 2000;6:40–45. |
Supplementary material
  | Figure S1 Risk of bias assessment. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







